Abstract
The global prevalence of childhood obesity highlights an urgent need to address its associated health complications. Cardiometabolic indicators, closely linked with obesity, can pose severe health risks, emphasizing the need for effective interventions. Among these, physical activity has shown many health benefits. However, a comprehensive understanding of the relationship between physical activity and cardiometabolic indicators in obese children remains somewhat unclear. This integrative review aims to fill this knowledge gap by critically examining relevant research over the past decade, thereby providing insights into evidence-based strategies to improve health outcomes in this vulnerable population. We conducted an integrative literature review of articles published between 2012 and 2022, retrieved from databases such as PubMed, Web of Science, Scopus, and EBSCO. Our focus was limited to Polish and English-language research with full text availability. We deployed keywords such as “physical activity”, “children”, “cardiometabolic indicators” and “BMI” linked using Boolean operators “and” and “or”. Methodological quality was independently assessed by two authors, and Rayyan software was utilized for review compilation. Out of the assessed articles, 55 met the inclusion criteria. The majority centered around programs and interventions targeting children, examining their impact on body composition, alterations in body fat content, waist circumference, body mass index, blood pressure, heart rate, lipoprotein, triglycerides, total cholesterol, glucose, and insulin levels. Interventions focusing on increasing physical activity and reducing sedentary behavior demonstrate positive effects on body composition, aerobic capacity, and select biochemical markers in children. This underscores the potential of physical activity as a valuable tool in managing obesity-related health risks among children.
Keywords: Obesity, Pediatric Obesity, Cardiometabolic Risk Factors, Activities of Daily Living, Body Mass 0Index
Background
Physical activity is seen as one of the key factors in the prevention of overweight and obesity and in the reduction of cardiometabolic risk in children and adolescents. Good physical fitness in childhood brings health benefits, especially cardiovascular and respiratory. According to literature, overweight and obesity in childhood also correlate with low levels of physical fitness [1]. Furthermore, higher levels of cardiometabolic activity significantly reduce the cardiometabolic risk in adult life, even among people with abdominal obesity that appeared during childhood [2].
Physical Activity in Children
The World Health Organization (WHO) defines physical activity as any movement of the body produced by skeletal muscles that requires energy expenditure [3]. Physical activity can be undertaken in many different ways: walking, cycling, sports, and active forms of recreation (eg, dancing, yoga, tai chi). Physical activity can also be undertaken at work and at home. All forms of physical activity can have health benefits if taken regularly, with appropriate duration and intensity [4]. Both moderate and intense physical activity improves health outcomes [3]. In children and adolescents, physical activity improves physical performance (cardiorespiratory and muscle efficiency), cardiometabolic health (blood pressure, dyslipidemia, glucose and insulin resistance), bone health, cognitive performance (learning outcomes, executive functions), and mental health (reduction of depression symptoms), and it reduces the risk of obesity [3]. It has been revealed that participation in organized sports improves cardiovascular performance and can reduce the risk of overweight/obesity and cardiovascular diseases [5].
Broad international data showed that 80% of adolescents do not achieve the recommended levels of physical activity [6]. Worldwide, 3 in 4 adolescents (11–17 years old) do not currently meet the WHO global physical activity guidelines [4]. Adolescent girls were less active than adolescent boys, although 85% vs 78%, respectively, did not meet WHO recommendations regarding at least 60 min of moderate or intense physical activity a day [3]. Physical activity is common in childhood but has a tendency to decrease with age [7]. There is strong evidence that high levels of physical activity, cardiovascular and muscle performance, and decreased time spent on sitting activities (eg, watching TV) are associated with a lower risk of cardiovascular diseases in youth [8,9] (Table 1).
Table 1.
The guidelines of the World Health Organization regarding the activation of selected groups of children for their overall wellbeing [3,10].
| Infants below 1 year (within 24 hours) |
|
| Children aged 1–2 years (within 24 hours) |
|
| Children aged 3–4 years (within 24 hours) |
|
| Children and adolescents aged 5–17 years, including children and adolescents with disabilities |
|
Team sports seem to be a good alternative to increasing youth participation in physical activity. Children are more interested in outdoor sports activities that are sociable and involve competition [11]. Participation in organized sports is in particular linked to improved mental health, reduced risk-taking behavior, and improved cardiovascular performance, and it can reduce the risk of overweight/obesity and cardiovascular diseases [5]. Regular physical activity has a positive effect on the body composition by increasing the loss of fat tissue, thus contributing to the maintenance of or increase in lean muscle mass [12]. Sedentary lifestyles, which are increasing among children, together with low physical activity and prolonged screen time, are among the major health threats to the pediatric population in developing and developed countries [7]. In various studies, relationships between physical activity and feeling no need to engage in it and cardiometabolic risk factors were identified [13]. What is more, evidence suggests that the ubiquitous lack of movement is associated with the following adverse health effects in children and adolescents: increased obesity (weight gain), decreased cardiometabolic health, physical fitness, and behavioral and prosocial behavior, and insufficient sleep [3]. Lifestyle changes, including public interventions, are needed to improve that situation. Future actions should focus on eliminating sedentary behaviors in children and encouraging physical activity from early life [13].
Physical Activity in Children and Cardiometabolic Indicators
Behaviors associated with physical activity are perceived as one of the key factors in the prevention of overweight and obesity and for the reduction of cardiometabolic risk [14]. Several mechanisms have been proposed to mediate the protective effect of physical activity and physical fitness on cardiometabolic risk, namely, anti-inflammatory effects, increased insulin sensitivity, higher glucose uptake independent of insulin, an improved lipid profile, and the function of hormones and enzymes involved in fat metabolism [15]. The lack of physical activity involves lower aerobic capacity and mental health, reduced glucose tolerance, higher fasting insulin levels, and increased risk factors for cardiovascular diseases [16]. Moderate or intense activity is associated with high cardiopulmonary capacity, a healthier lipid profile [17] and blood pressure, and lower insulin resistance [18]. It also affects hemodynamic variables along with psychological and socio-affective aspects [19]. High-intensity physical activity has a positive effect on metabolic profiles regardless of weight loss and energy expenditure [6]. It is suggested that exercise reduces blood pressure and serum glucose levels [17]. It can improve insulin sensitivity, functions of vascular endothelium, glycemic control, and blood pressure [20]. Also, exercise contributes to a reduced risk of non-alcoholic fatty liver disease and facilitate improvements in the event of chronic inflammation [21]. Regular exercise has a significant impact on cardiovascular capacity in people with type 2 diabetes. It reduces insulin resistance, and thus improves glycemic control and decreases cardiovascular risk factors [22]. In their meta-analysis, Ho et al point out that physical activity interventions and combined interventions (diet and exercise) can lead to weight loss and improved metabolism in the pediatric population with obesity or overweight. Combined interventions can determine greater improvements in high-density lipoprotein cholesterol (HDL-C), fasting glucose, and insulin levels within 6 months [21]. Researchers who have implemented those interventions in adolescents with overweight or obesity have reported an improvement in body composition, along with a decrease in systolic blood pressure (SBP), alanine transaminase, glucose, homeostasis model assessment of insulin resistance (HOMA-IR), glycated hemoglobin (HbA1c), total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglycerides, and an increase in HDL-C [23].
Childhood Obesity
With reference to the literature, obesity is not just the result of reversible poor eating habits, lack of movement, or other personal choices, although these undoubtedly effectively worsen this condition. Obesity is a chronic disease that results in changes in human anatomy, physiology, and metabolism [24,25]. The Obesity Medicine Association defines obesity as “a chronic, recurrent, multi-factor neurobehavioral disease in which adipose tissue growth promotes adipose tissue dysfunction and abnormal body fat mass resulting in adverse metabolic, biomechanical, and psychosocial (stigmatization) health consequences” [25,26]. The main factor is food, especially foods such as fat or sugar-sweetened drinks. An abundance of food, low physical activity, and several other environmental factors interact with the genetic susceptibility of humans to produce a positive energy balance. Most of this excess energy is stored as fat in enlarged and often more numerous fat cells, but some lipids can infiltrate other organs, such as the liver (ectopic fat). The formation of obesity is influenced by the interaction of genetics, epigenetics, metagenomics, and the environment [27]. The traditional treatment of obesity as a result of excessive or high-calorie consumption is insufficient [28]. The omission or depreciation of the role of the gastrointestinal tract in the etiology of obesity, which in turn is influenced by many factors, such as digestive enzymes, bile acids, microflora, intestinal hormones, and neural signals, none of which is under voluntary control, is a fairly common mistake [29]. Energy consumption through physical activity has been found to be relatively low [30]. Chamorro et al [31] point to circadian diseases and lack of sleep, and Claes et al [32] point to mental stress as contributing factors to obesity. Therefore, overeating and reduced physical activity may be symptoms rather than primary causes of obesity [33]. The complex causes of obesity are associated with lower biases related to weight disorders and less guilt [26]. Enlarged fat cells and ectopic fat produce and secrete a variety of metabolic, hormonal, and inflammatory products that cause damage to organs, such as the arteries, heart, liver, muscles, and pancreas [34].
There are significant individual differences in body weight and body fat within each environment, suggesting that obesity is influenced by complex interactions between genetic, developmental, behavioral, and environmental influences [27]. The prevalence of childhood obesity is increasing worldwide and is currently a global public health problem [35–37]. In 2020, a total of 39 million children under the age of 5 had overweight or obesity [38]. The problem of overweight and obesity also includes adolescence and adult life, causing a number of health problems, which are increasing with age [39]. Persons with obesity face not only increased risk of serious medical complications but also a pervasive and resilient form of social stigma. This phenomenon can cause significant harm to affected individuals, including physical and psychological results [26]. Childhood and adolescence are both critical moments, not only for the formation of habits in adult life. Behavior during this period is also associated with medical conditions at a later age [40]. Evidence suggests that obesity in children can persist throughout the whole life, reducing the quality of life and its expected length [19]. Preventing obesity and forming healthy habits at a young age is crucial for establishing long-term healthy behaviors. The acquisition of habits of a healthy lifestyle in childhood depends both on the individual and his or her socio-psychological environment [41].
Obesity in Children and Cardiometabolic Markers
Childhood obesity is associated with co-existing physical and mental diseases, including adverse cardiometabolic effects, such as high blood pressure, dyslipidemia, and insulin resistance [42,43]. Obesity is strongly connected with elevated concentrations of circulating inflammatory markers, such as C-reactive protein (CRP) [10]. Studies have shown that children and adolescents with low levels of cardiopulmonary capacity who have overweight or obesity have an increased risk of developing cardiovascular diseases or metabolic syndrome during puberty or in adulthood [1,14,44,45]. Children with overweight or obesity are more likely to develop cardiovascular problems in the form of type 2 diabetes, hypertension, myocardial infarction, coronary heart disease, dyslipidemia, and stroke [35,36,46]. Increased overweight and obesity is associated with hepatic steatosis, a high level of cholesterol, glucose intolerance, insulin resistance, interference with the menstrual cycle, and balance disorders [23,41,47]. Children with obesity are exposed to harmful short- and long-term health effects, thus showing metabolic syndrome components, such as changes in the lipid profile of the plasma [17], hypertension, insulin resistance, and impaired glucose metabolism [11,12,17,21]. Furthermore, their vascular structures deteriorate, which contributes to an adverse reconstruction of the heart, resulting in an abnormal cardiovascular response [41]. Damage to the endothelium, which leads to atherosclerosis, can develop during puberty [45]. Early endothelial dysfunction has been reported in children and adolescents with obesity, with a significantly greater thickness of the carotid median membrane than in persons with normal body mass [46].
