Abstract
Background
Our facility’s dental team consists of a full-time dentists and dental hygienists who work exclusively in the wards to implement best practices in oral healthcare. We executed the dental care system (DCS) that includes lectures and practical training for nurses conducted by dentists and dental hygienists, the introduction to oral assessment, standardization of oral care procedures, a process for nurses to request the dental team, and early bedside oral screening conducted by the dental team. This study investigated the DCS’s effects on the incidence of stroke-associated pneumonia (SAP).
Methods
This single-center retrospective cohort study included 2,771 acute stroke patients who were newly hospitalized between April 1, 2012, and March 31, 2020. The 8-year period was divided into four phases at two-year intervals as follows: Pre (N=632), Post-1 (N=642), Post-2 (N=716), and Post-3 (N=781). Pre was prior to DCS practice. Post-1 was an early introduction to DCS. Post-2 simplified dental team requests from nurses, and Post-3 added bedside oral screening within 72 hours of admission by the dental team. Statistical analysis was performed using the Cochran-Armitage trend test, followed by multivariate logistic regression.
Results
A decrease in SAP rates was observed across the four groups (P<0.0001). Logistic regression analysis revealed a significant difference for respiratory disease (odds ratio 7.74, 95% confidence interval 5.49–10.90), hypertension (2.28, 1.39–3.73), cardiac failure (1.72, 1.04–2.85), and diabetes (1.59, 1.11–2.26), 3-digit code on the Japan coma scale (3.57, 2.53–5.05 [reference ≤2-digit code]), age ≥90 years (2.34, 1.15–4.77 [reference 18–59 years]), male (1.86, 1.31–2.67), and the Post-1 (0.49, 0.31–0.76 [reference Pre]), Post-2 (0.38, 0.25–0.61 [reference Pre]), and Post-3 (0.24, 0.15–0.40 [reference Pre]) periods.
Conclusion
The suppression of SAP is effectively achieved through early intervention and education of nurses by dental professionals.
Keywords: hospital dental services, hospital dentistry, special care dentistry, oral health management, oral care, acute stroke, stroke-associated pneumonia, team approach
Plain Language Summary
Pneumonia is a dangerous disease that kills millions of individuals around the world every year. Stroke patients are susceptible to developing pneumonia because their swallowing reflex is sometimes impaired. This risk may be reduced by maintaining oral hygiene through advanced oral care involving dentists and dental hygienists. For this reason, our acute care hospital has a dental team consisting of a full-time dentists and dental hygienists dedicated to the ward. In 2014, the dental team launched a program for nurses on how to provide oral care to inpatients. Additionally, we have introduced a system that enables nurses to contact the dental team immediately if oral care becomes difficult for nurses due to problems in the patient’s mouth. Furthermore, the dental team began evaluating the oral health of all stroke patients within 72 hours after hospitalization. We studied the changes in the frequency of pneumonia in stroke patients at our hospital before the application of this approach and 6 years after its introduction. The rate of pneumonia decreased steadily during this period. Overall, the pneumonia rates in stroke patients at our hospital reduced by approximately 60%, suggesting that this system is an effective way to prevent pneumonia.
Background
The oral cavity is a reservoir of respiratory pathogens, and it is known that oral bacteria and the occurrence of respiratory diseases are associated.1–6 Oral health care improves oral hygiene, strengthens the sensitivity of the cough reflex,7 and increases the concentration of substance P in the saliva.8 Moreover, it is also effective in suppressing pneumonia.9–13 The American Heart Association/American Stroke Association recommend implementing oral hygiene protocols to prevent aspiration pneumonia.14 Similarly, the European Stroke Organisation and the European Society also support proactive oral health care interventions,15 and the Canadian Stroke Best Practice Recommendations for Acute Stroke Management16 suggested early referral to dentists if concerns regarding oral care are present. However, only a few facilities provide intensive dental interventions for acute stroke patients. In addition, hospital nurses in acute and post-acute settings tend not to prioritize oral care,17,18 and 92% of junior doctors are not confident in diagnosing dental diseases.19 Therefore, our hospital employs full-time dentists and dental hygienists who work exclusively in the wards to accomplish best practices in oral healthcare. They were affiliated with the rehabilitation department and collaborated with the department of nursing to establish the dental care system (DCS) to suppress hospital-acquired pneumonia.20 This DCS has been enhanced recently with the introduction of a simplified, computer-based system for nurses to pass requests to the dental team, and bedside oral screening of all stroke patients within 72 hours of admission by the dental team. In this study, we report the effects of these measures on the rate of stroke-associated pneumonia (SAP).
