Abstract
Purpose of review:
Hemophilia is an X-linked blood coagulation genetic disorder, which can cause significant disability. Replacement therapy for coagulation factor VIII (hemophilia A) or factor IX (hemophilia B) may result in the development of high affinity alloantibodies (“inhibitors”) to the replacement therapy, thus making it ineffective. Therefore, there is interest in directing immunological responses towards tolerance to infused factors.
Recent findings:
In this review we will discuss latest advancements in the development of potentially less immunogenic replacement clotting factors, optimization of current tolerance induction protocols (ITI), pre-clinical and clinical data of pharmacological immune modulation, hepatic gene therapy, and the rapidly advancing field of cell therapies. We will also evaluate publications reporting data from pre-clinical studies on oral tolerance induction using chloroplast-transgenic (transplastomic) plants.
Summary:
Until now, no clinical prophylactic immune modulatory protocol exists to prevent inhibitor formation to infused clotting factors. Recent innovative technologies provide hope improved eradication and perhaps even prevention of inhibitors.
Keywords: inhibitors, ITI, oral tolerance, gene therapy
Introduction
Hemophilia is an X-linked monogenic blood coagulation disorder with significant morbidity, affecting primarily males [1]. Mutations in F8 and F9 genes lead to development of hemophilia A and B, respectively, of which hemophilia A is more prevalent (1:5,000 male births) [2,3]. Severity of hemophilia is based on residual activity of coagulation factors, where levels of <1% are classified as severe, 1–5% moderate, and 5–40% mild [4]. Factor replacement is used for therapy and prophylaxis since the 1950’s. An increasing number of plasma-derived and recombinant FVIII and FIX products are available or in clinical development.
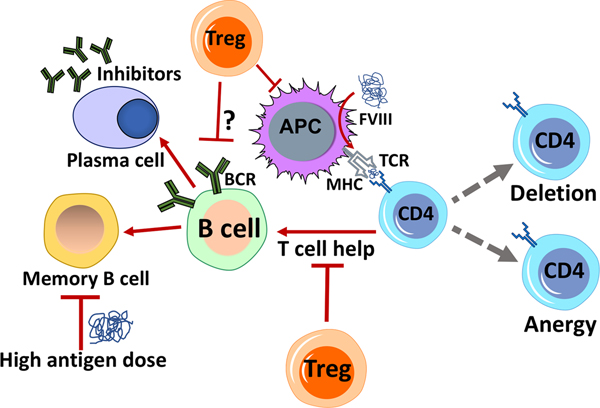
Development of inhibitory antibodies (inhibitors) is a major complication of replacement therapy, making it ineffective [5]. Inhibitors are high affinity alloantibodies directed against infused clotting factors, and are more common in hemophilia A patients [6]. Less than 5% of patients with severe hemophilia B develop inhibitors, mostly those with large F9 gene deletions [7]. Inhibitors are usually observed in previously untreated patients (PUP) within 50 days of exposure to factor product [6,8]. Bypassing agents are used to treat bleeding episodes, but the only way to eradicate inhibitors is through Immune Tolerance Induction (ITI). The mechanism of inhibitor formation is complex and not completely understood. Immune responses are initiated when antigen-presenting cells (APCs) encounter the “unfamiliar” replacement factor molecule and present it via HLA class II, marking it for elimination. Co-stimulatory signals via cell surface molecules such as cluster of differentiation 40 (CD40), or CD80/86 (which are found in dendritic cells and required for T cell survival), or ICOS (“inducible co-stimulator”), and secretion of pro-inflammatory cytokines are required for T cell activation [9]. CD4+ T helper cells enable B cell activation and differentiation into memory B cells or antibody producing plasma cells, which neutralize the infused clotting factor [10] (Figure 1).
Figure 1.
Overview of key cellular components that modulate inhibitor formation in hemophilia. Professional antigen presenting cells (APCs) such as dendritic cells present peptides derived from FVIII or FIX via their MHC II molecules to CD4+ T cells. These may differentiate into T helper cells and aid in the activation of B cells (which in turn produce the inhibitory antibodies). Peptide presentation by APCs to CD4+ T cells may also result in the induction of Tregs (regulatory CD4+ T cells) that actively suppress immune responses. Inhibitor development is largely dependent on CD4+ T cell help, which can, however, be suppressed by Tregs. Factors that lead to CD4+ T cell anergy (unresponsiveness) or their deletion also contribute to the prevention of the immune response. It is unclear whether Tregs can directly interact with and suppress activated B cells from differentiating into memory B cells or directly suppress inhibitor-producing plasma cells. High antigen doses directly inhibit memory B cells, leading to down-regulation of inhibitor formation.
