Abstract
LNPK encodes a conserved membrane protein that stabilizes the junctions of the tubular endoplasmic reticulum network playing crucial roles in diverse biological functions. Recently, homozygous variants in LNPK were shown to cause a neurodevelopmental disorder (OMIM#618090) in four patients displaying developmental delay, epilepsy and nonspecific brain malformations including corpus callosum hypoplasia and variable impairment of cerebellum. We sought to delineate the molecular and phenotypic spectrum of LNPK-related disorder. Exome or genome sequencing was carried out in 11 families. Thorough clinical and neuroradiological evaluation was performed for all the affected individuals, including review of previously reported patients. We identified 12 distinct homozygous loss-of-function variants in 16 individuals presenting with moderate to profound developmental delay, cognitive impairment, regression, refractory epilepsy and a recognizable neuroimaging pattern consisting of corpus callosum hypoplasia and signal alterations of the forceps minor (‘ear-of-the-lynx’ sign), variably associated with substantia nigra signal alterations, mild brain atrophy, short midbrain and cerebellar hypoplasia/atrophy. In summary, we define the core phenotype of LNPK-related disorder and expand the list of neurological disorders presenting with the ‘ear-of-the-lynx’ sign suggesting a possible common underlying mechanism related to endoplasmic reticulum-phagy dysfunction.
Keywords: endoplasmic reticulum, LNPK, ear-of-the-lynx sign, substantia nigra, corpus callosum hypoplasia
In this study, Accogli et al. delineate the molecular and clinical spectrum of LNPK-related neurodevelopmental disorder with degenerative course, characterized by a recognizable neuroimaging pattern consisting of corpus callosum hypoplasia and signal alterations of the forceps minor (‘ear-of-the-lynx’ sign), suggesting a pathomechanism related to endoplasmic reticulum-phagy dysfunction.
Graphical Abstract
Graphical Abstract.
Introduction
The endoplasmic reticulum (ER) is involved in diverse biological functions, including protein synthesis, folding and transport, carbohydrate metabolism, lipid and steroid synthesis and calcium homeostasis.1,2 The progressive understanding of ER structure and function in recent years has unravelled the role of ER dysfunction in several neurodegenerative disorders in humans, such as hereditary spastic paraplegia (SPG)3 and Parkinson disease.4
Lunapark (Lnp) is a conserved membrane protein that localizes preferentially to the three-way junctions connecting the tubular ER network.5,6 Through its ubiquitin ligase activity, it ubiquitinates atlastin-2 for the tubular network formation and stabilization of the junctions.7,8 In higher eukaryotes, phosphorylation of Lnp may contribute to the conversion of the ER from tubules to sheets during mitosis.8
Recently, three homozygous loss-of-function (LoF) variants in LNPK were shown to cause a neurodevelopmental disorder (OMIM#618090) in four patients displaying global developmental delay (GDD)/intellectual disability (ID), epilepsy, corpus callosum hypoplasia and variable impairment of cerebellar development.9,10
Here, we present 16 new individuals from 12 different families harbouring 11 novel homozygous LoF variants in LNPK, outlining the molecular and phenotypic spectrum of LNPK-related disorder.
Materials and methods
Patients and genetic analysis
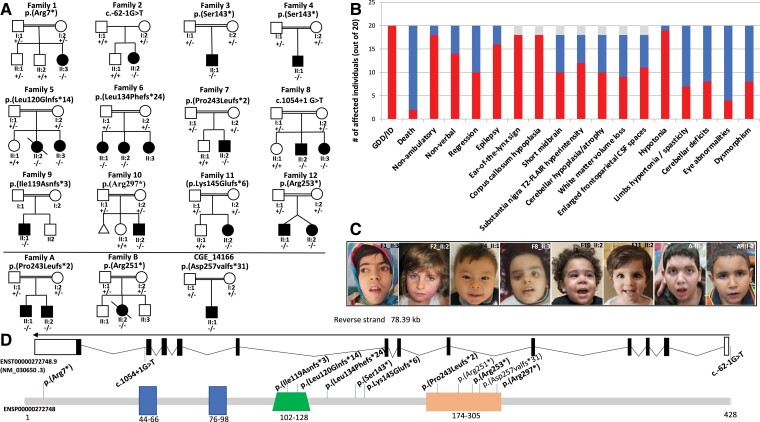
Sixteen previously unreported patients from 12 families of different ancestries (Egyptian, Iranian, Turkish, Saudi Arabian, Afghan, Pakistani and British) as well as additional follow-up data from four patients reported from three families (Egyptian, Pakistani and Turkish)9,10 were included in this study after obtaining written informed consent (Fig. 1A).