Study Population and Design
This was a retrospective cohort study of stroke patients newly admitted at the Ashikaga Red Cross Hospital, a tertiary emergency facility accredited by the Joint Commission International, conducted between 1st April 2012 and 31st March 2020. Our study was reviewed and approved by the Ethics Committee of the Ashikaga Red Cross Hospital (Authorization ID 2020-12). Written consent (or rejection) of the use of personal medical data for research purposes is obtained from all patients at our facility at admission. The principles outlined in the Declaration of Helsinki were adhered to throughout the course of the research. The hospital is located in Ashikaga City, Tochigi Prefecture, Japan, and has a population of approximately 140,000. Our hospital does not have a dedicated stroke care unit. Changes in the number of the medical staff during the survey period are presented in Supplementary Table 1. An increase in the number of therapists was observed between 2012 and 2013. However, no clear increase or decrease was detected among the other specialists. The hospital maintained 555 beds during the study. Among these 555 beds, 30 were in the high-care unit, which is used for the management of stroke patients. There was also no increase or decrease in the number of high-care unit beds.
Stroke patients were diagnosed with cerebral infarction (International Classification of Diseases, 10th edition [ICD-10], code I63x), cerebral hemorrhage (ICD-10, code I61x), or non-traumatic subarachnoid hemorrhage (ICD-10, code I60x), and 3,106 such cases were extracted from the Japanese Diagnosis Procedure Combination database,21 a part of the health payment system in Japan. Subsequently, the internal medical records (HOPE EGMAIN-GX system, FUJITSU, Tokyo, Japan) of the selected patients were evaluated, and those with a transient ischemic attack, no stroke, suspected stroke, unknown date of onset, or age<18 years were excluded. Thus, 2,771 stroke patients (1,527 males and 1,244 females, mean age±1SD 72±13 years) were analyzed in this study (Figure 1).
Figure 1.
Flowchart of the study.
I60x: non-traumatic subarachnoid hemorrhage, I61x: cerebral hemorrhage, I63x: cerebral infarction.
DCS comprises lectures and practical training for nurses presided over by dentists and dental hygienists and includes an introduction to oral assessment, standardization of oral care procedures, and a process for nurses to request dental interventions via in-hospital telephones.
The 8-year study was divided into four time periods (Table 1). The Pre period (April 1, 2012 to March 31, 2014; N=632) represented the term prior to DCS operation. Although the dental team provided treatment and care during this time, they did not conduct systematic oral management with the ward nurses. The Post-1 period (April 1, 2014 to March 31, 2016; N=642) exhibited duration of initial DCS practice. The Post-2 and 3 periods indicated phase of enhanced DCS. The Post-2 period (April1, 2016 to March 31, 2018; N=716) is characterized by the introduction of a simplified, computer-based system that enables nurses to request the service of the dental team. After the Post-2 period, the process of dental team request was streamlined such that only clicking a checkbox in the electronic medical records, rather than making in-hospital telephones. The Post-3 period (April 1, 2018 to March 31, 2020; N=781) expressed the phase during which bedside oral screening of all stroke patients was performed by the dental team within 72 hours of admission. Collaboration with the nurses at the patient flow center of the hospital, which manages admissions and discharges during the Post-3 period allowed the dental team to promptly monitor inpatient admissions.
Table 1.
Intervention contents in four periods.