Treatment of hemophilia – replacement therapies and bypassing strategies
Clotting factor replacement products can be used prophylactically or “on demand” in trauma or surgical intervention. Factor products have a relatively short half-life and require frequent administration [11,12,13]. One approach to increase systemic retention of clotting factors is pegylation. Pegylated factor products (Adynovate, Rebinyn) require less frequent administration than plasma derived or recombinant products [14]. IgG1 Fc conjugated clotting factor (Eloctate, Alprolix) can also prolong half life by binding to the neonatal Fc receptor (FcRn) and delaying lysosomal degradation [15–17]. There are also reports that Fc fusion products may be more tolerogenic, as suggested by pre-clinical studies and when used in ITI in a pediatric patient [18,19]. While the mechanism of tolerance by Fc-conjugated clotting factor is not completely understood, it has been suggested that upon degradation, the Fc portion is processed and presented by APCs (antigen presenting cells) by MHC II and promotes induction of regulatory T cells (Tregs) [20]. Fc fusion proteins also offer the ability for transplacental delivery of FVIII antigen, which can be exploited for perinatal tolerance induction. Transfer of maternal IgG to the fetal circulation is mediated by transcytosis upon binding to FcRn. Intravenous co-delivery of Fc fusions of A2 and C2 domains to pregnant hemophilia A mice during the third trimester suppressed inhibitor formation in the off-spring after challenge with FVIII at 2 months of age [21]. Furthermore, mechanistic studies with a model antigen revealed the ability to generate central tolerance and development of antigen-specific CD4+CD25+FoxP3+ Treg.
Bypassing agents can be used to restore hemostasis in patients with inhibitors. One experimental bypassing agent, which mimics the function of FVIII by binding to activated FIXa and FX, is the human, bispecific monoclonal antibody Emicizumab, recently approved as Hemlibra (Genentech Inc, San Francisco, CA). Emicizumab, when prophylactically administered once per week by the subcutaneous route, significantly reduced the number of bleeding episodes in patients with inhibitors [22,23]. No anti-drug antibodies were detected [24].
An alternative bypassing agent, Fitusiran (Alnylam Pharmaceuticals, Cambridge, MA) can be used to treat both hemophilia A and B [25]. Filtusiran is an RNA interference (RNAi) molecule that targets antithrombin, promoting blood coagulation, and is currently undergoing clinical trials.
Inhibitor formation and eradication - current ITI protocols
To date, the only clinically proven method to eradicate inhibitors and induce antigen specific tolerance to infused FVIII or FIX is ITI [26]. During ITI, patients receive daily, high dose (up to 200 IU/kg/day) infusions of clotting factors [27,28]. One of the working mechanistic theories of ITI is that repetitive, high doses of antigen can suppress activated T cell responses by overstimulation with antigen, followed by anergy and deletion [29], and has also been suggested to induce Tregs [10,30]. High antigen doses also eliminate antigen-specific memory B cells, as was demonstrated in hemophilia A mice [5,31,32]. Outcome of ITI is variable: it is successful in 60–70% of hemophilia A patients but only 30% of hemophilia B patients [5,33]. ITI in patients with FIX inhibitors often has to be discontinued due to severe allergic reactions to FIX or development of nephrotic syndrome [7,34,35,36]. The mechanism leading to nephrotic syndrome is poorly understood. This complication typically occurs 8–9 months into high-dose ITI in patients with previous allergic phenotype, and likely reflects an immunotoxicity. Even in patients with successful outcome of ITI, inhibitors can reappear once they are re-exposed to regular factor infusions when antigen-specific T and memory B cells become re-activated [37,38]. This review will explore new approaches of treating hemophilia through developing novel tolerance induction protocols.
Immune modulatory drugs
An obvious approach to reduce inhibitor formation is the use of immune suppressive drugs, similar to their use for autoimmune diseases or in organ or cell transplantation. However, more desirable than general immune suppression is a targeted intervention promoting immune tolerance. Various approaches, including transient blockage of different pathways that promote co-stimulation to CD4+ T cells, have been successfully employed in hemophilic mice [39].
Conceptually, shifting the balance of the T cell response from an effector to a Treg phenotype should induce immune tolerance (Figure 2). In this regard, the mTOR inhibitor rapamycin is ideally suitable, as antigen presentation in the presence of rapamycin causes programmed cell death of conventional CD4+ T cells, while CD4+CD25+FoxP3+ Treg are induced and expanded. Differential use of signaling pathways and metabolic requirements account for the ability of Treg to survive in the presence of rapamycin [40]. A 1-month regimen of FVIII and rapamycin tolerized hemophilia A mice to FVIII without compromising immune competence [41]. Unless peptide antigens are used, representing CD4+ T cell epitopes, FVIII or FIX protein antigen dose during the regimen is a critical parameter. The effect of rapamycin can be enhanced through combination with other drugs, for example to manipulate dendritic cells in a manner that augments Treg induction [40,42,43]. In an alternative method, co-administration of FVIII and rapamycin containing nanoparticles that are based on biodegradable polymers (Polylactic-co-glycolic acid, PLGA) prevented and reversed inhibitors in hemophilia A mice [44,45]. Interestingly, the antigen (peptide or protein) does not have to be co-packed with rapamycin into the nanoparticles.
Figure 2.
Experimental approaches to promote tolerance to clotting factor therapy that may serve to replace or supplement conventional ITI. In vivo gene transfer into the tolerogenic liver microenvironment using either AAV or lentiviral vectors promoted tolerance in several pre-clinical models. The immunomodulatory drug rapamycin, either alone or encapsulated in nanoparticles, is tolerogenic when co-administered with antigen. Fc-conjugation of FVIII/FIX may not only increase half-life in circulation but also facilitate tolerance induction. Oral administration of lettuce-encapsulated clotting factor leads to tolerance, which is mediated by the induction of at least two types of Tregs. Cellular therapy with either ex vivo expanded Tregs, engineered Tregs expressing specific TCRs, or CAR-Tregs that have receptor specificity to clotting factor without MHC restriction, have all been shown to promote tolerance in animal models of hemophilia.