Figure 1.
Pedigrees of the families, photos, clinical summary of the affected individuals and schematic representation of the gene and protein with all the pathogenic variants. (A) Pedigrees of Families 1–11. In the pedigrees, squares represent males, circles females and black shaded symbols denote patients harbouring biallelic LNPK variants. Plus (+) and minus (−) signs indicate absence or the presence of the LNPK variants [(+/+) wild-type, (+/−) heterozygote and (−/−) homozygote for the LNPK variant]. Pedigrees of previously reported patients are at the bottom of A separated by a line. (B) Bar graph showing the distribution of the most relevant clinical and radiological features among the total patients (20) identified so far with biallelic LNPK variants. Red: number of patients out of 18 showing each feature. Blue: number of patients without each specific feature. Grey: brain MRI not available for two individuals. GDD, global developmental delay; ID, intellectual disability (C) Clinical features of patients with homozygous LNPK variants showing subtle and nonspecific dysmorphic features such as medially flared eyebrows, long palpebral fissures, prominent philtrum, long chin in Patient II:3 of Family 1; bilateral infraorbital crease and thin upper lip vermilion in Patient II:2 of Family 2; almond-shaped eyes, anteverted nares and thin upper lip vermilion in Patient II:1 of Family 4; uplifted earlobes in Patient II:3 of Family 8; smooth philtrum, thin upper lip vermilion and uplifted earlobes in Patient II:2 of Family10; deep set eyes, thin upper lip vermilion and uplifted earlobes in Patient II:2 of Family 11; low frontal hairline and thick earlobes in Patients A-III-1 and A-III-2 previously published by Breuss et al.9 (D) Schematic depiction of transcript (ENST00000272748.9) and the full-length LNP protein (GenBank: NP_085153.1) showing two transmembrane domains (dark blue), a coiled coil region (green) and a zinc2+ finger domain (orange). The variants identified in the current cohort are displayed in bold. Note that the variant c.726del p.(Pro243Leufs*2) was also identified in the original manuscript of Breuss et al.9
Clinical data were collected using standardized pro forma from around 10 different hospitals and clinics, for all individuals. Brain magnetic resonance imaging (MRI) of these and of the previously reported patients9,10 were reviewed by an experienced neuroradiologist (M.S.). Exome sequencing (ES) or genome sequencing (Family 2) was performed in probands in the respective collaborating centres using slightly different analysis platforms according to the BWA/GATK-based pipelines. Sanger sequencing with standard methods was performed for candidate variants’ validation and familial segregation. All LNPK variants are reported according to the transcript NM_030650.3 and classified according to the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP) variant classification system. The study was approved by the University Colleague London (UCL) research ethics committee as well as institutional ethics committees of participating centres including medical research ethics of the National Research Centre (NRC) in Cairo, Egypt.
Results
Genetic findings
ES or genome sequencing revealed 12 novel or ultra-rare LNPK variants homozygous in affected individuals as follows: a nonsense variant c.19C>T p.(Arg7*) in Family 1, a splicing variant c.-62-1G>T in Family 2, a nonsense variant c.428C>A p.(Ser143*) in Families 3 and 4, a frameshift c.359_362del p.(Leu120Glnfs*14) in Family 5, a frameshift variant c.402_405del p.(Leu134Phefs*24) in Family 6, a frameshift variant c.726del p.(Pro243Leufs*2) in Family 7, the splicing variant c.1054+1G>T in Family 8, a frameshift variant c.355dup p.(Ile119Asnfs*3) in Family 9, a nonsense variant c.889C>T p.(Arg297*) in Family 10, a frameshift variant c.431dup p.(Lys145Glufs*6) in Family 11 and a nonsense variant c.757C>T p.(Arg253*) in Family 12 (Fig. 1A–D). Ten of these variants were novel, while the c.726del p.(Pro243Leufs*2) was previously reported in an unrelated family from Egypt.9
For Family 2, homozygosity was due to uniparental isodisomy involving the entire Chromosome 2, and only the mother was a heterozygous carrier. Sanger sequencing confirmed segregation of the variants with the phenotype within these families.