A sedentary lifestyle with other factors can cause the accumulation of adipose tissue [18]. A high percentage of visceral fat in the abdominal cavity has been shown to exacerbate hyperlipidemia and hypertension, and it considerably contributes to insulin resistance [35]. An extensive capillary network surrounding the fat tissue affects strain on the heart by increasing the blood volume and cardiac output [35]. When adipose tissue gathers in excessive amounts in the body, several metabolic changes begin to occur [15]. Research has shown that children with obesity are more likely to develop adverse lipid and glycemic profiles than are other children. It is additionally associated with an increase in fatty streaks in the endothelium of arteries in children, which can lead to the development of atherosclerotic plaques in adulthood [15]. Adipose tissue is a source of interleu-kin-6, which results in the formation of a chronic inflammation that can trigger acute coronary syndrome, as well as a source of lipoprotein lipase, estrogen, angiotensinogen, adiponectin, leptin, insulin-3 binding protein, and tumor necrosis factor α1. Fat tissue produces and secretes peptides and proteins called adipocytokines, which are involved in inflammation and immune responses [19]. In the case of obesity, the concentrations of different adipocytokines are high and are associated with hypertension (angiotensinogen), inhibition of fibrinolysis (plasminogen activator inhibitor-1), insulin resistance (tumor necrosis factor-α, interleukin-6, and resistine), and the onset or progression of atherosclerotic lesions (CRP). Adiponectin, which has anti-inflammatory and anti-atherosclerotic properties, is inversely proportional to the body mass index (BMI) and the percentage of adipose tissue [19]. Leptin, a hormone secreted by fat tissue, inhibits food intake, stimulating energy expenditure [11].
The main purpose of this review was to determine the existing relationship between physical activity, its forms, and selected cardiovascular indicators among children with obesity. This study includes publications from the last 10 years, which makes the accumulated knowledge current and helps the understanding of the research problem.
In this study, we assumed that the state of health among children perceived through the prism of measuring and analyzing cardiometabolic indicators is associated with various forms of physical activity with varying intensity and duration. We also state that the moderator of this compound is the BMI indicators and how the undertaken physical activity affects them, allowing noticeable changes in, among others, cholesterol and glucose levels. In addition, types of physical activity have a diverse impact on BMI.
The problem of childhood obesity is constantly increasing. It is important to try to counteract this. Therefore, the present study is based on an analysis of current studies that indicate the impact of activities among children with normal body weight and obesity on their cardiovascular health, as well as body weight, BMI, and biochemical parameters of the blood. To increase the understanding of childhood obesity and its importance in the relationship between physical activity and cardiometabolic indicators, the conceptual framework that guided the study is presented in Figure 1.
Figure 1.
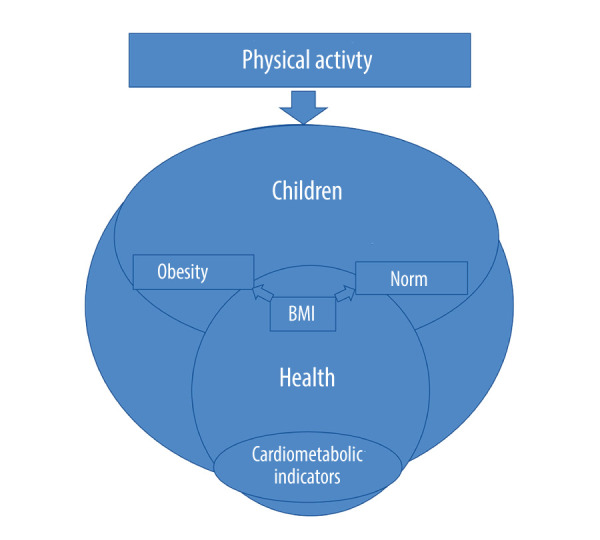
Conceptual research model (author’s elaboration).
The initial information forms the basis for the next parts of the article, in which the integration review method, detailed rules for its implementation, characterized results of tests qualified for basic analysis, as well as discussion, conclusions, limitations and gaps, broader context, and recommendations for future research will be presented.
Material and Methods
To comprehensively understand the analyzed problem, an integrative review was conducted. The integrative review method allows for combining data from both theoretical and empirical literature, and takes into account the results obtained during studies based on various research projects [48–50]. The use of different data sources enable us to specifying current knowledge on a certain subject and contribute to the holistic understanding of the research subject [50] through identifying, analyzing, and synthesizing the results of independent studies [49].
The review was based on an integrative review framework developed by Whittemore and Knafl [48], made up of 5 stages: problem identification, literature search, data evaluation, data analysis, and the presentation of results [48]. The integrative review begins with a description of the problem, which is addressed in the review, as well as with a definition of the purpose of the review [48]. At this stage, it is important to consider the concept, target population, healthcare problem, and types of empirical research. In the next stage, the literature necessary for a deeper understanding of the problem was searched, having regard for a variety of bibliographical databases and the table of contents review of journals containing publications about a given subject [48]. Subsequently, data obtained from several publications and literature were evaluated in terms of methodological quality. Whittemore and Knafl propose the coding of sources in terms of methodological or theoretical rigor, along with the usefulness of data on a 2-point scale (high or low) [48]. The findings relevant for the review should be distinguished from the reports obtained at a later stage. The results are compared and synthesized, whereas at the final stage they are presented with the conclusions. To draw conclusions, the results should be synthesized. The conclusions of the integration reviews can be presented in the form of a table or scheme. By providing clear evidence to support the conclusions, a logical chain of evidence is created, which allows the reader to make sure that the conclusions of the review do not go beyond evidence [48].
The quality assessment was carried out by the lead author, and the results were discussed with two other investigators who had experience with the review methodology. Discrepancies between authors were resolved during discussions. The summary and content of the article were re-analyzed, so that the authors could exchange comments with each other. Critical evaluation of the studies considered issues such as the compatibility of the study design with its research objective, the risk of bias, if applicable, the quality of reporting, generalizability, and possible repeatability. The different types of studies required the application of separate criteria for each of them. Critically evaluated studies were classified as strong and moderately strong. There were no studies that were assessed as weak. The review mainly qualified articles with significant or moderate methodological quality; however, several works with lower percentages (50–58%) were separated. The authors of the review thus wanted to ensure high reliability of the results and final conclusions.
Electronic databases, including PubMed, Scopus, Web of Science and EBSCO, were searched in accordance with the guidelines of Preferred Reporting Items for Systematic Review and Meta Analyses (PRISMA). During searching for keywords, there should be a balance between a search that will be comprehensive enough to cover everything about the theme defined in the research question, and precise enough to capture only these results that are particularly important. The presented project used Medical Subject Headings (MeSH), which is a thesaurus of the controlled vocabulary of the National Library of Medicine, used to index articles in the MEDLINE®/PubMed MeSH database. The search used a combination of the MeSH terms (or equivalents) and the following keywords: “physical activity”, “children”, “BMI”, and “cardiometabolic indicators”, which were connected by the Boolean operators “and” and “or”. The criteria for the inclusion and exclusion of articles are shown in Table 2.
Table 2.
Criteria for the inclusion and exclusion of research articles in the analysis.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
We used Rayyan software to review and select the publications [51], which is a tool designed for conducting literature reviews. Articles were verified independently by two authors (MG & AF), and any discrepancies were discussed. The selection of articles for the analysis lasted a total of 2747 min (110 sessions). During the review, various types of research were identified, such as randomized control studies, quasi-experimental studies, case series, and cohort studies; hence, the we assessed the methodological quality by using the following check-lists: Critical Appraisal of a Meta-analysis or Systematic Review (Center of Evidence-Based Management, CEBMa), Critical Appraisal of a Cross-Sectional Study (survey, CEBMa), Critical Appraisal of a Cohort or Panel Study (CEBMa), Randomized Controlled Trial Standard Checklist (Critical Appraisal Skills Programme, Critical Appraisal Check-list for Quasi-Experimental Studies (non-randomized experimental studies) (Joanna Briggs Institute), and Critical Appraisal Checklist for Case Series (Joanna Briggs Institute). Based on the points obtained from individual check-lists, methodological quality was presented in the form of a percentage assessment. The selection of checklists was guided by the theoretical assumptions of David Sackett as the basis for their preparation, along with the experience of the team of the developing institution (ie, Oxford CEBM, Joanna Briggs Institute), clarity of detailed criteria, and their adequacy to the type of scientific evidence assessed. Furthermore, the option of free access to check-lists was recognized as a criterion.
We prepared special timetable to show the process of making an integrative review (each stage has a date assigned; Figure 2).
Figure 2.
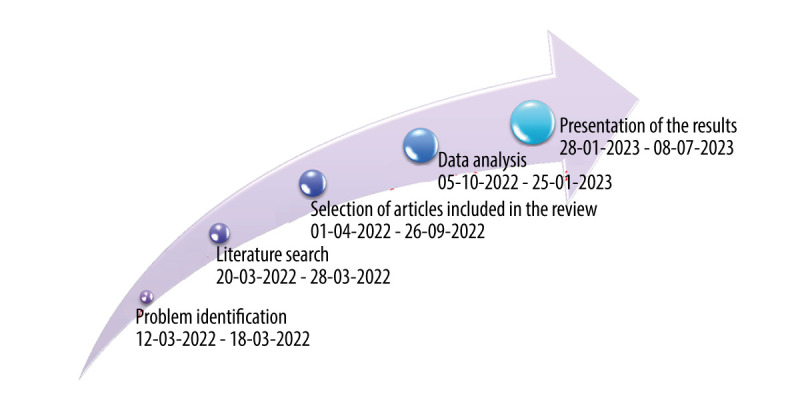
Timeline of each stage of an integrative review (author’s elaboration).
The assessment of methodological quality was determined using recognised checklist criteria, to help to determine the confidence with which the study results could be interpreted in the synthesis of this review. The minimum threshold to qualify a publication for further analysis was 50% of the possible points, although most of the qualified publications are studies of higher methodological quality. It should be noted, however, that these figures are intended only to illustrate the comparative quality of the articles, recognizing that items do not necessarily carry equal weight and are prone to subjectivity. In summary, strengths of all articles included clear aims and rationale for the research, rigorous analysis of the data appropriate to its quality, often involving multiple analysts, and discussion of results based on empirical findings. A major weakness of the studies was the underrepresentation of designs with a blinded control group.
Assessment of the reliability of the sources was made during analysis of the full texts of the articles, particularly when there were discrepancies in their assessment (MG, AF). In determining the final consensus on the eligibility of publications for analysis, the following were considered: the authors’ qualifications to present the information, whether the source of the data was peer-reviewed, and whether the information was subject to risk of error.
Results
A preliminary search yielded 1874 records, 856 of which were duplicates. The results came from 4 databases: PubMed (253 articles), Scopus (476 articles), Web of Science (552 articles), and EBSCO (593 articles). The database of articles was not complemented with manual searches. After further analysis of 1018 records, 673 summaries and articles were excluded, including 514 irrelevant articles, as well as 103 articles that were removed because of wrong research group, 44 articles due to the lack of full text, and 12 articles owing to more than 10 years from publication. Authors during the first selection were based on titles and abstracts. Moreover, at a subsequent stage, whole articles were read. Finally, the subject of the analysis was a total of 345 articles, and 55 articles were included in the review. The schedule for the selection of records and articles according to PRISMA [52] is shown in Figure 3.
Figure 3.
Schedule for the selection of records and works according to PRISMA.
Among the qualified articles, 20 concerned cross-sectional studies; 10 were clinical trials; 6, randomized clinical trials (RCT); 3, multicenter clinical trials; 5, cohort studies; 3, systematic reviews; 3, meta-analyses; 2, longitudinal studies; 1, a quasi-experimental study; 1, a pilot study; 1, a feasibility study; 1, a latent profile analysis; and 1, a comparative study.