Direct targeting of B cells to eliminate anti-FVIII formation has been tested in humans in a Phase II clinical study. However, the B cell depleting monoclonal anti-CD20 (Rituximab) showed only a modest effect in reducing existing inhibitors and preventing anamnesis after repeated exposure. [46]. One limitation may be that antibody-producing plasma cells do not express CD20 and are therefore not targeted. Addition of a specific tolerance-inducing step may also be necessary to prevent inhibitor relapse. A recent study in hemophilia A mice found that anti-CD20 and rapamycin can be combined for more effective reversal of inhibitors [47]. This approach was superior to using either drug alone or the combination of rapamycin and Treg therapy.
Tolerance induction via gene therapy
Gene therapy with adeno-associated viral (AAV) vectors has resulted in sustained correction of monogenic disorders in multiple recent gene therapy trials The first FDA approved gene therapy drug for a genetic disease (Luxturna), to correct an inherited form of blindness, is based on an AAV vector [48,49]. AAV is a small, non-pathogenic, non-integrating, replication deficient parvovirus with vast tissue tropism. It has a packaging capacity ~5kb, and can transduce non-dividing cells in vivo [50]. The liver is an ideal target for gene delivery for hemophilia A and B. Both FVIII and FIX is synthetized and secreted by the liver; FIX is produced by hepatocytes while FVIII is primarily made in endothelial cells [51,52]. Due to the small size of the F9 cDNA, a powerful expression cassette F9 can be easily packaged when incorporated into the AAV genome. Several clinical trials for hemophilia B with liver directed AAV delivery either ongoing or completed [49,53,54] [55,56]. Follow-up in both long-term and a recent trial by Spark Therapeutics demonstrated sustained expression of near normal FIX activity after gene transfer [48,53]. By using the hyperactive FIX-Padua variant, it was possible to use a vector dose 5–10-fold lower compared to previous trials without compromising therapy [53]. Packaging of FVIII into AAV is more challenging due to its large size. Nonetheless, use of the B-domain deleted FVIII transgene, optimization of small promoters and use of codon-optimization substantially improved gene expression. In a first successful clinical trial by BioMarin for severe hemophilia A, seven patients received IV injection of a high dose (6 × 1013 vector genomes [vg]/kg) of such a cassette packaged into AAV5. All patients showed sustained expression of functional FVIII one year after gene transfer [58], with no spontaneous bleeds. Rather than targeting the natural site of FVIII expression (endothelial cells), gene expression in this approach occurs in hepatocytes from a cell type-specific promoter.
Importantly, the liver represents a tolerogenic site of gene expression, as it is constantly exposed to foreign proteins from the gut and circulation [59]. Immune tolerance to FVIII and FIX induced by hepatic gene transfer depends on induction and expansion of antigen-specific CD4+CD25+FoxP3+ Tregs, which actively suppress antibody and T cell responses [60,61]. At the same time, activation induced cell death is required to induce tolerance [61]. Thus, tolerance is maintained upon subsequent IV delivery of factor product or secondary gene transfer [62–64]. Multiple studies in hemophilic dogs demonstrate that hepatic AAV gene transfer suppresses inhibitor formation and can even eradicate pre-existing inhibitors [65–67]. This was also successful and safe in hemophilia B mice with pre-existing IgE/anaphylactic reactions against FIX [63]. Tregs were found to be critical for both induction and maintenance of tolerance. Tolerance can also be achieved by hepatic gene transfer using lentiviral vectors with optimized regulation of gene expression using microRNA targets that eliminate expression in certain antigen presenting cells and/or cell-type-specific promoters [68–70]. Interestingly, tolerance is established by targeted expression to hepatocytes, liver sinusoidal endothelial cells, or myeloid cells alone. Avoiding expression in plasmacytoid dendritic cells appears crucial to the success of these approaches [70,71].
Tolerogenic cell therapies
Dramatic advances have been made in recent years in cancer immunotherapy using gene modified lymphocytes. Similar concepts may apply to tolerance induction in hemophilia [72]. For example, primary B cells can be engineered to induce tolerance to FVIII or FIX [73–75]. However, production of vectors that efficiently transduce human B cells remains a limitation. Alternatively, engineering of Treg is an emerging and promising concept [76,77]. Substantial progress has been made with ex vivo expansion of human Treg, and gene transfer technologies for their engineering are available and approved for clinical use [78–81]. In hemophilic mice, transplant of autologous ex vivo expanded Treg promoted tolerance induction to FVIII and FIX, likely by reducing the ability of dendritic cells to provide co-stimulation and by promoting induction of endogenous Treg [82]. Nonetheless, an antigen-specific approach, requiring fewer Treg for transplant, would be more desirable. Antigen specificity of Tregs can be re-directed to FVIII by transducing them with T cell receptors (TCR) specific for a FVIII MHC II-peptide complex. Tregs expressing an HLA-DRA DRB1*0101 restricted T cell epitope specific to the C2 protein of FVIII were indeed shown to suppress antibody formation against FVIII in vivo upon transplant into “humanized” hemophilia A mice [83]. Utilizing chimeric artificial receptors (CARs) would eliminate MHC restrictions and therefore the need to customize the receptor for individual patients. In a first proof-of-principle study, a single-chain variable fragment (scFv) of a FVIII-A2 domain epitope immunoglobulin was isolated from a phage library and linked to 2nd generation intracellular signal transduction domains to direct a response to FVIII upon gene transfer to human CD4+CD25+CD127lo Treg. Effectiveness of this approach was demonstrated in hemophilia A mice [77] (Figure 2). In vitro studies suggest that these CAR-Treg still require antigen presenting cells for stimulation although they do not rely on recognition of peptide-MHC complexes [77]. Since these studies on engineered FVIII-specific Treg utilized human cells transplanted into mice for in vivo evaluation, durability of this approach awaits further study, as does evaluation in pre-immune animals compared to prevention of inhibitor formation.