| Intervention Details | Pre: April 1, 2012 to March 31, 2014 (N=632) | Post–1: April 1, 2014 to March 31, 2016 (N=642) | Post–2: April 1, 2016 to March 31, 2018 (N=716) | Post–3: April 1, 2018 to March 31, 2020 (N=781) | |
|---|---|---|---|---|---|
| Dental care system (to nurses) | Lectures and practical training | No | Yes | Yes | Yes |
| Unified oral assessments | No | Yes | Yes | Yes | |
| Standardized oral care procedures | No | Yes | Yes | Yes | |
| Dental team request by in-hospital telephone | No | Yes | Yes | Yes | |
| Enhanced dental care system 1 (to nurses) | Simplify requests to the dental team using electronic medical records | No | No | Yes | Yes |
| Enhanced dental care system 2 (to the dental team) | Early bedside oral screening of stroke patients by the dental team within 72 hours of admission | No | No | No | Yes |
Data Collection
Data extracted from medical records included sex, age (18–59, 60–69, 70–79, 80–89, ≥90 years), classification of stroke (cerebral infarction, cerebral hemorrhage, non-traumatic subarachnoid hemorrhage), hospitalized from home, body mass index (BMI; <18.5, 18.5–24.9, ≥25.0), the Japan Coma Scale22 (JCS) score (single/double-digit or triple-digit code), and medical history (previous stroke, hypertension, diabetes, dyslipidemia, respiratory disease, dementia, heart failure, renal failure, and liver disease). Stroke was diagnosed by neurologists or neurosurgeons using computed tomography and/or magnetic resonance imaging. The JCS score is widely used to evaluate the consciousness level in Japan, and its correlation with the Glasgow Coma Scale has been reported.22 The interventions performed during hospitalization consisted the use of tissue-type plasminogen activator (tPA), surgery for stroke, tracheostomy, gastrostomy, rehabilitation, and dental treatment and/or care. SAP was defined as the occurrence of pneumonia within the first seven days after the onset of stroke in unventilated patients.23 SAP was determined by the attending physician according to the CDC/NHSN surveillance definition for healthcare-associated infection in acute care medical environments and the criteria for specific types of pneumonia infection (PNU1).24 Moreover, the duration of hospital stay and the number of deaths were also recorded.
Sample Size
A previous study described the prevalence of pneumonia as 5% in the dental intervention group and 12% in the control group.20 Therefore, we assessed whether the rates were equal among the Pre, Post-1, Post-2, and Post-3 periods using Fisher’s exact test. The significance level was set at 0.05. The sample size for each group calculated using the Monte Carlo method with a power of 0.80 was 219.
Statistical Analysis
Wilcoxon rank-sum test was used for the duration of hospital stay, whereas Pearson’s chi-square test was conducted for all other variables. Bonferroni correction was performed for all multiple comparisons. The Cochran-Armitage trend test detected trends in the event of SAP, and a multivariate logistic regression model was applied. The Cochran-Armitage trend test was carried out to evaluate the Pre, Post-1, Post-2, and Post-3 periods. Subsequently, a multivariate logistic regression model was performed with SAP as the objective variable and sex; age (60–69, 70–79, 80–89, ≥90 years); BMI (<18.5, ≥25.0); the JCS score (3 digit code); hospitalization from home; previous stroke, hypertension, diabetes, respiratory disease, dementia, and heart failure; and the Post-1, Post-2, and Post-3 periods as the explanatory variables. Explanatory variables were selected from the risk factors for pneumonia identified in previous studies and comorbidities that showed significant differences in univariate analysis. Missing BMI values were processed using a multivariate normal imputation. JMP (version 14.3 for Macintosh; SAS Institute Inc., Cary, NC, USA) was used for statistical analysis, and a P-value of <0.05 was considered statistically significant.
Results
Supplementary Table 2 presents the admission-related information. The number of stroke patients significantly tended to increase (P<0.0001: Pre vs. Post-3, Post-1 vs. Post-3; P<0.001: Post-2 vs. Post-3), and significant differences were noted in the 18–59 (P=0.001: Pre vs. Post-1, P<0.001: Post-1 vs. Post-2) and 60–69 (P=0.002: Pre vs. Post-3) age groups. Furthermore, significant differences were confirmed in patients with a previous stroke (P=0.001: Pre vs. Post-2, P<0.001: Post-1 vs. Post-2), hypertension (P<0.0001: Pre vs. Post-3), respiratory disease (P<0.0001: Pre vs. Post-1, Post-2; P=0.004: Pre vs. Post-3; P=0.001: Post-2 vs. Post-3), and liver disease (P=0.008: Pre vs. Post-1). No significant differences were shown in the other variables. Table 2 indicates the details of the clinical interventions during hospitalization. The use of tPA (P=0.002: Pre vs. Post-3), rehabilitation (P=0.003: Post-2 vs. Post-3), dental treatment/care (P<0.001: Pre vs. Post-1, Post-2, and Post-3; P=0.004: Post-1 vs. Post-3), and dental interventions within 7 days of admission (P<0.001: Pre vs. Post-1, Post-2, and Post-3; Post-1 vs. Post-3; and Post-2 vs. Post-3) presented significant differences.
Table 2.