The type of study has an important impact on the results and their interpretation. Among the primary studies, the most valuable results were those from a RCT, which demonstrate the effect of an experimental agent under controlled conditions over a specified period of time. Of the studies selected for review, 19 out of 55 are based on RCT methodology. The study of processes (eg, of illness or recovery) was possible from a prospective perspective, which was ensured by cohort studies, longitudinal studies, which account for 5 out of 55 papers analyzed. Retrospective studies based on the recollection of circumstances and events by the respondents have a higher risk of error, accounting for 2 studies out of 55 analyzed. Cross-sectional studies make it possible to assess the state of affairs at a given point in time and, depending on the methods used to evaluate variables (objective, subjective) and the methods used to qualify people for the study, the results are of varying value. A number of cross-sectional surveys can be used to make inferences about a process, but this is not the same as continuous surveys. This type of outcome dominates (20 out of 55) in this integrative review. The results of studies based on the opinions of respondents require very careful interpretations.
The results of secondary studies, namely, systematic reviews and especially meta-analyses, are a source of integrated knowledge with the highest probability of being consistent with reality. This type of study accounts for 6 of the 55 papers included in the integrative review. In summary, this paper considers the type of studies whose results provide a moderate to high level of credibility [53].
The results of the analyzed research came from 29 countries: Spain (8), USA (8), Brazil (7), United Kingdom (4), Germany (4), Greece (4), Italy (4), Iran (3), Australia (3), Chile (3), Hungary (3), Poland (2), Sweden (2), Norway (2), Austria (2), Canada (2), South Africa (2), Nigeria (1), Ghana (1), Egypt (1), Netherlands (1), Belgium (1), France (1), Serbia (1), New Zealand (1), Turkey (1), Portugal (1), Romania (1), and Japan (1). More than half of the studies analyzed (35/55) were conducted in Europe, indicating a metabolic direction in the empirical search to exemplify selected health behaviors. On the other hand, the wide geographical distribution of the studies allows for a greater diversity of results, including the consideration of many factors related to physical activity, such as cultural or economic factors.
The analysis and synthesis of the empirical information contained in the articles shortlisted for the review was based on the approach described by Thomas and Harden [54]. The thematic synthesis performed by the author of the article (DZ) who had not previously participated in the literature review involved coding “line by line” information related to the research question posed earlier. Subsequently, the extracted key information was combined into related topic areas that were unique to the baseline content, but were within the defined scope of the review, namely, relationships between physical activity (or lack thereof), forms of physical activity, and cardiometabolic factors in relation to the obese child population. To minimize bias, the authors MG and AF met to discuss the proposed themes of the detailed synthesis of the information contained in the 55 articles, and the reliability, accuracy, and relevance of the information qualified for the final analysis was assessed. An iterative process was used to reach consensus on the final synthesis sub-themes. Another author (DZ) independently extracted and reviewed data from 5 randomly selected studies to ensure consistency with the primary studies. DZ also blindly assessed the fit between the baseline content and the unique directions extracted. There was 100% agreement.
The present integrative review looked for an association between physical activity and cardiometabolic indices in children with obesity. The conducted analysis made it possible to capture many factors related to physical activity. The results of the cited studies were analyzed in terms of the topic of the paper, the method used, the reliability, the group size and age of the study participants, and the time of publication, defining, on the basis of the aforementioned thematic synthesis, the main relationships of physical activity and diet with cardiometabolic factors in children with obesity.
The present integrative review looked for an association between physical activity and cardiometabolic indices in children with obesity, which, to clarify the presentation of results, was included in a recommended question with a population, intervention, comparison, outcome (PICO) structure [55]. In other words, in children with obesity, how does physical activity versus no physical activity affect cardiometabolic indices? The conducted analysis made it possible to capture many factors related to physical activity:
An assessment of the effectiveness of interventions taken to increase physical activity and to improve physical performance in children and adolescents (Table 3, articles 1 [56], 5, 15 [57], 25 [58], 33, 34 [59], 37, 42 [60], 44, 45, 46). There is evidence that sports intervention programs affect body mass as well as improve body composition and cardiorespiratory fitness. Maximum oxygen uptake (VO2 max), blood lipid profile, and insulin sensitivity become optimized in children, reducing the risk of cardiovascular diseases. At the same time, a slight decrease in BMI and BMI-z-score was noted;
The impact of physical exercise and dietary advice on the cardiovascular risk profile (Table 3, articles 9, 13, 19, 21, 24, 27, 30, 40, 43 [61]). Nutritional and motor interventions significantly lowered the anthropometric and biochemical parameters, such as BMI, BMI standard deviation score (BMI-SDS), waist circumference (WC), waist-to-height ratio (WtHR), glucose and insulin during oral glucose tolerance tests, and insulin sensitivity, and they showed tendencies to lower blood pressure and LDL-C levels. A decrease in SBP was also confirmed;
The relationship between a sedentary lifestyle and physical activity, and cardiometabolic risk factors (Table 3, articles 14 [62], 20 [63], 22, 32, 36, 39, 48). Children and adolescents with low physical activity/high screen time were observed to have the following parameters at less favorable levels: BMI scores, WC, LDL-C, SBP and diastolic blood pressure (DBP), and lower HDL-C levels than non-exercising groups. Active lifestyle should be promoted to avoid, for example, abdominal obesity and hypertension;
The role of physical activity in the prevention of obesity and its impact on aerobic capacity, muscle strength, body composition, hemodynamic variables, biochemical markers, and endothelial function (Table 3, articles 3, 4, 16, 17, 18, 23, 26, 29, 35 [64], 38 [65], 41 [66], 46, 47, 49, 52). It was shown that due to long-term physical activity, changes in fat, WC, SBP, insulin, LDL-C, and total cholesterol, as well as minor changes in DBP and glucose, were achieved. Apart from anthropometric parameters, children with lower physical activity values were found to display a worse psychosocial and physical condition;
The relationship between physical training, glycemic control and cardiovascular risk factors in adolescents with type 1 diabetes (Table 3, article 6 [67]) and type 2 diabetes (Table 3, articles 54, 55). Exercise training improves VO2 max, thus also contributing to lower BMI-SDS) and HbA1c and a higher level of HDL-C. Regular exercise is recommended as an element of therapy for children and adolescents with type 2 diabetes;
Determining the role of intensity and duration of physical activity in modulating the relationships between physical activity and markers of cardiometabolic risk (Table 3, articles 2, 8, 28, 31, 50, 51 [68]). Research results have shown some associations between physical activity and a variety of improvements connected with metabolic health, such as lower a BMI value, WC, and ratio of total cholesterol to LDL-C;
The relationship between anthropometric indicators, body composition, cardiorespiratory fitness, and physical activity (Table 3, articles 7, 10 [69], 11, 12, 52, 53). Moderate or intense physical activity, cardiovascular and respiratory performance, and anthropometric variables are important predictors of blood pressure variability in children. The lower the physical activity, the worse the blood pressure and BMI values of a child. Physical activity in childhood has a marginal effect on obesity or blood pressure in the later years of a child’s life.
Table 3.
Qualitative analysis of the articles included in the review.
| Author | Title of the article | Research method research tool sample | Analysis of the results | Literature number/ range | Methodological quality | |
|---|---|---|---|---|---|---|
| Review articles | ||||||
| 1 | Neil-Sztramko SE, et al. (2021) | School-based physical activity programs for promoting physical activity and fitness in children and adolescents aged 6 to 18 | Method: systematic review Tools: CENTRAL, MEDLINE, Embase, CINAHL, PsycINFO, BIOSIS, SPORTDiscus, Sociological Abstracts Sample: 89 articles |
School-based physical activity interventions may improve physical fitness reported as VO2 max; they may result in a minor decrease in BMI z-scores, and may not affect BMI expressed in kg/m2 | 307 1985–2020 |
90% |
| 2 | Dietz P, et al. (2012) | Influence of exclusive resistance training on body composition and cardiovascular risk factors in overweight or obese children: A systematic review | Method: systematic review Tools: MEDLINE, SPORTDiscus Sample: 6 articles |
Individually planned and supervised whole body resistance training of moderate or submaximal intensity in children appears to be safe and tends to have a positive effect on body composition | 49 1978–2011 |
60% |
| 3 | Vasconcellos F, et al. (2014) | Physical activity in overweight and obese adolescents: A systematic review of the effects on physical fitness components and cardiovascular risk factors | Method: systematic review Tools: PubMed, LILACS, Web of Science, Scopus (including Embase), SPORTDiscus Sample: 24 articles |
The results indicate a positive effect of physical activity on changes in fat, waist circumference, SBP, insulin, LDL-C and total cholesterol, as well as minor changes in DBP and glucose | 89 1985–2013 |
80% |
| Meta-analysis | ||||||
| 4 | Busnatu SS, et al (2022) | Effects of exercise in improving cardiometabolic risk factors in overweight children: A systematic review and meta-analysis | Method: meta-analysis, systematic review Tools: PubMed/Medline, Cochrane Library, and Google Scholar Sample: 12 articles |
Physical interventions significantly improved several cardiometabolic risk factors, such as BMI, LDL, blood pressure and blood sugar | 83 1982–2021 |
90% |
| 5 | Cesa CC, et al. (2022) | Physical activity and cardiovascular risk factors in children: Meta-analysis of randomized clinical trials | Method: meta-analysis of randomized clinical trials Tools: PubMed, EMBASE I Cochrane CENTRAL Sample: 17 studies |
Since sports intervention programmes lasting more than 6 months are associated with lower blood pressure and triglyceride levels, they should be included in the programmes for the prevention of cardiovascular diseases in school-age children | 48 1995–2020 |
80% |
| 6 | Ostman C, et al. (2017) | Clinical outcomes of exercise training in type 1 diabetes: A systematic review and meta-analysis | Method: meta-analysis, systematic review Tools: MEDLINE search strategy, Cochrane Controlled Trials Registry, CINAHL, SPORTDiscus and Science Citation Index Sample: 15 studies |
Exercise training improves insulin doses, waist circumference, LDL and triglycerides in children | 36 1980–2016 |
90% |
| Original articles | ||||||
| 7 | Pinheiro G, et al. (2021) | Blood pressure in children: Association with anthropometric indicators, body composition, cardiorespiratory fitness and physical activity | Method: cross-sectional study Tools: Guidelines for Measurement and Tests of PROESP-Br Sample: 215 children aged 6–12 years |
Moderate or intense physical activity, cardiovascular and respiratory performance, anthropometric variables, age, gender and maturity are important predictors of blood pressure variability in children | 28 2002–2021 |
75% |
| 8 | Tarp J, et al. (2018) | Physical activity intensity, bout duration, and cardiometabolic risk markers in children and adolescents | Method: cross-sectional study Tools: secondary data from the International Physical Activity Database for Children (ICAD) Sample: 38 306 observations in 29 734 persons aged 4–18 years) |