Oral tolerance
Perhaps more readily acceptable as a prophylactic regimen to prevent inhibitor formation in PUPs could be to induce systemic immune tolerance for hemophilia A and B by delivering the antigen orally [84,85]. This concept, relies on the ability of the gut to control unwanted responses to food antigens through a complex immune regulatory mechanism [86–88]. A growing body of evidence now supports the usefulness of oral tolerance to combat food allergies [89,90]. Transgenic plant cells producing high amounts of clotting-factor in chloroplasts (“transplastomic” plants) provides low production costs and bioencapsulation via the plant cell wall [91]. Commensal bacteria in the gut are able to degrade cell wall components, thus releasing the antigen. Expression of a FVIII or FIX fusion protein that includes a transmucosal carrier such as CTB (cholera toxin B subunit) assures effective delivery across the gut epithelium [92].
This concept was successfully employed to prevent inhibitor formation and anaphylaxis against FIX in hemophilia B mice using initially tobacco, and subsequently lettuce plants [93,94], upon repeated oral delivery (2x/week for 2 months) over a wide dose range. Intact antigen could be stored for at least 2 years in form of powder of freeze-dried plant cells. Production of clinical grade material was efficient and could be scaled-up in a hydroponic system [94]. Repeated oral delivery to hemophilia B dogs suppressed antibody formation against FIX, so that correction of coagulation was consistently achieved upon weekly IV delivery of human FIX over an 8-week period [95]. Experiments in mice also showed the ability to reverse the pathogenic antibody response against FIX [87]. Mechanistic studies showed increases in subsets of dendritic cells in the immune system of the small intestine that are known to promote Treg induction, antigen-specific up-regulation of immune suppressive cytokines in response, and induction of CD4+CD25+FoxP3+ as well as latency-associated peptide (LAP)+ Tregs that actively suppressed antibody formation upon adoptive transfer [87]. Oral delivery of combination of either heavy chain and C2 or heavy chain and light chain antigens (derived from B domain deleted, BDD, FVIII) suppressed inhibitor formation against FVIII in different strains of hemophilia A mice, again at low antigen doses [96,97].
Conclusion/Summary:
In conclusion, a number of recent innovative and diverse approaches have been developed to induce immune tolerance to coagulation factors. These include fusion proteins, oral tolerance, immune modulatory drugs, in vivo gene therapies, cell therapies, among others. Until now, no clinical prophylactic immune tolerance protocol exists to prevent inhibitor formation in PUPs, which may change with the emergence of these new technologies, pending further studies and risk-benefit analysis. Complexity and costs also vary substantially for these diverse approaches, representing additional factors that may affect feasibility for routine clinical use.
Key points:
Formation of inhibitory antibodies against coagulation factors is a major problem in the treatment of hemophilia
Diverse and innovative approaches to tolerance induction have emerged in recent years
Gene therapies are being developed that can simultaneously achieve correction of hemophilia and immune tolerance
Immunomodulatory drugs and oral tolerance may accomplish prophylactic tolerance induction
Tolerogenic lymphocyte therapies, foe example using engineered regulatory T cells, hold promise
Acknowledgements:
We like to thanks Drs. David Markusic and Sandeep Kumar for suggestions.
Financial support and sponsorship:
Some of the work reviewed in this article has been supported by NIH grants R01 HL133191, R01 HL131093, R01 HL097088, and R01 AI51390 to RWH. MB was supported by a Scientist Development Grant from the Bayer Hemophilia Awards Program.
Footnotes
Conflicts of interest: RWH is member of the scientific advisory board of AGCT (Applied Genetic Technologies Corporation) and has received royalty payments from Spark Therapeutics as well as grant support from Novo Nordisk and Bayer in the past.