Clinical interventions during hospitalization
| Overall (N=2,771) | Pre (N=632) | Post-1 (N=642) | Post-2 (N=716) | Post-3 (N=781) | P-value | |
|---|---|---|---|---|---|---|
| April 1, 2012 to March 31, 2020; N (%) | April 1, 2012 to March 31, 2014; N (%) | April 1, 2014 to March 31, 2016; N (%) | April 1, 2016 to March 31, 2018; N (%) | April 1, 2018 to March 31, 2020; N (%) | ||
| *tPA use (% of infarcted patients) | 95 (5.7) | 31 (8.5) | 21 (5.5) | 28 (6.6) | 15 (3.0) | 0.018a |
| Stroke-related surgery | 357 (12.9) | 105 (16.6) | 74 (11.5) | 86 (12.0) | 92 (11.8) | 0.017 |
| Tracheostomy | 50 (1.8) | 15 (2.4) | 16 (2.5) | 9 (1.3) | 10 (1.3) | 0.152 |
| Gastrostomy | 54 (1.9) | 14 (2.2) | 17 (2.6) | 15 (2.1) | 8 (1.0) | 0.143 |
| Rehabilitation | 2525 (91.1) | 572 (90.5) | 587 (91.4) | 637 (89.0) | 729 (93.3) | 0.026b |
| Dental treatment/care | 944 (34.1) | 141 (22.3) | 214 (33.3) | 270 (37.7) | 319 (40.8) | P<0.0001c |
| Intervention within 7 days of hospitalization | 527 (19.0) | 47 (7.4) | 123 (19.2) | 139 (19.4) | 218 (27.9) | P<0.0001d |
Notes: *tPA: tissue-type plasminogen activator. All comparisons used the Pearson’s chi-square test, and multiple comparisons used Bonferroni’s adjustment. aP=0.002: Pre vs. Post-3. bP=0.003: Post-2 vs. Post-3. cP<0.001: Pre vs. Post-1, Post-2, and Post-3; P=0.004: Post-1 vs. Post-3. dP<0.001: Pre vs. Post-1, Post-2, and Post-3; Post 1 vs. Post-3; and Post-2 vs. Post-3.
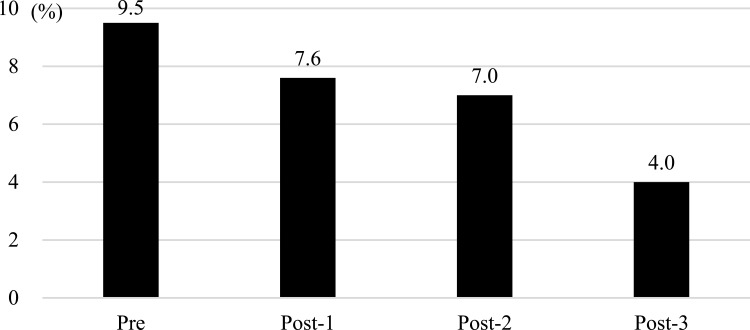
A decreasing trend was viewed in the frequency of SAP (Figure 2; P<0.0001). The median duration of hospital stay was as follows: overall, 21 days (interquartile range 11–41); Pre, 18 days (11–36); Post-1, 21 days (11–38); Post-2, 20 days (11–42); and Post-3, 22 days (12–48). The number of deaths was as follows: overall, 306 (11%); Pre, 71 (11%); Post-1, 79 (12%); Post-2, 65 (9%); and Post-3, 91 (12%). Thus, no significant differences were spotted in the duration of hospital stay and death rate. Table 3 displays the results of the logistic regression analysis. History of respiratory disease (odds ratio 7.74, 95% confidence interval 5.49–10.90), hypertension (2.28, 1.39–3.73), heart failure (1.72, 1.04–2.85), and diabetes (1.59, 1.11–2.26); JCS 3-digit code (3.57, 2.53–5.05 [reference ≤2-digit codes]); age≥90 years (2.34, 1.15–4.77 [reference 18–59 years]); male (1.86, 1.31–2.67); and the Post-1 (0.49, 0.31–0.76 [reference Pre]), Post-2 (0.38, 0.25–0.61 [reference Pre]), and Post-3 (0.24, 0.15–0.40 [reference Pre]) periods showed significant differences.
Figure 2.