Time spent on increasing physical activity is favourably associated with markers of cardiometabolic risk in adolescents | 60 1985–2018 |
67% |
| 9 | Grace J, et al. (2021) | Effect of physical activity and nutrition education on body mass index, blood pressure and biochemical variables in overweight and obese adolescents | Method: quasi-experiment Tools: Borg RPE scale, Calculator of American Academy of Paediatrics Sample: 129 children aged 13–16 years |
10 weeks of physical activity and nutritional education in adolescents with overweight and obesity significantly lowered BMI and showed tendencies to lower blood pressure and LDL-C | 40 1985–2020 |
100% |
| 10 | Macdonald-Wallis C, et al. (2017) | A longitudinal study of the associations of children’s body mass index and physical activity with blood pressure | Method: longitudinal study Tools: − Sample: 2047 children aged 6 and 9 years and one or both parents |
BMI in children may be a risk factor for the development of hypertension | 47 1990–2016 |
75% |
| 11 | Sadoh WE, et al. (2016) | Physical activity, body mass index and blood pressure in primary school pupils attending private schools | Method: cross-sectional study Tools: questionnaire assessing physical activity among children Sample: 353 primary school students |
Physical activity may lead to lower average blood pressure in physically active students compared to students who are not independent of body mass index. Obesity and overweight students were more likely to have hypertension than healthy students |
21 1985–2016 |
58% |
| 12 | Wiersma R, et al. (2020) | Adiposity and high blood pressure during childhood: A prospective analysis of the role of physical activity intensity and sedentary time in the GECKO Drenthe Cohort | Method: cohort study Tools: ActiGraph GT3X Sample: 947 children aged 5–6 years and 10–11 years |
Physical activity in childhood has a marginal effect on obesity or blood pressure in the later years of a child’s life | 64 1981–2020 |
83% |
| 13 | Poeta LS, et al. (2013) | Effects of physical exercises and nutritional guidance on the cardiovascular risk profile of obese children | Method: clinical control study Tools: − Sample: 44 children aged 8–11 years |
The physical exercise and nutritional guidance programme, in addition to the regular clinical treatment, was effective in reducing BMI, total cholesterol, LDL-V, diastolic arterial pressure and carotid intima-media thickness | 30 1962–2010 |
70% |
| 14 | Pepera G, et al. (2022) | Associations between cardiorespiratory fitness, fatness, hemodynamic characteristics, and sedentary behavior in primary school-aged children | Method: clinical study Tools: HELENA questionnaoire Sample: 105 children aged 6–12 years |
Children with optimal BMI tend to present better CRF results than obese and overweight children. Sedentary lifestyle is associated with lower CRF in school-age children | 45 1984–2021 |
90% |
| 15 | Martínez-Vizcaíno V, et al. (2014) | Gender differences on the effectiveness of a school-based physical activity intervention for reducing cardiometabolic risk: a cluster randomized trial | Method: randomized control study Tools: MOVI-2 programme Sample: 712 children aged 8–10 years |
A safe and effective way to reduce obesity in both sexes and improve the cardiometabolic risk profile in girls is an out-of-school intervention based on uncompetitive physical activity | 48 2003–2012 |
82% |
| 16 | Nqweniso S, et al. (2021) | Physical activity and risk factors of cardio-metabolic diseases in South African children | Method: cross-sectional study Tools: results of KaziAfya research Sample: 832 children aged 5–8 years and 9–13 years |
Low levels of physical activity are associated with increased cardiovascular risk factors. Higher CRF, VPA and MVPA were negatively associated with lower body fat percentage and a lower risk of concentrated cardiovascular disease | 45 1983–2020 |
75% |
| 17 | Ramezani A, et al. (2017) | Effects of three methods of exercise training on cardiovascular risk factors in obese boys | Method: clinical trial Tools: Exercise Training Protocol Sample: 60 children with obesity aged 8–12 years |
The results of this study show that three routines of regular exercise over 8 weeks, including resistance training (50–75% 1RM) and endurance training (50–75% of target heart rate) had a desired effect on BMI, serum glucose and lipid profile risk factors | 39 2000–2016 |
70% |
| 18 | Moschonis G, et al. (2013) | “Leaner and less fit” children have a better cardiometabolic profile than their “heavier and more fit” peers: The Healthy Growth Study | Method: cross-sectional study Tools: standardised questionnaire of free time physical activity, endurance 20-m shuttle run test (ERT) Sample: 1222 boys and 1188 girls aged 9–13 years |
Slender and less fit boys and girls had better cardiometabolic risk profiles than their heavier and more athletic peers, which probably suggests a greater importance of a slim figure over fitness in children in terms of cardiometabolic health benefits | 40 1955–2012 |
67% |
| 19 | Pablos A, et al. (2017) | Effectiveness of a school-based program focusing on diet and health habits taught through physical exercise | Method: clinical study Tools: Inventory of Healthy Habits (IHH) questionnaire Sample: 158 primary school students |
The School Physical Activity Program (HHP) has initiated a significant improvement in the incidence of normal total cholesterol levels, blood pressure and BMI | 52 1989–2017 |
60% |
| 20 | Fridolfsson J, et al. (2021) | High-intensity activity is more strongly associated with metabolic health in children compared to sedentary time: a cross-sectional study of the I.Family cohort | Method: cross-sectional study Tools: IDEFICS Metabolic Syndrome Score Tool Sample: 2592 children aged 8–11 years |
The results suggest a higher physical actibity corresponding to the intensity of at least brisk walking with the inclusion of intense exercise, rather than limited sitting time, which is of greater importance for metabolic health in children | 48 1984–2021 |
67% |
| 21 | Delgado-Floody P, et al. (2019) | Influence of Mediterranean diet adherence, physical activity patterns, and weight status on cardiovascular response to cardiorespiratory fitness test in Chilean school children | Method: cross-sectional study Tools: Multistage Fitness (Beep) test, standing long jump test Sample: 605 children aged 12.00±1.23 years |
Overweight children had lower physical fitness, and a higher percentage of them had high blood pressure. Decreased SBP was associated with cardiovascular strength and performance | 41 1988–2017 |
75% |
| 22 | Carson V, et al. (2019) | Compositional analyses of the associations between sedentary time, different intensities of physical activity, and cardiometabolic biomarkers among children and youth from the United States | Method: cross-sectional study Tools: National Health and Nutrition Examination Survey Sample: 2544 children and adolescents aged 6–17 years |
The composition of ST, LPA, MPA and VPA in this large representative sample of children and adolescents was significantly related to many aspects of cardiometabolic health, also suggesting that compositions with more time for more intense activities may be superior in some aspects of cardiometabolic health | 31 2002–2019 |
67% |
| 23 | Kamal NN, Ragy MM (2012) | The effects of exercise on CRP, insulin, leptin and some cardiometabolic risk factors in Egyptian children with or without metabolic syndrome | Method: clinical trial Tools: absent Sample: 93 children aged od 8 do 12 years |
After 12 weeks of exercise, both groups of children with obesity, with and without metabolic syndrome, showed reduced body weight, BMI and CRP levels, and increased HDL-C levels | 41 1972–2009 |
60% |
| 24 | Brzeziński M, et al. (2020) | “PoZdro!” as an example of a successful multicenter programme for obesity management and healthy lifestyle promotion in children and adolescents – programme protocol and preliminary results from the first intervention site | Method: multicentre clinical trial Tools: diet diaries, approved structured interview Sample: 603 children aged 13 years |
Preliminary results show that the participants observed a noticeable change in the body weight index and body fat content during the two-year intervention process | 10 1992–2018 |
75% |
| 25 | D’Agostino EM, et al. (2018) | Effect of participation in a park-based afterschool programme on cardiovascular disease risk among severely obese youth | Method: cohort study Tools: fitness tests and a test of knowledge about health and well-being Sample: children aged 6–14 years |
The findings provide evidence of a significant improvement in the risk of cardiovascular disease in adolescents with obesity after attending an after-school programme at the park for at least a year | 32 1986–2018 |
83% |
| 26 | Fam B, et al. (2013) | Association between Physical activity and metabolic risk factors in adolescents: Tehran lipid and glucose study | Method: cross-sectional study Tools: interview questionnaire Sample: 777 adolescents aged 12–18 years |
The results of this study confirm the association between physical activity and some individual MetS components, such as waist circumstance and HDL-C | 27 1996–2012 |
67% |
| 27 | Plavsic L, et al. (2020) | Effects of high-intensity interval training and nutrition advice on cardiometabolic markers and aerobic fitness in adolescent girls with obesity | Method: randomized control clinical trial Tools: medical records Sample: 44 adolescents with obesity aged 13–19 years |
The 12-week HIIT intervention combined with nutritional counseling led to improvements in some anthropometric and biochemical parameters such as BMI, BMI-SDS, WC, WtHR, glucose and insulin during OGTT and insulin sensitivity compared to the non-training group | 43 1984–2019 |
73% |
| 28 | Howie EK, et al. (2020) | Physical activity trajectories from childhood to late adolescence and their implications for health in young adulthood | Method: cohort study Tools: International Physical Activity Questionnaire (IPAQ), the Short-Form 12 Health Survey (SF-20) Sample: 2868 participants aged respectively 8, 10, 14, 17, 20 and 22 |
Selected directions of physical activity show clear associations with a variety of physical and mental health interventions in early adulthood, including obesity, cardiometabolic health, depression and cognitive performance | 35 1998–2019 |
75% |
| 29 | Gallardo-Escribano C, et al. (2021) | Lifestyle modification improves insulin resistance and carotid intima-media thickness in a metabolically healthy obese prepubescent population | Method: cross-sectional study Tools: food questionnaires relating to food frequency, questionnaires of compliance MedDiet Sample: 131 children aged 4–9 years |
Results show a decrease in both body weight and BMI in the whole population after 12 months of intervention. The most effective interventions to reduce weight in the pediatric population are those that involve dietary modification and regular physical activity | 33 2003–2020 |
83% |
| 30 | Cuenca-García M, et al. (2012) | Combined influence of healthy diet and active lifestyle on cardiovascular disease risk factors in adolescents | Method: cross-sectional study Tools: HELENA-DIAT (Dietary Assessment Tool), Young Adolescents’ Nutrition Assessment on Computer (YANA-C), Diet Quality Index for Adolescents (DQI-A) Sample: 1513 children aged 12.5–17.5 |
The combination of a healthy diet and an active lifestyle is associated with a healthier level of key cardovascular disease risk factors, such as cardiovascular and respiratory performance, obesity and blood lipid profile. Physical activity can reduce the harmful effects of an unhealthy diet | 44 1976–2013 |
67% |
| 31 | Larouche R, et al. (2014) | Active transportation and adolescents’ health: The Canadian Health Measures Survey | Method: cross-sectional study Tools: modified Canadian Aerobic Fitness Test (mCAFT) Sample: 1016 adolescents aged 12–19 |
Compared to adolescents who did not cycle, those who cycled ≥1 hour a week accumulated more light physical activity, had greater aerobic fitness and lower BMI, WC and ratio of total cholesterol to LDL-C; those who reported cycling <1 hour a week had lower total cholesterol | 71 1979–2014 |
83% |
| 32 | Barker AR, et al. (2018) | Physical activity, sedentary time, TV viewing, physical fitness and cardiovascular disease risk in adolescents: The HELENA study | Method: cross-sectional study Tools: Family Affluence Scale (FAS), validated self-report sedentary behavior questionnaire, 20 m shuttle run test Sample: 534 adolescents aged 12.5–17.5 years |
Physical activity affects body composition and cardiometabolic risk | 42 1976–2016 |
67% |