References:
- 1.Young G: New challenges in hemophilia: long-term outcomes and complications. Hematology Am Soc Hematol Educ Program 2012, 2012:362–368. [DOI] [PubMed] [Google Scholar]
- 2.Franchini M, Mannucci PM: Past, present and future of hemophilia: a narrative review. Orphanet J Rare Dis 2012, 7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stonebraker JS, Bolton-Maggs PH, Michael Soucie J, Walker I, Brooker M: A study of variations in the reported haemophilia B prevalence around the world. Haemophilia 2012, 18:e91–94. [DOI] [PubMed] [Google Scholar]
- 4.White GC 2nd, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J, Factor V, Factor IXS: Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost 2001, 85:560. [PubMed] [Google Scholar]
- 5.Kempton CL, Meeks SL: Toward optimal therapy for inhibitors in hemophilia. Blood 2014, 124:3365–3372. [DOI] [PubMed] [Google Scholar]
- 6.Bertamino M, Riccardi F, Banov L, Svahn J, Molinari AC: Hemophilia Care in the Pediatric Age. J Clin Med 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castaman G, Bonetti E, Messina M, Morfini M, Rocino A, Scaraggi FA, Tagariello G, Italian Association of Hemophilia C: Inhibitors in haemophilia B: the Italian experience. Haemophilia 2013, 19:686–690. [DOI] [PubMed] [Google Scholar]
- 8.Miller CH: Laboratory testing for factor VIII and IX inhibitors in haemophilia: A review. Haemophilia 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lillicrap D: The role of immunomodulation in the management of factor VIII inhibitors. Hematology Am Soc Hematol Educ Program 2006:421–425. [DOI] [PubMed] [Google Scholar]
- 10.Astermark J: FVIII inhibitors: pathogenesis and avoidance. Blood 2015, 125:2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, Ingram JD, Manco-Johnson ML, Funk S, Jacobson L, et al. : Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 2007, 357:535–544. [DOI] [PubMed] [Google Scholar]
- 12.Young G, Mahlangu JN: Extended half-life clotting factor concentrates: results from published clinical trials. Haemophilia 2016, 22 Suppl 5:25–30. [DOI] [PubMed] [Google Scholar]
- 13.Balkaransingh P, Young G: Novel therapies and current clinical progress in hemophilia A. Ther Adv Hematol 2018, 9:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arruda VR, Doshi BS, Samelson-Jones BJ: Novel approaches to hemophilia therapy: successes and challenges. Blood 2017, 130:2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufman RJ, Powell JS: Molecular approaches for improved clotting factors for hemophilia. Blood 2013, 122:3568–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannucci PM, Mancuso ME: Investigational drugs for coagulation disorders. Expert Opin Investig Drugs 2013, 22:945–953. [DOI] [PubMed] [Google Scholar]
- 17. Mahlangu J, Powell JS, Ragni MV, Chowdary P, Josephson NC, Pabinger I, Hanabusa H, Gupta N, Kulkarni R, Fogarty P, et al. : Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood 2014, 123:317–325. *First clincial experience with an Fc-conjugated clotting factor that may have enhanced potential for toelrance induction.
- 18.Krishnamoorthy S, Liu T, Drager D, Patarroyo-White S, Chhabra ES, Peters R, Josephson N, Lillicrap D, Blumberg RS, Pierce GF, et al. : Recombinant factor VIII Fc (rFVIIIFc) fusion protein reduces immunogenicity and induces tolerance in hemophilia A mice. Cell Immunol 2016, 301:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groomes CL, Gianferante DM, Crouch GD, Parekh DS, Scott DW, Lieuw K: Reduction of Factor VIII Inhibitor Titers During Immune Tolerance Induction With Recombinant Factor VIII-Fc Fusion Protein. Pediatr Blood Cancer 2016, 63:922–924. [DOI] [PubMed] [Google Scholar]
- 20.Blumberg RS, Lillicrap D: Tolerogenic properties of Fc portion of IgG and its relevance to the treatment and management of hemophilia. Blood 2018, 131:2205–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gupta N, Culina S, Meslier Y, Dimitrov J, Arnoult C, Delignat S, Gangadharan B, Lecerf M, Justesen S, Gouilleux-Gruart V, et al. : Regulation of immune responses to protein therapeutics by transplacental induction of T cell tolerance. Sci Transl Med 2015, 7:275ra221. *Study demonstrates the potnetial for in utero tolerance induction via maternal ransfer of clotting factor antigen.
- 22.Kitazawa T, Esaki K, Tachibana T, Ishii S, Soeda T, Muto A, Kawabe Y, Igawa T, Tsunoda H, Nogami K, et al. : Factor VIIIa-mimetic cofactor activity of a bispecific antibody to factors IX/IXa and X/Xa, emicizumab, depends on its ability to bridge the antigens. Thromb Haemost 2017, 117:1348–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchida N, Sambe T, Yoneyama K, Fukazawa N, Kawanishi T, Kobayashi S, Shima M: A first-in-human phase 1 study of ACE910, a novel factor VIII-mimetic bispecific antibody, in healthy subjects. Blood 2016, 127:1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oldenburg J, Mahlangu JN, Kim B, Schmitt C, Callaghan MU, Young G, Santagostino E, Kruse-Jarres R, Negrier C, Kessler C, et al. : Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med 2017. **Novel bypassing agent that can treat patients with inhibitors.
- 25. Pasi KJ, Rangarajan S, Georgiev P, Mant T, Creagh MD, Lissitchkov T, Bevan D, Austin S, Hay CR, Hegemann I, et al. : Targeting of antithrombin in hemophilia A or B with RNAi therapy. N Engl J Med 2017, 377:819–828. **Novel bypassing agent that can treat patients with inhibitors.