Incidence of stroke-associated pneumonia during study periods (P<0.0001; Cochran-Armitage test for trend). Pre: Before introduction of the dental care system (DCS), 4/1/2012 to 3/31/2014. Post-1: Initial DCS practice, 4/1/2014 to 3/31/2016. Post-2: Enhanced DCS 1 (simplify requests to the dental team using electronic medical records), 4/1/2016 to 3/31/2018. Post-3: Enhanced DCS 2 (bedside oral screening of stroke patients by the dental team within 72 hours of admission), 4/1/2018 to 3/31/2020.
Table 3.
Stroke-associated pneumonia and explanatory variables (logistic regression analysis)
| Odds ratio (95% Confidence Interval) | P-value | ||
|---|---|---|---|
| Male | 1.86 (1.31–2.67) | 0.001 | |
| Age (reference 18–59 years) | 60–69 | 0.91 (0.50–1.66) | 0.757 |
| 70–79 | 1.11 (0.63–1.94) | 0.725 | |
| 80–89 | 1.43 (0.81–2.52) | 0.215 | |
| ≥90 | 2.34 (1.15–4.77) | 0.019 | |
| Body mass index (reference 18.5–24.9 kg/m2) | <18.5 | 1.21 (0.73–2.02) | 0.458 |
| ≥25 | 0.74 (0.50–1.14) | 0.172 | |
| Japan coma scale 3-digit code (reference ≤2-digit codes) | 3.57 (2.53–5.05) | <0.0001 | |
| Resided at home (reference other than home) | 0.66 (0.37–1.19) | 0.165 | |
| Previous stroke | 0.81 (0.51–1.27) | 0.361 | |
| Hypertension | 2.28 (1.39–3.73) | 0.001 | |
| Diabetes | 1.59 (1.11–2.26) | 0.011 | |
| Respiratory disease | 7.74 (5.49–10.90) | <0.0001 | |
| Dementia | 1.43 (0.85–2.40) | 0.175 | |
| Heart failure | 1.72 (1.04–2.85) | 0.033 | |
| Study period (reference Pre) | Post-1 | 0.49 (0.31–0.76) | 0.002 |
| Post-2 | 0.38 (0.25–0.61) | <0.0001 | |
| Post-3 | 0.24 (0.15–0.40) | <0.0001 |
Discussion
Our previous study20 reported that the DCS, which included oral care lectures and practical training for nurses presided over by dental team, the introduction of oral assessment, the standardization of oral care procedures, and protocols for requesting dental interventions lowered the occurrence of SAP. The enhanced DCS in this study, which included bedside oral screening by the dental team within 72 hours of admission and the simplification of the procedure for nurses to request dental interventions, have further decreased the frequency of SAP.
The admission data showed that the rate of respiratory diseases was higher in the Post groups compared to the Pre group. Previous studies have suggested that patients with chronic obstructive pulmonary disease are more susceptible to developing pneumonia.25,26 Therefore, the Post groups may have been at a disadvantage compared with the Pre group in terms of reducing the event of pneumonia.
When contrasting our cohort with patients from a Japanese multi-center survey,27 the average age (73 years for the multi-center survey vs. 72 years for ours); rates of cerebral infarction, cerebral haemorrhage, and subarachnoid hemorrhage (61%, 29%, and 9% vs. 60%, 30%, and 10%); 3-digit code for JCS score (14% vs. 16%); and the history of hypertension (75% vs. 81%), diabetes (26% vs. 30%), and dyslipidemia (28% vs. 30%) were alike. In addition, when comparing the content of clinical interventions, the duration of hospital stay, and the mortality rate with another Japanese multi-center survey,28 our study showed differences in the use of tPA (17% in the multi-center survey vs. 6% in our study), surgery (10% vs. 13%), rehabilitation (73% vs. 91%), the median duration of hospital stay (24 days vs. 21 days), and mortality rate (16% vs. 11%). These findings hint that our stroke patient was typical for a Japanese hospital, with the exception of rehabilitation interventions. And, the fact that rehabilitation was accomplished in approximately 90% of our patients may have contributed favorably to pneumonia suppression. There have been reports of pneumonia control through dysphagia screening,29,30 and physical activity31 in stroke patients.
Lyons et al32 noted a lack of evidence regarding the benefits of oral care after stroke in their narrative review and indicated the need for oral assessment and the optimisation of training from dental specialists. One of these factors may be the absence of full-time dental professionals in hospitals that handle acute stroke patients. The medical system of many countries makes it difficult to assign full-time dental staff to acute care hospitals. However, dentists and dental hygienists can be exclusively designated to hospital wards in Japan. The average hospital stay for acute care is 6.9 days in the UK, 6.1 days in the US, and 16 days in Japan.33 Consequently, in Japan, where hospital stays are longer, there is a possibility of a need to provide dental services for hospitalized patients.