| 33 | Momoniyi MM, et al. (2020) | “AMPE” exercise programme has positive effects on anthropometric and physiological parameters of school children: A pilot study | Method: pilot study Tools: Sample: 78 children aged 9–12 years |
The AMPE exercise program is effective because it reduces body weight and body mass index and improves the following parameters: SBP, DPB and heart rate | 20 2001–2019 |
78% |
| 34 | Chansavang Y, et al. (2015) | Feasibility of an after-school group-based exercise and lifestyle programme to improve cardiorespiratory fitness and health in less-active Pacific and Maori adolescents | Method: feasibility study Tools: International Physical Activity Questionnaire (IPAQ) Sample: 18 secondary school students |
The programme improved the results of VO2 max, SBP, HbA1c and intense and moderate physical activity | 26 1985–2014 |
60% |
| 35 | Gonçalves R, et al. (2014) | Association of body mass index and aerobic physical fitness with cardiovascular risk factors in children | Method: cross-sectional study Tools: 20 m shuttle run test Sample: 290 children aged 6–10 years |
Significant associations and an increased likelihood of cardiovascular risk factors have been shown in children with poorer oxygen performance and higher BMI | 29 1984–2010 |
58% |
| 36 | Bustos-Barahona R, et al. (2020) | Lifestyle associated with physical fitness related to health and cardiometabolic risk factors in Chilean school children | Method: cross-sectional study Tools: Kreece plus test Sample: 582 children aged 10–13 years |
Lifestyles of school-aged children are linked to CMR (i.e., abdominal obesity and hypertension) and health-related physical fitness, which requires promoting interventions to promote active lifestyles, including more physical activity weekly and less screen time during the day | 39 1988–2020 |
67% |
| 37 | Kokkvoll AS, et al. (2019) | No additional long-term effect of group vs individual family intervention in the treatment of childhood obesity – a randomized trial | Method: randomized control clinical trial Tools: validated Andersen intermittent running test Sample: 97 children aged 6–12 years |
Analysis results from the combination of data from both intervention groups showed a decrease in BMI-SDS, improvements in total and LDL-C, CRF, and the incidence of hypertension | 30 1985–2018 |
82% |
| 38 | Willis EA, et al. (2015) | Length of moderate-to-vigorous physical activity bouts and cardio-metabolic risk factors in elementary school children | Method: latent profile analysis Tools: Progressive Aerobic Cardiovascular Endurance Run (PACER), growth charts CDC Sample: 396 second- and third-grade students aged 7.6±0.6 years |
Longer rather than shorter MVPA seizures are associated with reduced cardiovascular risk factors, especially lower BMI percentiles and WC in children | 40 1963–2013 |
67% |
| 39 | Heshmat R, et al. (2016) | Joint association of screen time and physical activity with cardiometabolic risk factors in a national sample of Iranian adolescents: The CASPIANIII Study | Method: cross-sectional study Tools: author’s questionnaire Sample: 5625 children and adolescents aged 10–18 years |
Adolescents with low physical activity/high screen time were shown to have higher BMI scores with, WC, LDL-C, SBP and DBP and lower HDL-C levels compared to other physical activity/screen time combinations. In contrast, patients with high physical activity/low screen time had the highest mean total serum cholesterol | 63 1987–2014 |
67% |
| 40 | Verduci E, et al. (2015) | Change in metabolic profile after 1-year nutritional-behavioral intervention in obese children | Method: clinical study Tools: standardized interview questionnaire, Feeding Frequency Questionnaire (FFQ) Sample: 90 children with obesity, age ≥6 years |
Nutritional-behavioral interventions may improve the blood lipid profile and insulin sensitivity in children with obesity, and may benefit the metabolic syndrome | 49 1985–2015 |
70% |
| 41 | Delgado-Floody P, et al. (2018) | Psychosocial, physical and anthropometric variables in Chilean school children. A comparative study according to physical activity levels | Method: comparative study Tools: PAQ-C questionnaire for children, TAE-Alumno test, The Body Shape Questionnaire Sample: 605 children aged 11–14 years |
The main result of this study was that the results of children with lower physical activity values were noticeable in psychosocial, physical and anthropometric variables compared to those that performed physical activity | 40 1988–2018 |
60% |
| 42 | D’Agostino EM, et al. (2017) | Longitudinal analysis of cardiovascular disease risk profiles in neighbourhood poverty subgroups: 5-year results from an afterschool fitness programme in the USA | Method: longitudinal study Tools: Fit2Play study protocol, the Presidential Youth Fitness Programme testing protocol, modified sit and reach test, PACER test Sample: 2264 children and adolescents (average age: 9.4 age) |
Results show that the youth programme can maintain or improve cardiovascular health in at-risk teens over 5 years of age, including blood pressure, weight status, and physical fitness | 41 1982–2017 |
75% |
| 43 | Nişancı-Kılınç F, Çağdaş DN (2013) | Diet and physical activity interventions do have effects on body composition and metabolic syndrome parameters in overweight and obese adolescents and their mothers | Method: clinical trial Tools: Ozturk’s growth charts, Harpenden Skinfold Caliper, the charts of fat percentage percentiles Sample: 19 adolescents with an average age of 12.52±2.85 years and their mothers |
Nutritional and motor interventions affect body composition and metabolic syndrome parameters in adolescents with obesity and their mothers with overweight and obesity | 38 1993–2013 |
60% |
| 44 | Vasconcellos F, et al. (2015) | Health markers in obese adolescents improved by a 12-week recreational soccer program: a randomised controlled trial | Method: randomized control clinical trial Tools: recreational soccer programme (RSP) Sample: 30 adolescents aged 12–17 years |
Recreational soccer performer 3 times a week during 12 weeks resulted in beneficial changes in body mass and composition, VOzpeak, resting blood pressure, autonomic activity, plasma lipid and glukose profile, CRP and endothelial-dependent vasodilation | 44 1962–2014 |
73% |
| 45 | Messiah SE, et al. (2016) | Impact of a park-based afterschool program replicated over five years on modifiable cardiovascular disease risk factors | Method: cohort study Tools: The Presidential Youth Fitness Programme protocol; the modified sit and reach test, PACER test. EmpowerMe4Life 9-item scale Sample: children aged 6–14 years |
People of normal body weight maintained a healthy BMI, overweight/obese people had reduced both BMI z-score and percentiles, all weight groups lowered blood pressure, improved fitness and knowledge about health and wellbeing | 52 1965–2016 |
75% |
| 46 | Antunesn B de MM, et al. (2015) | Effect of concurrent training on gender-specific biochemical variables and adiposity in obese adolescents | Method: clinical trial Tools: Sample: 25 adolescents aged 12–15 years |
Significant increase in height and decrease in BMI (P=0.002 and P=0.017), BMI z-score (P=0.033 and P=0.004), FM% (P=0.002 and P=0.002), TFM% (P=0.009 and P=0.018), total cholesterol (P=0.042 and P=0.001) and LDL-C (P=0.006 and P=0.001) was found in the male and female groups, respectively, after 20 weeks of intervention when compared with baseline | 32 1999–2015 |
60% |
| 47 | Horner K, et al. (2015) | Effect of aerobic vs resistance exercise on pulse wave velocity, intima media thickness and left ventricular mass in obese adolescents | Method: clinical trial Tools: graded treadmill test Sample: 66 children aged 12–18 years |
In adolescents with obesity, CRF was significantly associated with cIMT and LVMI at baseline. Also, total fat and improvements in CRF after 3 months of aerobic and resistance exercise were noticed | 43 1988–2014 |
70% |
| 48 | Moura BP, et al. (2019) | Effects of isotemporal substitution of sedentary behavior with light-intensity or moderate-to-vigorous physical activity on cardiometabolic markers in male adolescents | Method: cross-sectional study Tools: interview-based questionnaire, ActiGraph GT3X+ Sample: 140 adolescents aged 14–18 years |
Replacing sedentary behavior with light-intensity physical activity showed positive results on metabolic (HDL-C and HOMA2-S) and physiological (SBP) indicators, while replacing sedentary behavior with MVPA was only associated with one obesity indicator (BF%) | 49 1972–2019 |
75% |
| 49 | Ogawa M, et al. (2021) | Comparative evaluation of obesity-related parameters in junior sumo wrestlers and children with obesity | Method: clinical study Tools: Sample: 70 children aged 9–17 years |
The BMI z-score, obesity rate, WC (P<0.05, along with the non-exercising group), and sedentary behavior were significantly higher and the HDL-C level was lower in the sumo group than in the other sports group (P<0.05). WtHR was significantly higher in the non-exercising group than in other sports group | 30 1976–2021 |
70% |
| 50 | Mendoza JA, et al. (2012) | General vs central adiposity and relationship to pediatric metabolic risk | Method: cross-sectional study Tools: National Health and Nutrition Examination Survey (NHANES) Sample: 2155 participants aged 6–19 years |
Participants with more minutes of MVPA had lower SBP and higher HDL-C, daily minutes of MVPA were significantly associated with SBP, HDL-C and WC. WC significantly mediated the relationship between minutes of MVPA and CRP and HDL-C, and was independently associated with several metabolic risk factors, such as higher CRP, glycohemoglobin, fasting TG, fasting insulin, and lower HDL-C | 40 1986–2011 |
83% |
| 51 | Aadland E, et al. (2020) | Accelerometer epoch setting is decisive for associations between physical activity and metabolic health in children | Method: cross-sectional study Tools: Andersen intermittent running test, ActiGraph GT3X+ Sample: 841 children |
The explained variance in models of metabolic health improved when epoch durations decreased | 38 1972–2018 |
75% |
| 52 | Gopinath B, et al. (2014) | Activity behaviors in school children and subsequent 5-year change in blood pressure | Method: cohort study Tools: activity questionnaires Sample: 821 children aged 6–11 years |
Adhering to the recommended guidelines of 60 minutes or more a day of physical activity in the long term was associated with an appreciable reduction in blood pressure 5 years later among school children. In contrast, higher levels of total screen time were prospectively associated with higher levels of blood pressure | 29 2008–2018 |
73% |
| 53 | Aguilar-Cordero MJ, et al. (2020) | Influence of physical activity on blood pressure in children with overweight/obesity: A randomized clinical trial | Method: randomized control clinical trial Tools: clinical history Sample: 98 children |
An intervention based on physical activity and nutritional recommendations is shown to be effective in reducing hypertension in children with overweight or obesity | 29 2008–2018 |
73% |
| 54 | Herbst A, et al. (2014) | Impact of regular physical activity on blood glucose control and cardiovascular risk factors in adolescents with type 2 diabetes mellitus – a multicenter study of 578 patients from 225 centres | Method: multicentre clinical trial Tools: Pediatric Quality Initiative (DPV) Sample: 578 children aged 10–20 years with type 2 diabetes |
An intervention based on physical activity and nutritional recommendations is shown to be effective in reducing hypertension in children with overweight or obesity | 39 1991–2013 |
90% |
| 55 | Nightingale CM, et al. (2018) | The contribution of physical fitness to individual and ethnic differences in risk markers for type 2 diabetes in children: The Child Heart and Health Study in England (CHASE) | Method: cross-sectional study Tools: 8-minute submaximal step test Sample: 1445 children aged 9–10 years |
Higher VO2 max was associated with lower FMI, insulin, HOMA-IR, HbA1c, glucose, urate, CRP, triglycerides, LDL-C, blood pressure and higher HDL-C. Physical activity is associated with risk markers for T2D and cardiovascular diseases, which persist after adjustment for adiposity | 32 1980–2016 |
75% |
BMI – body mass index; CRP – C-reactive protein; CRF – cardio-metabolic risk factors; DBP – diastolic blood pressure; HOMA-IR – homeostasis model assessment of insulin resistance; SBP – systolic blood pressure; LDL-C – low-density lipoprotein cholesterol; HDL-C – high-density lipoprotein cholesterol; LIPA – light-intensity physical activity; MVPA – moderate to vigorous physical activity; WC – waist circumference.