- 26.Brackmann HH, Oldenburg J, Schwaab R: Immune tolerance for the treatment of factor VIII inhibitors--twenty years’ ‘bonn protocol’. Vox Sang 1996, 70 Suppl 1:30–35. [DOI] [PubMed] [Google Scholar]
- 27.DiMichele DM: Immune tolerance in haemophilia: the long journey to the fork in the road. Br J Haematol 2012, 159:123–134. [DOI] [PubMed] [Google Scholar]
- 28.Ljung RCR: How I manage patients with inherited haemophilia A and B and factor inhibitors. Br J Haematol 2018, 180:501–510. [DOI] [PubMed] [Google Scholar]
- 29.Waters B, Lillicrap D: The molecular mechanisms of immunomodulation and tolerance induction to factor VIII. J Thromb Haemost 2009, 7:1446–1456. [DOI] [PubMed] [Google Scholar]
- 30.Reipert BM, van Helden PM, Schwarz HP, Hausl C: Mechanisms of action of immune tolerance induction against factor VIII in patients with congenital haemophilia A and factor VIII inhibitors. Br J Haematol 2007, 136:12–25. [DOI] [PubMed] [Google Scholar]
- 31.Reipert BM: B-cell memory against factor VIII. Cell Immunol 2016, 301:49–58. [DOI] [PubMed] [Google Scholar]
- 32.Oldenburg J, Austin SK, Kessler CM: ITI choice for the optimal management of inhibitor patients - from a clinical and pharmacoeconomic perspective. Haemophilia 2014, 20 Suppl 6:17–26. [DOI] [PubMed] [Google Scholar]
- 33.Hay CR, DiMichele DM, International Immune Tolerance S: The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood 2012, 119:1335–1344. [DOI] [PubMed] [Google Scholar]
- 34.DiMichele D: Inhibitor development in haemophilia B: an orphan disease in need of attention. Br J Haematol 2007, 138:305–315. [DOI] [PubMed] [Google Scholar]
- 35.Bon A, Morfini M, Dini A, Mori F, Barni S, Gianluca S, de Martino M, Novembre E: Desensitization and immune tolerance induction in children with severe factor IX deficiency; inhibitors and adverse reactions to replacement therapy: a case-report and literature review. Ital J Pediatr 2015, 41:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorland EC, Drost JB, Lusher JM, Warrier I, Shapiro A, Koerper MA, Dimichele D, Westman J, Key NS, Sommer SS: Anaphylactic response to factor IX replacement therapy in haemophilia B patients: complete gene deletions confer the highest risk. Haemophilia 1999, 5:101–105. [PubMed] [Google Scholar]
- 37.Allacher P, Baumgartner CK, Pordes AG, Ahmad RU, Schwarz HP, Reipert BM: Stimulation and inhibition of FVIII-specific memory B-cell responses by CpG-B (ODN 1826), a ligand for Toll-like receptor 9. Blood 2011, 117:259–267. [DOI] [PubMed] [Google Scholar]
- 38.Pordes AG, Baumgartner CK, Allacher P, Ahmad RU, Weiller M, Schiviz AN, Schwarz HP, Reipert BM: T cell-independent restimulation of FVIII-specific murine memory B cells is facilitated by dendritic cells together with toll-like receptor 7 agonist. Blood 2011, 118:3154–3162. [DOI] [PubMed] [Google Scholar]
- 39.Scott DW, Pratt KP, Miao CH: Progress toward inducing immunologic tolerance to factor VIII. Blood 2013, 121:4449–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nayak S, Cao O, Hoffman BE, Cooper M, Zhou S, Atkinson MA, Herzog RW: Prophylactic immune tolerance induced by changing the ratio of antigen-specific effector to regulatory T cells. J Thromb Haemost 2009, 7:1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moghimi B, Sack BK, Nayak S, Markusic DM, Mah CS, Herzog RW: Induction of tolerance to factor VIII by transient co-administration with rapamycin. J Thromb Haemost 2011, 9:1524–1533. *Ability to tolerize to fcator VIII using a transient course of the immune suppressive drug ramapmycin.
- 42.Nayak S, Sarkar D, Perrin GQ, Moghimi B, Hoffman BE, Zhou S, Byrne BJ, Herzog RW: Prevention and reversal of antibody responses against factor IX in gene therapy for hemophilia B. Front Microbiol 2011, 2:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biswas M, Sarkar D, Kumar SR, Nayak S, Rogers GL, Markusic DM, Liao G, Terhorst C, Herzog RW: Synergy between rapamycin and FLT3 ligand enhances plasmacytoid dendritic cell-dependent induction of CD4+CD25+FoxP3+ Treg. Blood 2015, 125:2937–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang AH, Rossi RJ, Yoon J, Wang H, Scott DW: Tolerogenic nanoparticles to induce immunologic tolerance: Prevention and reversal of FVIII inhibitor formation. Cell Immunol 2016, 301:74–81. *Ability to tolerize to factor VIII using rapamycin packaged into nanoparticles.
- 45.Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, Griset AP, O’Neil C, Altreuter DH, Browning E, et al. : Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci U S A 2015, 112:E156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leissinger C, Josephson CD, Granger S, Konkle BA, Kruse-Jarres R, Ragni MV, Journeycake JM, Valentino L, Key NS, Gill JC, et al. : Rituximab for treatment of inhibitors in haemophilia A. A Phase II study. Thromb Haemost 2014, 112:445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Biswas M, Rogers GL, Sherman A, Byrne BJ, Markusic DM, Jiang H, Herzog RW: Combination therapy for inhibitor reversal in haemophilia A using monoclonal anti-CD20 and rapamycin. Thromb Haemost 2017, 117:33–43. *Combination immune modulatory therapy to reverse inhibitors.
- 48.Nathwani AC, Davidoff AM, Tuddenham EGD: Advances in gene therapy for haemophilia. Hum Gene Ther 2017. [DOI] [PubMed] [Google Scholar]
- 49.Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, Della Peruta M, Lheriteau E, Patel N, Raj D, et al. : Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014, 371:1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berns KI, Muzyczka N: AAV: An overview of unanswered questions. Hum Gene Ther 2017, 28:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pierce GF, Pipe SW: A cornucopia of therapies under study for hemophilia. Mol Ther 2017, 25:2429–2430. *Highlights the diverse treatment options that are currently emerging for patients with hemophilia. Some of these are effective in inhibitor patients, while others may induce immune tolerance.