A study in which dentists evaluated patients with cerebral infarction and cerebral hemorrhage in the intensive care unit found that 78% of patients exhibited features of periodontitis and 79% had dental caries.34 Moreover, conditions that hinder oral care, such as oral hemorrhage, oral candidiasis, mobile teeth, and difficulty in opening the mouth, have been demonstrated in acute and subacute stroke patients.20 Thus, early diagnosis and management by dental team are essential for creating an environment in which nurses can perform optimal oral care practices. These practices inhibit the aspiration of oral bacterial and improve cough and swallowing reflexes.7,8 As a result, it can be expected to have a preventive effect against pneumonia. Aoki et al12 mentioned a reduction in the incidence of hospital-acquired pneumonia in acute stroke patients due to the professional care provided by dentists and dental hygienists who were members of a multidisciplinary swallowing team. Bellissimo-Rodrigues et al35 conducted a randomized clinical trial in which dentists intervened in the ICU. In the group receiving dental treatment, which included tooth brushing, tongue cleaning, plaque removal, tooth extraction, and caries treatment performed 4–5 times a week, a reduction in the frequency of lower respiratory tract infections was observed. The findings of these surveys support our recommendations for dental interventions during the acute stage.
A previous study stated that the prevalence of hospital-acquired pneumonia could be reduced through educational dental interventions alone. Quinn et al36 analyzed the state of oral care and developed an oral care protocol that reflected the opinions of on-site staff. Dentists contributed to both the creation of this protocol and the execution of practical training, including lectures, mitigated the rate of hospital-acquired pneumonia. Likewise, our study also built the DCS that incorporated nurses’ perspectives. Yuan et al37 documented that dental professional-led nurse education diminished the incidence of pneumonia in male stroke patients with high severity.
In the future, it will be important to involve dental specialists in acute stroke medical teams, to supply oral health education and dental treatment/care in different medical settings, and to collect evidence. Therefore, dental professionals need to understand the impact of dental diseases on overall health and should receive training in dental treatment and care for hospitalized patients. Additionally, ensuring effective oral care in the ward necessitates interdisciplinary communication and management skills. These highlights the challenge of human resource development for dental staff.
Limitations
This study was a single-center retrospective cohort study. Furthermore, data about pneumococcal vaccination, severity of stroke other than consciousness level, details of comorbidities, smoking history, use of medications excluding tPA, nutrition-related data other than BMI, oral status, and specifics of rehabilitation could not be investigated. Additionally, we did not verify the oral care skills, awareness, and knowledge of the nurses.
Conclusions
The control of SAP was achieved through the synergistic effect of high-quality oral care delivered by nurses trained by dentists and dental hygienists, along with early bedside oral screening and treatment/care provided by dental practitioners.
Acknowledgement
The authors would like to thank the nursing, rehabilitation and dental staff of Japanese Red Cross Ashikaga Hospital, who enthusiastically contributed to this study, and the Medical Information Division for providing ready access to the relevant clinical information. We would also like to express our gratitude to dental hygienists Etsuyo Horikoshi and Mayu Motoki for their oral care.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.El-Solh AA, Pietrantoni C, Bhat A, et al. Colonization of dental plaques: a reservoir of respiratory pathogens for hospital-acquired pneumonia in institutionalized elders. Chest. 2004;126:1575–1582. doi: 10.1378/chest.126.5.1575 [DOI] [PubMed] [Google Scholar]