Discussion
Physical activity in childhood is associated with many health benefits. This issue seems to be relevant especially today, with an increasing proportion of children with limited physical activity, which predisposes them to the development of chronic diseases. Furthermore, increased screen-time use reduces children’s opportunities to develop healthy social skills. Because technology use interrupts the daily activities of children, it can result in decreased physical activity, which is essential, especially to maintain optimal body weight and cardiometabolic health. The purpose of this study was to examine the relationship between physical activity and cardiometabolic indicators in children with obesity. Physical activity has an impact on many aspects of children’s and young people’s lives, including the composition of their bodies [7,19,70], as shown by our results. Also, there is scientific evidence that sports interventions affect body mass and improve body composition and cardiorespiratory fitness in children. In summary, it can be concluded that physical activity provides a protective health potential in a low-cost, valuable way by preventing and managing current childhood obesity, among high-risk groups in particular.
Among the planned interventions related to physical activity, attention is paid especially to aerobic training of moderate and intense activity. Beneficial modifications apart from body weight and composition are peak oxygen uptake (VO peak), blood pressure, autonomic activity of the heart, lipid and glucose profile in plasma, and CRP, which occur even by playing football (soccer) recreationally [71]. Similarly, other team sports as well as walking, jogging, skating, cycling or dancing, practiced 3 to 5 times a week, up to 60 min a day, bring similar changes [1,5]. This dependence was also confirmed by Barker et al, who examined adolescents practicing cycling ≥1 h a week and found that they had better aerobic capacity and lower BMI, WC, and ratio of total cholesterol to HDL than those who did not ride a bike [8]. In addition, aerobic exercise programs have been proven to lower levels of LDL and triglycerides, and combined exercise programs also improve the concentration of HDL [17,22], which is important particularly for children with obesity, potentially positively affecting cardiovascular disease risk later in life.
However, it has been shown that in order to improve the lipid profile physical training should be below the anaerobic threshold [15]. Therefore, the duration and intensity of exercise training should be considered. There are also other factors that matter, such as the lipid profile and weight condition of a child, which has been confirmed in studies [72]. According to Busnat et al and Cesa et al, interventions in the form of physical activity would have to be conducted for at least 6 months to significantly affect blood pressure or triglyceride levels [19,66]. In turn, the results of other studies recommend any duration of the activity, regardless of an increase in its intensity [23]. In conclusion, any form of physical activity is generally used for children and adolescents, but its impact on cardiovascular parameters is quite diverse, depending on the type of activity, frequency, and circumstances of use. Therefore, we suggest that future studies focus on the specific type of physical activity and its short- and long-term benefits.
The present research also indicates that moderate or intense physical activity, cardiovascular and respiratory activity, and anthropometric variables are important predictors of blood pressure variability in children. The lower physical activity is, the higher blood pressure and BMI values are [73]. The impacts of physical activity on blood pressure in children with obesity or overweight were assessed by Aguilar-Cordero et al. The results of a randomized clinical trial confirmed that the element of physical activity is not only effective but, first and foremost, necessary to maintain optimal blood pressure [74,75]. Convincing evidence is also provided by the analysis conducted by Kılınç et al, which showed a significant difference in pre- and post-exercise SBP and DBP, and several biochemical parameters of adolescents from the risk group [61]. Also, higher values of SBP and DBP were presented by children and adolescents with a low level of physical activity, similar to higher BMI z-scores, WC, and LDL-C [21,76,77]. This may happen due to increased dependency on technology. However, according to literature, physical activity in childhood has a marginal effect on obesity or blood pressure in the later years of a child’s life, which leads to following conclusion: the earlier we start to stimulate physical activity in a child, the better it is for children’s motor and cognitive development [14]. The results of the studies indicate the importance of physical activity characteristics in its effectiveness in modeling blood pressure and selected cardiometabolic parameters. Therefore, it may provide an important first step for incorporating exercise programs, for instance, at schools for prevention of obesity and cardiometabolic diseases.
Furthermore, an active lifestyle combined with some necessary nutritional intervention in the research by Pinheiro et al ensured significantly improved cardiorespiratory endurance among the participants [43]. For this reason, physical training in addition to dietary changes is recommended in particular for patients with metabolic diseases, such as diabetes mellitus. According to the meta-analysis by Larouche et al, physical training and dietary changes have a positive impact on LDL and triglyceride concentrations, WC, and insulin levels, which represent only a few indicators of the severity of type 1 diabetes [16,44]. Young people should engage in regular physical activity, which is confirmed by the results obtained by Herbst et al. The examined participants presented lower HbA1c and BMI-SDS, as well as higher HDL-C due to physical activity. Also, triglycerides, LDL-C. and HDL-C are the most strongly associated measures with indicators such as HbA1c and BMI in children with diabetes [22]. These results justify the conclusion that regular exercise is a useful therapeutic recommendation for children and adolescents with diabetes mellitus. Thus, physical activity, but also diet, has a normalizing effect on cholesterol levels and its fractions. It may be an indication to evaluate the clinical relevance and long-term effectiveness of nutritional-behavioral interventions based on normocaloric diet and physical activity.
Cardiovascular and metabolic risk can be adjusted by reducing visceral fat. The results of a study verifying the impact of moderate and high-intensity interval training on cardiovascular indicators suggest WC as a significant correlate of children under the metabolic risk [78]. Also, it was shown that body fat percentage, body mass, and WtHR are related to HOMA-IR [45,79]. Insulin resistance is a fundamental abnormality of metabolic syndrome. Exercise increases insulin sensitivity and, accordingly, higher-intensity activity is considered a basic therapeutic tool in the treatment of metabolic syndrome in childhood. This allows for the conclusion that regular physical activity is a significant non-pharmacological measure for cardiometabolic risk reduction in children.
Some studies have illustrated that it is possible that the higher BMI, the more adipose tissue becomes a direct source of pro-inflammatory cytokines. The percentage of body fat decreases significantly after using the physical activity intervention, thus improving the cardiometabolic risk profile in girls and reducing obesity in both sexes. These discoveries are consistent with those of other studies developed in the context of physical activity in the pediatric population [11,40,80–82].
Analysis of available studies indicates an increasing trend in developing systematic reviews and meta-analyses of the relationship between physical activity and cardiometabolic indicators in children with obesity [35,83]. However, the added value of the present review is the selection of databases, scope of searches and, finally, subject of the analysis, which was a total of 345 articles. Fifty-five articles were included in the review, and their analysis covered different types of studies, namely, reviews, meta-analyses, randomized, cross-sectional, cohort, longitudinal, clinical-control and feasibility studies, and latent profile analyses. The results of the studies cited above indicate that blood pressure and BMI measurements are the best way to identify increased cardiovascular risk.
Limitations and Gaps
This integrative review has limitations due to the varying methodological quality of the included studies. Studies with a lack of blinding can be considered one of the main limitations among these studies, although this is quite common in non-pharmacological studies. Studies that include physical activity and no comparative intervention bring other relevant information regarding, for example, body weight, BMI, triglycerides, blood LDL-cholesterol and glucose, HbA1c, CRP, and HOMA-IR index. The demonstration of similar associations/relationships in selected articles provides data for the analysis of several components, even in a small sample. In our integrative review, several studies considering the relationship between physical activity and blood pressure were excluded, as they were ultimately considered not thematically relevant to the topic at hand. In addition, children and adolescents were included regardless of baseline BMI or the way physical activity was measured, which may have a distracting effect on the results of studies classified for analysis.
This integrative review also has strengths, which are worth mentioning. The process of selecting articles was done according to strict criteria, taking into account different types of studies, in order to be sensitive to preliminary research reports carried out in a less strong research protocol. In addition, the issue under consideration is part of a contemporary world health problem: the growing phenomenon of child and adolescent obesity and its significant health consequences. In the future, funding and infrastructure for the development of organized and spontaneous physical activity should be provided at the national and supranational level, beyond the narrow scope of sports policies. Broad and personally targeted promotion of daily physical activity with reference to scientific research demonstrating the importance of AP for health and well-being is needed. In addition, it seems necessary to develop a strategy at a global level to return to pre-pandemic activity, taking into account the stigma left by the pandemic on children and adolescents.
Broader Context
Evidence-based knowledge of the impact of physical activity on individual health [84], as well as understanding of its impact on public health [85–87] has increased recently. Evidence is emerging to suggest that participation in physical activity can have benefits beyond physical health [88]. However, among child and adolescent populations, there is a global epidemic of insufficient physical activity [89,90] and obesity [91], further exacerbated by the effects of the COVID-19 pandemic [92]. Given the importance of physical activity for physical as well as mental health, and the emerging evidence that exercise can have beneficial outcomes for many diseases including COVID-19, it is recommended that governments be more involved in promoting ways to increase physical activity and reduce sedentary behavior, and to consider the broad context of epidemiological restriction decisions [93].
Participation in organized team and individual physical activity plays an important role, primarily in supporting vigorous physical activity, but also in community-wide physical activity among children. The negative effects of pandemics should be mitigated by increasing the participation of young people in various forms of organized physical activity, with an emphasis on structured individual physical activity [94], revitalizing supportive peer relationships, increasing motivation for physical activity through its attractiveness, flexibility regarding place and time of implementation, and increasing recreational infrastructure in the immediate environment [95], or implementing the use of family-based behavioral interventions [96].
Recommendations for Future Research
The integrative review showed the need for a standardized scheme based on measurable indicators to monitor the effectiveness of physical activity promotion programs. We should continuously search for new, specific, and highly differentiated laboratory markers indicating the impact of physical activity on cardiovascular function, the endocrine system, or the well-being of children and adolescents. It also seems reasonable and cognitively quite intriguing to search for cultural [97] and genetic influences on physical activity and health consequences in children.
Conclusions
Healthy behaviors undertaken in childhood are essential for the improvement of physical fitness, bone condition, cardiometabolic health, cognitive performance, and mental health. Additionally, healthy habits are important in preventing chronic diseases in adulthood. The analysis of the thematic literature allowed for identifying factors related to physical activity. As presented, numerous studies describing the relationships between physical activity and cardiometabolic indicators, as well as overweight, require constant monitoring in the changing conditions of the socio-economic environment, as they have a profound impact on all spheres of human life and health.