- 52.Arruda VR: The search for the origin of factor VIII synthesis and its impact on therapeutic strategies for hemophilia A. Haematologica 2015, 100:849–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. George LA, Sullivan SK, Giermasz A, Rasko JEJ, Samelson-Jones BJ, Ducore J, Cuker A, Sullivan LM, Majumdar S, Teitel J, et al. : Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med 2017, 377:2215–2227. *Successful liver-directed gene therapy for hemophilia B.
- 54.Miesbach W, Meijer K, Coppens M, Kampmann P, Klamroth R, Schutgens R, Tangelder M, Castaman G, Schwable J, Bonig H, et al. : Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood 2018, 131:1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herzog RW: Complexity of immune responses to AAV transgene products - Example of factor IX. Cell Immunol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.High KA, Anguela XM: Adeno-associated viral vectors for the treatment of hemophilia. Hum Mol Genet 2016, 25:R36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, et al. : Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011, 365:2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rangarajan S, Walsh L, Lester W, Perry D, Madan B, Laffan M, Yu H, Vettermann C, Pierce GF, Wong WY, et al. : AAV5-Factor VIII Gene Transfer in Severe Hemophilia A. N Engl J Med 2017, 377:2519–2530. *Successful liver-directed gene therapy for hemophilia A.
- 59.Perrin GQ, Zolotukhin I, Sherman A, Biswas M, de Jong YP, Terhorst C, Davidoff AM, Herzog RW: Dynamics of antigen presentation to transgene product-specific CD4+ T cells and of Treg induction upon hepatic AAV gene transfer. Mol Ther Methods Clin Dev 2016, 3:16083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dobrzynski E, Fitzgerald JC, Cao O, Mingozzi F, Wang L, Herzog RW: Prevention of cytotoxic T lymphocyte responses to factor IX-expressing hepatocytes by gene transfer-induced regulatory T cells. Proc Natl Acad Sci U S A 2006, 103:4592–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, Arruda VR, High KA, Herzog RW: Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest 2003, 111:1347–1356. **Tolerance induction to coagulation factor IX by hepatic adeno-associated viral (AAV) gene transfer.
- 62.Sack BK, Merchant S, Markusic DM, Nathwani AC, Davidoff AM, Byrne BJ, Herzog RW: Transient B cell depletion or improved transgene expression by codon optimization promote tolerance to factor VIII in gene therapy. PLoS One 2012, 7:e37671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Markusic DM, Hoffman BE, Perrin GQ, Nayak S, Wang X, LoDuca PA, High KA, Herzog RW: Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol Med 2013, 5:1698–1709. *Effective reversal of pathogenic antibodies against factor IX by tolerance-inducing gene therapy.
- 64.Hoffman BE, Dobrzynski E, Wang L, Hirao L, Mingozzi F, Cao O, Herzog RW: Muscle as a target for supplementary factor IX gene transfer. Hum Gene Ther 2007, 18:603–613. [DOI] [PubMed] [Google Scholar]
- 65. Finn JD, Ozelo MC, Sabatino DE, Franck HW, Merricks EP, Crudele JM, Zhou S, Kazazian HH, Lillicrap D, Nichols TC, et al. : Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood 2010, 116:5842–5848. *Effective reversal of antibodies against factor VIII in large animal model of hemophilia A by a tolerance-inducing gene therapy.
- 66.Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, Zhou S, Wright JF, Jiang H, Pierce GF, et al. : Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood 2007, 110:2334–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arruda VR, Samelson-Jones BJ: Gene therapy for immune tolerance induction in hemophilia with inhibitors. J Thromb Haemost 2016, 14:1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown BD, Cantore A, Annoni A, Sergi LS, Lombardo A, Della Valle P, D’Angelo A, Naldini L: A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood 2007, 110:4144–4152. [DOI] [PubMed] [Google Scholar]
- 69. Annoni A, Cantore A, Della Valle P, Goudy K, Akbarpour M, Russo F, Bartolaccini S, D’Angelo A, Roncarolo MG, Naldini L: Liver gene therapy by lentiviral vectors reverses anti-factor IX pre-existing immunity in haemophilic mice. EMBO Mol Med 2013, 5:1684–1697. *Effective reversal of pathogenic antibodies against factor IX by tolerance-inducing gene therapy.
- 70. Merlin S, Cannizzo ES, Borroni E, Bruscaggin V, Schinco P, Tulalamba W, Chuah MK, Arruda VR, VandenDriessche T, Prat M, et al. : A novel platform for immune tolerance induction in hemophilia A mice. Mol Ther 2017, 25:1815–1830. *Tolerance induction to factor VIII by gene transfer to different liver cell types, including liver sinusoidal endothelial cells
- 71.Jobson J, Brown BD: Micromanaging tolerance in hemophilia A gene therapy. Mol Ther 2017, 25:1739–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sherman A, Biswas M, Herzog RW: Innovative approaches for immune tolerance to Factor VIII in the treatment of hemophilia A. Front Immunol 2017, 8:1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lei TC, Scott DW: Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins. Blood 2005, 105:4865–4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X, Moghimi B, Zolotukhin I, Morel LM, Cao O, Herzog RW: Immune tolerance induction to factor IX through B cell gene transfer: TLR9 signaling delineates between tolerogenic and immunogenic B cells. Mol Ther 2014, 22:1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X, Herzog RW, Byrne BJ, Kumar SRP, Zhou Q, Buchholz CJ, Biswas M: Immune modulatory cell therapy for hemophilia B based on CD20-targeted lentiviral gene transfer to primary B cells. Mol Ther Methods Clin Dev 2017, 5:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biswas M, Kumar SRP, Terhorst C, Herzog RW: Gene therapy with regulatory T cells: a beneficial alliance. Front Immunol 2018, 9:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yoon J, Schmidt A, Zhang AH, Konigs C, Kim YC, Scott DW: FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood 2017, 129:238–245. **Tolerance induction to factor VIII using CAR (chimeric antigen receptor) expressing Treg.