- 2.Sumi Y, Miura H, Michiwaki Y, Nagaosa S, Nagaya M. Colonization of dental plaque by respiratory pathogens in dependent elderly. Arch Gerontol Geriatr. 2007;44:119–124. doi: 10.1016/j.archger.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 3.Tan L, Wang H, Li C, Pan Y. 16S rDNA-based metagenomic analysis of dental plaque and lung bacteria in patients with severe acute exacerbations of chronic obstructive pulmonary disease. J Periodontal Res. 2014;49:760–769. doi: 10.1111/jre.12159 [DOI] [PubMed] [Google Scholar]
- 4.Rivas Caldas R, Le Gall F, Revert K, et al. Pseudomonas aeruginosa and periodontal pathogens in the oral cavity and lungs of cystic fibrosis patients: a case-control study. J Clin Microbiol. 2015;53:1898–1907. doi: 10.1128/JCM.00368-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaeckle NT, Heyman B, Criner AJ, Criner GJ. Markers of dental health correlate with daily respiratory symptoms in COPD. Chronic Obstr Pulm Dis. 2018;5(2):97–105. doi: 10.15326/jcopdf.5.2.2017.0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watando A, Ebihara S, Ebihara T, et al. Daily oral care and cough reflex sensitivity in elderly nursing home patients. Chest. 2004;126(4):1066–1070. doi: 10.1378/chest.126.4.1066 [DOI] [PubMed] [Google Scholar]
- 8.Yoshino A, Ebihara T, Ebihara S, Fuji H, Sasaki H. Daily oral care and risk factors for pneumonia among elderly nursing home patients. JAMA. 2001;286(18):2235–2236. doi: 10.1001/jama.286.18.2235 [DOI] [PubMed] [Google Scholar]
- 9.Yoneyama T, Yoshida M, Matsui T, Sasaki H. Oral care and pneumonia. Oral Care Working Group. Lancet. 1999;354(9177):515. doi: 10.1016/S0140-6736(05)75550-1 [DOI] [PubMed] [Google Scholar]
- 10.Sjogren P, Nilsson E, Forsell M, Johansson O, Hoogstraate J. A systematic review of the preventive effect of oral hygiene on pneumonia and respiratory tract infection in elderly people in hospitals and nursing homes: effect estimates and methodological quality of randomized controlled trials. J Am Geriatr Soc. 2008;56(11):2124–2130. doi: 10.1111/j.1532-5415.2008.01926.x [DOI] [PubMed] [Google Scholar]
- 11.Takahata H, Tsutsumi K, Baba H, Nagata I, Yonekura M. Early intervention to promote oral feeding in patients with intracerebral hemorrhage: a retrospective cohort study. BMC Neurol. 2011;11:6. doi: 10.1186/1471-2377-11-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoki S, Hosomi N, Hirayama J, et al. The multidisciplinary swallowing team approach decreases pneumonia onset in acute stroke patients. PLoSOne. 2016;11:e0154608. doi: 10.1371/journal.pone.0154608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray J, Scholten I. An oral hygiene protocol improves oral health for patients in inpatient stroke rehabilitation. Gerodontology. 2018;35:18–24. doi: 10.1111/ger.12309 [DOI] [PubMed] [Google Scholar]
- 14.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 15.Dziewas R, Michou E, Trapl-Grundschober M, et al. European Stroke Organisation and European Society for Swallowing Disorders guideline for the diagnosis and treatment of post-stroke dysphagia. Eur Stroke J. 2021;6(3):LXXXIX–CXV. doi: 10.1177/23969873211039721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulanger JM, Lindsay MP, Gubitz G, et al. Canadian stroke best practice recommendations for Acute Stroke Management: prehospital, emergency department, and acute inpatient stroke care, 6th edition, update 2018. Int J Stroke. 2018;13(9):949–984. doi: 10.1177/1747493018786616 [DOI] [PubMed] [Google Scholar]
- 17.Salamone K, Yacoub E, Mahoney AM, Edward KL. Oral care of hospitalised older patients in the acute medical setting. Nurs Res Pract. 2013;2013:827670. doi: 10.1155/2013/827670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coker E, Ploeg J, Kaasalainen S, Carter N. Nurses’ oral hygiene care practices with hospitalised older adults in postacute settings. IntJ Older People Nurs. 2017;12. doi: 10.1111/opn.12124 [DOI] [PubMed] [Google Scholar]
- 19.Doshi M, Weeraman M, Mann J. A survey of the knowledge of junior doctors in managing oral conditions in adult inpatients. Br Dent J. 2019;227:393–398. doi: 10.1038/s41415-019-0666-z [DOI] [PubMed] [Google Scholar]