Footnotes
Conflict of interest: None declared
Department and Institution Where Work Was Performed
Department of Pediatric and Pediatric Nursing and Department of Integrated Nursing Care, Faculty of Health Sciences, Medical University of Lublin, Lublin, Poland.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: The study received support from the Medical University of Lublin within the framework of grant number DS 514/2022
References
- 1.Dietz P, Hoffmann S, Lachtermann E, et al. Influence of exclusive resistance training on body composition and cardiovascular risk factors in overweight or obese children: A systematic review. Obes Facts. 2012;5:546–60. doi: 10.1159/000341560. [DOI] [PubMed] [Google Scholar]
- 2.Bustos-Barahona R, Delgado-Floody P, Martínez-Salazar C. Lifestyle associated with physical fitness related to health and cardiometabolic risk factors in Chilean schoolchildren. Endocrinol Diabetes Nutr. 2020;67:586–93. doi: 10.1016/j.endinu.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (Switzerland) Guidelines on physical activity and sedentary behavior. Geneva: World Health Organization; 2020. [Google Scholar]
- 4.World Health Organization (Switzerland) Global action plan on physical activity 2018–2030: More active people for a healthier world. Geneva: World Health Organization; 2018. [Google Scholar]
- 5.Logan K, Lloyd RS, Schafer-Kalkhoff T, et al. Youth sports participation and health status in early adulthood: A 12-year follow-up. Prev Med Rep. 2020;19:101–7. doi: 10.1016/j.pmedr.2020.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plavsic L, Knezevic OM, Sovtic A, et al. Effects of high-intensity interval training and nutrition advice on cardiometabolic markers and aerobic fitness in adolescent girls with obesity. Appl Physiol Nutr Metab. 2020;45:294–300. doi: 10.1139/apnm-2019-0137. [DOI] [PubMed] [Google Scholar]
- 7.Moura BP, Rufino RL, Faria RC, Amorim PRS. Effects of isotemporal substitution of sedentary behavior with light-intensity or moderate-to-vigorous physical activity on cardiometabolic markers in male adolescents. PLoS One. 2019;14(11):e0225856. doi: 10.1371/journal.pone.0225856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker AR, Gracia-Marco L, Ruiz JR, et al. Physical activity, sedentary time, TV viewing, physical fitness and cardiovascular disease risk in adolescents: The HELENA study. Int J Cardiol. 2018;254:303–9. doi: 10.1016/j.ijcard.2017.11.080. [DOI] [PubMed] [Google Scholar]
- 9.Chaput JP, Willumsen J, Bull F, et al. 2020 WHO guidelines on physical activity and sedentary behavior for children and adolescents aged 5–17 years: Summary of the evidence. Int J Behav Nutr Phys Act. 2020;17(1):141. doi: 10.1186/s12966-020-01037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization (Switzerland) Guidelines on physical activity, sedentary behavior and sleep for children under 5 years of age. Geneva: World Health Organization; 2019. [PubMed] [Google Scholar]
- 11.Vasconcellos F, Seabra A, Cunha F, et al. Health markers in obese adolescents improved by a 12-week recreational soccer program: A randomised controlled trial. J Sports Sci. 2016;34:564–75. doi: 10.1080/02640414.2015.1064150. [DOI] [PubMed] [Google Scholar]
- 12.Nqweniso S, Walter C, du Randt R, et al. Physical activity, cardiorespiratory fitness and clustered cardiovascular risk in south african primary schoolchildren from disadvantaged communities: A cross-sectional study. Int J Environ Res Public Health. 2021;18(4):2080. doi: 10.3390/ijerph18042080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heshmat R, Qorbani M, Shahr Babaki AE, et al. Joint association of screen time and physical activity with cardiometabolic risk factors in a national sample of Iranian adolescents: The CASPIANIII study. PLoS One. 2016;11(5):e0154502. doi: 10.1371/journal.pone.0154502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiersma R, Hartman E, Boezen HM, Corpeleijn E. Adiposity and high blood pressure during childhood: A prospective analysis of the role of physical activity intensity and sedentary time in the GECKO Drenthe Cohort. Int J Environ Res Public Health. 2020;17(24):9526. doi: 10.3390/ijerph17249526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moschonis G, Mougios V, Papandreou C, et al. “Leaner and less fit” children have a better cardiometabolic profile than their “heavier and more fit” peers: The Healthy Growth Study. Nutr Metab Cardiovasc Dis”. 2013;23:1058–65. doi: 10.1016/j.numecd.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Larouche R, Faulkner GEJ, Fortier M, et al. Active transportation and adolescents’ health: The Canadian health measures survey. Am J Prev Med. 2014;46:507–15. doi: 10.1016/j.amepre.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Ramezani A, Gaeini AA, Hosseini M, et al. Effects of three methods of exercise training on cardiovascular risk factors in obese boys. Iran J Pediatr. 2017;27(5):e7145. [Google Scholar]
- 18.Cuenca-García M, Ortega FB, Ruiz JR, et al. Healthy lifestyle and cardiovascular diseases. Scand J Med Sci Sports. 2012;24:553–62. doi: 10.1111/sms.12022. [DOI] [PubMed] [Google Scholar]
- 19.Vasconcellos F, Seabra A, Katzmarzyk PT, et al. Physical activity in overweight and obese adolescents: Systematic review of the effects on physical fitness components and cardiovascular risk factors. Sports Med. 2014;44:1139–52. doi: 10.1007/s40279-014-0193-7. [DOI] [PubMed] [Google Scholar]
- 20.Kamal NN, Ragy MM. The effects of exercise on C-reactive protein, insulin, leptin and some cardiometabolic risk factors in Egyptian children with or without metabolic syndrome. Diabetol Metab Syndr. 2012;4(1):27. doi: 10.1186/1758-5996-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verduci E, Lassandro C, Giacchero R, et al. Change in metabolic profile after 1-year nutritional-behavioral intervention in obese children. Nutrients. 2015;7:10089–99. doi: 10.3390/nu7125520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbst A, Kapellen T, Schober E, et al. Impact of regular physical activity on blood glucose control and cardiovascular risk factors in adolescents with type 2 diabetes mellitus – a multicenter study of 578 patients from 225 centres. Pediatr Diabetes. 2014;16:204–10. doi: 10.1111/pedi.12144. [DOI] [PubMed] [Google Scholar]
- 23.Grace J, Biggs C, Naicker A, Moss S. Effect of physical activity and nutrition education on body mass index, blood pressure and biochemical variables in overweight and obese adolescents. Ann Glob Health. 2021;87(1):9. doi: 10.5334/aogh.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bray GA, Kim KK, Wilding JPH World Obesity Federation. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18:715–23. doi: 10.1111/obr.12551. [DOI] [PubMed] [Google Scholar]
- 25.Cuda S, Censani M, O’Hara V, et al. Pediatric obesity algorithm. Obesity Medicine Association; 2020–2022. Available from: www.obesitymedicine.org/childhood-obesity. [Google Scholar]
- 26.Rubino F, Puhl RM, Cummings DE, et al. Joint international consensus statement for ending stigma of obesity. Nat Med. 2020;26:485–97. doi: 10.1038/s41591-020-0803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thaker VV. Genetic and epigenetic causes of obesity. Adolesc Med State Art Rev. 2017;28:379–405. [PMC free article] [PubMed] [Google Scholar]
- 28.Morton GJ, Cummings DE, Baskin DG, et al. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 29.Busnatu SS, Serbanoiu LI, Lacraru AE, et al. Effects of exercise in improving cardiometabolic risk factors in overweight children: A systematic review and meta-analysis. Healthcare (Basel) 2022;10(1):82. doi: 10.3390/healthcare10010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall KD, Heymsfield SB, Kemnitz JW, et al. Energy balance and its components: Implications for body weight regulation. Am J Clin Nutr. 2012;95:989–94. doi: 10.3945/ajcn.112.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chamorro R, Jouffe C, Oster H, et al. When should I eat: A circadian view on food intake and metabolic regulation. Acta Physiol (Oxf) 2023;237(3):e13936. doi: 10.1111/apha.13936. [DOI] [PubMed] [Google Scholar]
- 32.Claes L, Jeannin R, Braet C. Obesity: Etiology, assessment and treatment. Comprehensive Clinical Psychology. 2022;8:388–405. [Google Scholar]
- 33.Sharma AM, Padwal R. Obesity is a sign-over-eating is a symptom: An aetiological framework for the assessment and management of obesity. Obes Rev. 2010;11:362–70. doi: 10.1111/j.1467-789X.2009.00689.x. [DOI] [PubMed] [Google Scholar]
- 34.Hampl SE, Hassink SG, Skinner AC, et al. Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics. 2023;151(2):e2022060640. doi: 10.1542/peds.2022-060640. [DOI] [PubMed] [Google Scholar]
- 35.Busnatu SS, Serbanoiu LI, Lacraru AE, et al. Effects of exercise in improving cardiometabolic risk factors in overweight children: A systematic review and meta-analysis. Healthcare (Basel) 2022;10(1):82. doi: 10.3390/healthcare10010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadoh WE, Sadoh AE, Onyiriuka AN. Physical activity, body mass index and blood pressure in primary school pupils attending private schools. Afr Health Sci. 2016;16:947–53. doi: 10.4314/ahs.v16i4.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delgado-Floody P, Alvarez C, Caamaño-Navarrete F, et al. Influence of Mediterranean diet adherence, physical activity patterns, and weight status on cardiovascular response to cardiorespiratory fitness test in Chilean school children. Nutrition. 2020;71:110621. doi: 10.1016/j.nut.2019.110621. [DOI] [PubMed] [Google Scholar]
- 38.Obesity and overweight Key facts. World Health Organization; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [Google Scholar]
- 39.Aguilar-Cordero MJ, Rodríguez-Blanque R, Leon-Ríos X, et al. Influence of physical activity on blood pressure in children with overweight/obesity: A randomized clinical trial. Am J Hypertens. 2020;33:131–36. doi: 10.1093/ajh/hpz174. [DOI] [PubMed] [Google Scholar]
- 40.Howie EK, McVeigh JA, Smith AJ, et al. Physical activity trajectories from childhood to late adolescence and their implications for health in young adulthood. Prev Med. 2020;139:106224. doi: 10.1016/j.ypmed.2020.106224. [DOI] [PubMed] [Google Scholar]
- 41.Gallardo-Escribano C, Vargas-Candela A, Vilches-Perez A, et al. Lifestyle modification improves insulin resistance and carotid intima-media thickness in a metabolically healthy obese prepubescent population. J Pediatr Gastroenterol Nutr. 2021;72:127–34. doi: 10.1097/MPG.0000000000002901. [DOI] [PubMed] [Google Scholar]
- 42.Kokkvoll AS, Grimsgaard S, Flægstad T, et al. No additional long-term effect of group vs individual family intervention in the treatment of childhood obesity – a randomised trial. Acta Paediatr. 2020;109:183–92. doi: 10.1111/apa.14916. [DOI] [PubMed] [Google Scholar]
- 43.de Antunes BM, Christofaro DG, Monteiro PA, et al. Effect of concurrent training on gender-specific biochemical variables and adiposity in obese adolescents. Arch Endocrinol Metab. 2015;59(4):303–9. doi: 10.1590/2359-3997000000095. [DOI] [PubMed] [Google Scholar]
- 44.Pinheiro G, Mello J, Gaya A, et al. Blood pressure in children: Association with anthropometric indicators, body composition, cardiorespiratory fitness and physical activity. Arq Bras Cardiol. 2021;116:950–56. doi: 10.36660/abc.20190520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarp J, Child A, White T, et al. International Children’s Accelerometry Database (ICAD) Collaborators. Physical activity intensity, bout-duration, and cardiometabolic risk markers in children and adolescents. Int J Obes. 2018;42:1639–50. doi: 10.1038/s41366-018-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poeta LS, de Duarte MF, Caramelli B, et al. [Effects of physical exercises and nutritional guidance on the cardiovascular risk profile of obese children]. Rev Assoc Med Bras (1992) 2013;59(1):56–63. [PubMed] [Google Scholar]
- 47.Ogawa M, Sagayama H, Tamai S, et al. Comparative evaluation of obesity-related parameters in junior sumo wrestlers and children with obesity. Phys Act Nutr. 2021;25:36–43. doi: 10.20463/pan.2021.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oermann MH, Knafl KA. Strategies for completing a successful integrative review. Nurse Author Ed. 2021;31:65–68. [Google Scholar]
- 49.Souza MT, Silva MD, Carvalho Rd. Integrative review: What is it? How to do it? Einstein (Sao Paulo) 2010;8:102–6. doi: 10.1590/S1679-45082010RW1134. [DOI] [PubMed] [Google Scholar]
- 50.Systematic vs Scoping vs Integrative Review. Duquesne University; 2022. Available from: https://guides.library.duq.edu/c.php?g=1055475&p=7725920. [Google Scholar]
- 51.Rayyan AI powered tool for systematic literature reviews. Available from: https://www.rayyan.ai.