- 78.Fraser H, Safinia N, Grageda N, Thirkell S, Lowe K, Fry LJ, Scotta C, Hope A, Fisher C, Hilton R, et al. : A rapamycin-based GMP-compatible process for the isolation and expansion of regulatory T cells for clinical trials. Mol Ther Methods Clin Dev 2018, 8:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seay HR, Putnam AL, Cserny J, Posgai AL, Rosenau EH, Wingard JR, Girard KF, Kraus M, Lares AP, Brown HL, et al. : Expansion of human Tregs from cryopreserved umbilical cord blood for GMP-Compliant autologous adoptive cell transfer therapy. Mol Ther Methods Clin Dev 2017, 4:178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Levine BL, Miskin J, Wonnacott K, Keir C: Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev 2017, 4:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Milone MC, Bhoj VG: The pharmacology of T cell therapies. Mol Ther Methods Clin Dev 2018, 8:210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sarkar D, Biswas M, Liao G, Seay HR, Perrin GQ, Markusic DM, Hoffman BE, Brusko TM, Terhorst C, Herzog RW: Ex vivo expanded autologous polyclonal regulatory T cells suppress inhibitor formation in hemophilia. Mol Ther Methods Clin Dev 2014, 1:14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim YC, Zhang AH, Su Y, Rieder SA, Rossi RJ, Ettinger RA, Pratt KP, Schevach EM, Scott DW: Engineered antigen-specific human regulatory T cells: immunosuppression of FVIII-specific T- and B-cell responses. Blood 2015, 125:1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang X, Sherman A, Liao G, Leong KW, Daniell H, Terhorst C, Herzog RW: Mechanism of oral tolerance induction to therapeutic proteins. Adv Drug Deliv Rev 2013, 65:759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuhn C, Weiner HL: Immunology. How does the immune system tolerate food? Science 2016, 351:810–811. [DOI] [PubMed] [Google Scholar]
- 86.Rezende SM, Pinheiro K, Caram C, Genovez G, Barca D: Registry of inherited coagulopathies in Brazil: first report. Haemophilia 2009, 15:142–149. [DOI] [PubMed] [Google Scholar]
- 87.Wang X, Su J, Sherman A, Rogers GL, Liao G, Hoffman BE, Leong KW, Terhorst C, Daniell H, Herzog RW: Plant-based oral tolerance to hemophilia therapy employs a complex immune regulatory response including LAP+CD4+ T cells. Blood 2015, 125:2418–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X, Terhorst C, Herzog RW: In vivo induction of regulatory T cells for immune tolerance in hemophilia. Cell Immunol 2016, 301:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yanagida N, Sato S, Ebisawa M: Clinical aspects of oral immunotherapy for the treatment of allergies. Semin Immunol 2017, 30:45–51. [DOI] [PubMed] [Google Scholar]
- 90.Du Toit G, Sampson HA, Plaut M, Burks AW, Akdis CA, Lack G: Food allergy: Update on prevention and tolerance. J Allergy Clin Immunol 2018, 141:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kwon KC, Verma D, Singh ND, Herzog R, Daniell H: Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells. Adv Drug Deliv Rev 2013, 65:782–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao Y, Kwon KC, Hoffman BE, Kamesh A, Jones NT, Herzog RW, Daniell H: Low cost delivery of proteins bioencapsulated in plant cells to human non-immune or immune modulatory cells. Biomaterials 2016, 80:68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verma D, Moghimi B, LoDuca PA, Singh HD, Hoffman BE, Herzog RW, Daniell H: Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc Natl Acad Sci U S A 2010, 107:7101–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Su J, Zhu L, Sherman A, Wang X, Lin S, Kamesh A, Norikane JH, Streatfield SJ, Herzog RW, Daniell H: Low cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B. Biomaterials 2015, 70:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Herzog RW, Nichols TC, Su J, Zhang B, Sherman A, Merricks EP, Raymer R, Perrin GQ, Hager M, Wiinberg B, et al. : Oral tolerance induction in hemophilia B dogs fed with transplastomic lettuce. Mol Ther 2017, 25:512–522. *Plant-based oral tolerance induction in a large animal model of hemophilia.
- 96. Sherman A, Su J, Lin S, Wang X, Herzog RW, Daniell H: Suppression of inhibitor formation against FVIII in a murine model of hemophilia A by oral delivery of antigens bioencapsulated in plant cells. Blood 2014, 124:1659–1668. **Plant-based oral tolerance induction for factor VIII.
- 97.Kwon KC, Sherman A, Chang WJ, Kamesh A, Biswas M, Herzog RW, Daniell H: Expression and assembly of largest foreign protein in chloroplasts: oral delivery of human FVIII made in lettuce chloroplasts robustly suppresses inhibitor formation in haemophilia A mice. Plant Biotechnol J 2018, 16:1148–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]