- 20.Ozaki K, Teranaka S, Tohara H, Minakuchi S, Komatsumoto S. Oral management by a full-time resident dentist in the hospital ward reduces the incidence of pneumonia in patients with acute stroke. Int J Dentistry. 2022. doi: 10.1155/2022/6193818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang K, Li P, Chen L, Kato K, Kobayashi M, Yamauchi K. Impact of the Japanese diagnosis procedure combination-based payment system in Japan. JMed Syst. 2010;34:95–100. doi: 10.1007/s10916-008-9220-2 [DOI] [PubMed] [Google Scholar]
- 22.Ono K, Wada K, Takahara T, Shirotani T. Indications for computed tomography in patients with mild head injury. Neurol Med Chir. 2007;47:291–297; discussion 297–298. doi: 10.2176/nmc.47.291 [DOI] [PubMed] [Google Scholar]
- 23.Smith CJ, Kishore AK, Vail A, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in Stroke Consensus Group. Stroke. 2015;46:2335–2340. doi: 10.1161/strokeaha.115.009617 [DOI] [PubMed] [Google Scholar]
- 24.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 25.Sellars C, Bowie L, Bagg J, et al. Risk factors for chest infection in acute stroke: a prospective cohort study. Stroke. 2007;38:2284–2291. doi: 10.1161/STROKEAHA.106.478156 [DOI] [PubMed] [Google Scholar]
- 26.Masiero S, Pierobon R, Previato C, Gomiero E. Pneumonia in stroke patients with oropharyngeal dysphagia: a six-month follow-up study. Neurol Sci. 2008;29:139–145. doi: 10.1007/s10072-008-0925-2 [DOI] [PubMed] [Google Scholar]
- 27.Nishimura A, Nishimura K, Kada A, Iihara K. Status and future perspectives of utilizing big data in neurosurgical and stroke research. Neurol Med Chir. 2016;56:655–663. doi: 10.2176/nmc.ra.2016-0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takashima N, Arima H, Kita Y, et al. Incidence, management and short-term outcome of stroke in a general population of 1.4 million Japanese- Shiga stroke registry. Circ J. 2017;81:1636–1646. doi: 10.1253/circj.CJ-17-0177 [DOI] [PubMed] [Google Scholar]
- 29.Titsworth WL, Abram J, Fullerton A, et al. Prospective quality initiative to maximize dysphagia screening reduces hospital-acquired pneumonia prevalence in patients with stroke. Stroke. 2013;44:3154–3160. doi: 10.1161/strokeaha.111.000204 [DOI] [PubMed] [Google Scholar]
- 30.Schrock JW, Lou L, Ball BAW, Van Etten J. The use of an emergency department dysphagia screen is associated with decreased pneumonia in acute strokes. Am J Emerg Med. 2018;36:2152–2154. doi: 10.1016/j.ajem.2018.03.046 [DOI] [PubMed] [Google Scholar]
- 31.Cuesy PG, Sotomayor PL, Piña JO. Reduction in the incidence of poststroke nosocomial pneumonia by using the “turn-mob” program. J Stroke Cerebrovasc Dis. 2010;19:23–28. doi: 10.1016/j.jstrokecerebrovasdis.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 32.Lyons M, Smith C, Boaden E, et al. Oral care after stroke: where are we now? Eur Stroke J. 2018;3:347–354. doi: 10.1177/2396987318775206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Organisation for Economic Co-operation and Development. Health at a Glance 2021, OECD INDICATORS. Paris: OECD Publishing; 2021. [cited 2023 May 30; Available from: https://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-2021_ae3016b9-en [Google Scholar]
- 34.Sinha RK, Singh A, Kishor A, Richa S, Kumar R, Kumar A. Evaluation of oral hygiene status in patients with hemorrhagic and ischemic stroke. J Pharm Bioallied Sci. 2021;13:S233–S236. doi: 10.4103/jpbs.JPBS_698_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellissimo-Rodrigues WT, Menegueti MG, Gaspar GG, et al. Is it necessary to have a dentist within an intensive care unit team? Report of a randomised clinical trial. Int Dent J. 2018;68:420–427. doi: 10.1111/idj.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quinn B, Giuliano KK, Baker D. Non-ventilator health care-associated pneumonia (NV-HAP): best practices for prevention of NV-HAP. Am J Infect Control. 2020;48:A23–A27. doi: 10.1016/j.ajic.2020.03.006 [DOI] [PubMed] [Google Scholar]
- 37.Yuan D, Zhang J, Wang X, Chen S and Wang Y. (2020). Intensified Oral Hygiene Care in Stroke-Associated Pneumonia: A Pilot Single-Blind Randomized Controlled Trial. INQUIRY, 57: 004695802096877. doi: 10.1177/0046958020968777 [DOI] [PMC free article] [PubMed] [Google Scholar]