- 52.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ingham-Broomfield R. A nurses’ guide to the hierarchy of research designs and evidence. Aust J Adv Nurs. 2011;33(3):38–43. [Google Scholar]
- 54.Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol. 2008;8:45. doi: 10.1186/1471-2288-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tawfik GM, Dila KAS, Mohamed MYF, et al. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop Med Health. 2019;47:46. doi: 10.1186/s41182-019-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dobbins M, Husson H, DeCorby K, LaRocca RL. School-based physical activity programs for promoting physical activity and fitness in children and adolescents aged 6 to 18. Cochrane Database Syst Rev. 2013;2013(2):CD007651. doi: 10.1002/14651858.CD007651.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martínez-Vizcaíno V, Sánchez-López M, Notario-Pacheco B, et al. Gender differences on effectiveness of a school-based physical activity intervention for reducing cardiometabolic risk: A cluster randomized trial. Int J Behav Nutr Phys Act. 2014;11:154. doi: 10.1186/s12966-014-0154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D’Agostino EM, Patel HH, Hansen E, et al. Effect of participation in a park-based afterschool program on cardiovascular disease risk among severely obese youth. Public Health. 2018;159:137–43. doi: 10.1016/j.puhe.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 59.Chansavang Y, Elley CR, McCaffrey B, et al. Feasibility of an after-school group-based exercise and lifestyle programme to improve cardiorespiratory fitness and health in less-active Pacific and Maori adolescents. J Prim Health Care. 2015;7:57–64. [PubMed] [Google Scholar]
- 60.D’Agostino EM, Patel HH, Hansen E, et al. Longitudinal analysis of cardiovascular disease risk profile in neighbourhood poverty subgroups: 5-year results from an afterschool fitness programme in the USA. J Epidemiol Community Health. 2018;72:193–201. doi: 10.1136/jech-2017-209333. [DOI] [PubMed] [Google Scholar]
- 61.Kılınç FN, Çağdaş DN. Diet and physical activity interventions do have effects on body composition and metabolic syndrome parameters in overweight and obese adolescents and their mothers. Turk J Pediatr. 2013;55:292–99. [PubMed] [Google Scholar]
- 62.Pepera G, Hadjiandrea S, Iliadis I, et al. Associations between cardiorespiratory fitness, fatness, hemodynamic characteristics, and sedentary behavior in primary school-aged children. BMC Sports Sci Med Rehabil. 2022;14(1):16. doi: 10.1186/s13102-022-00411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fridolfsson J, Buck C, Hunsberger M, et al. High-intensity activity is more strongly associated with metabolic health in children compared to sedentary time: A cross-sectional study of the I.Family cohort. Int J Behav Nutr Phys Act. 2021;18(1):90. doi: 10.1186/s12966-021-01156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonçalves R, Szmuchrowski LA, Damasceno VO, et al. [Association of body mass index and aerobic physical fitness with cardiovascular risk factors in children]. Rev Paul Pediatr. 2014;32:208–14. doi: 10.1590/0103-0582201432310. [in Portuguese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willis EA, Ptomey LT, Szabo-Reed AN, et al. Length of moderate-to-vigorous physical activity bouts and cardio-metabolic risk factors in elementary school children. Prev Med. 2015;73:76–80. doi: 10.1016/j.ypmed.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delgado-Floody P, Palomino-Devia C, Zulic-Agramunt C, et al. Psychosocial, physical and anthropometric variables in Chilean schoolchildren. A comparative study according to physical activity levels. Arch Med Deporte. 2019;36:151–56. [Google Scholar]
- 67.Ostman C, Jewiss D, King N, et al. Clinical outcomes to exercise training in type 1 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2018;139:380–91. doi: 10.1016/j.diabres.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 68.Aadland E, Andersen LB, Anderssen SA, et al. Accelerometer epoch setting is decisive for associations between physical activity and metabolic health in children. J Sports Sci. 2020;38:256–63. doi: 10.1080/02640414.2019.1693320. [DOI] [PubMed] [Google Scholar]
- 69.Macdonald-Wallis C, Solomon-Moore E, Sebire SJ, et al. A longitudinal study of the associations of children’s body mass index and physical activity with blood pressure. PLoS One. 2017;12(12):e0188618. doi: 10.1371/journal.pone.0188618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mendoza JA, Nicklas TA, Liu Y, et al. General versus central adiposity and relationship to pediatric metabolic risk. Metab Syndr Relat Disord. 2012;10:128–36. doi: 10.1089/met.2011.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Messiah SE, Vidot DE, Hansen E, et al. Impact of a park-based afterschool program replicated over five years on modifiable cardiovascular disease risk factors. Prev Med. 2017;95:66–73. doi: 10.1016/j.ypmed.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 72.Stoner L, Beets MW, Brazendale K, et al. Exercise dose and weight loss in adolescents with overweight-obesity: A meta-regression. Sports Med. 2019;49:83–94. doi: 10.1007/s40279-018-01040-2. [DOI] [PubMed] [Google Scholar]
- 73.Mazur A, Zachurzok A, Baran J, et al. Childhood obesity: Position statement of Polish Society of Pediatrics, Polish Society for Pediatric Obesity, Polish Society of Pediatric Endocrinology and Diabetes, the College of Family Physicians in Poland and Polish Association for Study on Obesity. Nutrients. 2022;14(18):3806. doi: 10.3390/nu14183806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gopinath B, Hardy LL, Kifley A, et al. Activity behaviors in schoolchildren and subsequent 5-yr change in blood pressure. Med Sci Sports Exerc. 2014;46:724–29. doi: 10.1249/MSS.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 75.Fam B, Amouzegar A, Arzhan S, et al. Association between physical activity and metabolic risk factors in adolescents: Tehran Lipid and Glucose Study. Int J Prev Med. 2013;4:1011–17. [PMC free article] [PubMed] [Google Scholar]
- 76.Plavsic L, Knezevic OM, Sovtic A, et al. Effects of high-intensity interval training and nutrition advice on cardiometabolic markers and aerobic fitness in adolescent girls with obesity. Appl Physiol Nutr Metab. 2020;45:294–330. doi: 10.1139/apnm-2019-0137. [DOI] [PubMed] [Google Scholar]
- 77.Carson V, Tremblay MS, Chaput JP, et al. Compositional analyses of the associations between sedentary time, different intensities of physical activity, and cardiometabolic biomarkers among children and youth from the United States. PLoS One. 2019;14(7):e0220009. doi: 10.1371/journal.pone.0220009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Racil G, Coquart JB, Elmontassar W, et al. Greater effects of high- compared with moderate-intensity interval training on cardio-metabolic variables, blood leptin concentration and ratings of perceived exertion in obese adolescent females. Biol Sport. 2016;33:145–52. doi: 10.5604/20831862.1198633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pablos A, Nebot V, Vañó-Vicent V, et al. Effectiveness of a school-based program focusing on diet and health habits taught through physical exercise. Appl Physiol Nutr Metab. 2018;43:331–37. doi: 10.1139/apnm-2017-0348. [DOI] [PubMed] [Google Scholar]
- 80.Brzeziński M, Korzeniowska K, Szarejko K, et al. “PoZdro!” as an example of a successful multicenter programme for obesity management and healthy lifestyle promotion in children and adolescents – programme protocol and preliminary results from the first intervention site. Pediatr Endocrinol Diabetes Metab”. 2020;26:22–26. doi: 10.5114/pedm.2020.94393. [DOI] [PubMed] [Google Scholar]
- 81.Cuenca-García M, Ortega FB, Ruiz JR. Combined influence of healthy diet and active lifestyle on cardiovascular disease risk factors in adolescents. Scand J Med Sci Sports. 2014;24:553–62. doi: 10.1111/sms.12022. [DOI] [PubMed] [Google Scholar]
- 82.Horner K, Kuk JL, Barinas-Mitchell E, et al. Effect of aerobic versus resistance exercise on pulse wave velocity, intima media thickness and left ventricular mass in obese adolescents. Pediatr Exerc Sci. 2015;27:494–502. doi: 10.1123/pes.2015-0067. [DOI] [PubMed] [Google Scholar]
- 83.Cesa CC, Molino GOG, Lima J, et al. Physical activity and cardiovascular risk factors in children: A meta-analysis update. Int J Cardiovasc Sci. 2022;35:304–15. [Google Scholar]
- 84.Chaput JP, Willumsen J, Bull F, et al. 2020 WHO guidelines on physical activity and sedentary behavior for children and adolescents aged 5–17 years: Summary of the evidence. Int J Behav Nutr Phys Act. 2020;17:141. doi: 10.1186/s12966-020-01037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Candio P, Meads D, Hill AJ, et al. Modelling the impact of physical activity on public health: A review and critique. Health Policy. 2020;124:1155–64. doi: 10.1016/j.healthpol.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 86.Gebreslassie M, Sampaio F, Nystrand C, et al. Economic evaluations of public health interventions for physical activity and healthy diet: A systematic review. Prev Med. 2020;136:106100. doi: 10.1016/j.ypmed.2020.106100. [DOI] [PubMed] [Google Scholar]
- 87.Laine J, Kuvaja-Köllner V, Pietilä E, et al. Cost-effectiveness of population-level physical activity interventions: A systematic review. Am J Health Promot. 2014;29(2):71–80. doi: 10.4278/ajhp.131210-LIT-622. [DOI] [PubMed] [Google Scholar]
- 88.Valkenborghs SR, Noetel M, Hillman CH, et al. The impact of physical activity on brain structure and function in youth: A systematic review. Pediatrics. 2019;144(4):e20184032. doi: 10.1542/peds.2018-4032. [DOI] [PubMed] [Google Scholar]
- 89.Guthold R, Stevens GA, Riley LM, Bull FC. Global trends in insufficient physical activity among adolescents: A pooled analysis of 298 population-based surveys with 1·6 million participants. Lancet Child Adolesc Health. 2020;4(1):23–35. doi: 10.1016/S2352-4642(19)30323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zembura P, Korcz A, Nałęcz H, Cieśla E. Results from Poland’s 2022 Report Card on Physical Activity for Children and Youth. Int J Environ Res Public Health. 2022;19(7):4276. doi: 10.3390/ijerph19074276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spinelli A, Buoncristiano M, Kovacs VA, et al. Prevalence of severe obesity among primary school children in 21 European countries. Obes Facts. 2019;12:244–58. doi: 10.1159/000500436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nogueira-de-Almeida CA, Del Ciampo LA, Ferraz IS, et al. COVID-19 and obesity in childhood and adolescence: A clinical review. J Pediatr. 2020;96(5):546–58. doi: 10.1016/j.jped.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stockwell S, Trott M, Tully M, et al. Changes in physical activity and sedentary behaviors from before to during the COVID-19 pandemic lockdown: A systematic review. BMJ Open Sport Exerc Med. 2021;7(1):e000960. doi: 10.1136/bmjsem-2020-000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Groffik D, Fromel K, Ziemba M, et al. Trends in physical activity in adolescents participating and not participating in organized team or individual physical activity. Ann Agric Environ Med. 2023:aaem162040. doi: 10.26444/aaem/162040. [DOI] [PubMed] [Google Scholar]
- 95.Bronikowska M, Krzysztoszek J, Łopatka M, et al. Comparison of physical activity levels in youths before and during a pandemic lockdown. Int J Environ Res Public Health. 2021;18(10):5139. doi: 10.3390/ijerph18105139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Epstein LH, Wilfley DE, Kilanowski C, et al. Family-based behavioral treatment for childhood obesity implemented in pediatric primary care: A randomized clinical trial. JAMA. 2023;329(22):1947–56. doi: 10.1001/jama.2023.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mozaffarian D. Implications of the New recommendation on behavioral counseling interventions to promote healthy eating and physical activity. JAMA. 2022;328(4):334–35. doi: 10.1001/jama.2022.10801. [DOI] [PubMed] [Google Scholar]