All variants were classified as pathogenic according to the ACMG/AMP criteria and are extremely rare in human population variant databases (allele frequency ranging from 0 to 0.000003995 in gnomAD, UK Biobank and Queen Square genomics database). None of the variants were reported in a homozygous state in healthy individuals.
All nonsense and frameshift variants are predicted to result in a premature truncation of the transcript, likely leading to nonsense-mediated mRNA decay. The variants c.-62-1G>T and c.1054+1G>T are predicted to severely impair the protein structure through aberrant mRNA splicing (acceptor loss 0.98 score and donor loss 0.99 score, according to the SpliceAI tool).11 No other pathogenic/likely pathogenic variants were identified in the currently known neurodevelopmental or neurodegenerative disorder (NDD)–related genes.
Clinical and neuroradiological findings
All 16 patients (9 females, 7 males; mean age 8.2, range 2–19) had GDD and moderate-to-profound ID (moderate = 5; severe = 8; profound = 3). Only one individual was able to walk with support at the last follow-up visit, and all were mostly nonverbal. Developmental regression was observed in seven, mostly occurring after seizure onset. One individual (II:2 of Family 5) died at the age of 9.5 years due to status epilepticus in the context of respiratory infection. Twelve individuals had epilepsy, experiencing different seizure types with a predominance of myoclonic and tonic–clonic seizures, and the age of onset was between 2 months and 6 years. For nine of them, epilepsy was refractory to antiseizure medications. Review of available EEG for 11 patients (including 2 previously reported) did not identify a specific electrographic pattern. Additional details about EEG findings are available in Supplemental Table 1 and other Supplementary material. Two patients were diagnosed with autism spectrum disorder while no major behavioural abnormalities were noted in other children. Neurological exam demonstrated axial hypotonia (n = 16), hyporeflexia (n = 6), limb hypertonia (n = 4), cerebellar tremor (n = 3) and ataxic gait in one of the two patients who were able to walk prior to regression. Ophthalmological findings included strabismus (n = 5), nystagmus (n = 4), bilateral cataracts (n = 2) and optic atrophy (n = 1). Two individuals had postnatal microcephaly, and another two showed mild macrocephaly, while the majority had normal head circumference. Subtle and nonspecific dysmorphic features were noticed in those individuals for whom photos were available (Fig. 1C).
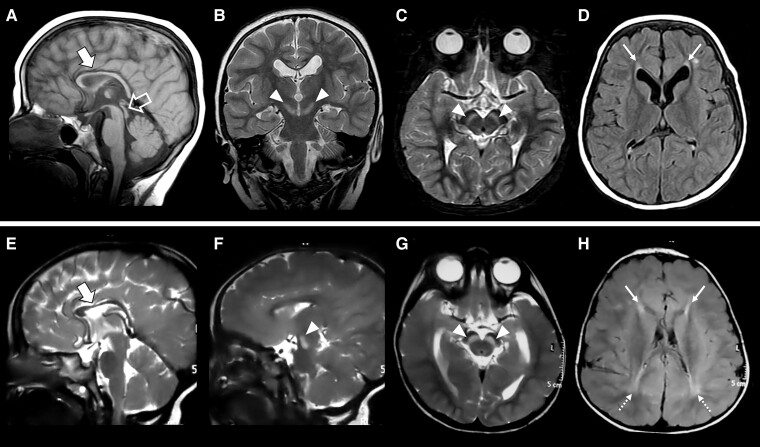
Brain MRI studies were available for review in 18 patients (14 from the present cohort and 4 from previous publications9,10; mean age at MRI: 4.6 years, range 1–14 years) (Supplementary Fig. 1). In all patients (18/18, 100%), we found callosal hypoplasia with prevalent anterior involvement and focal signal changes of the forceps minor of the corpus callosum reminiscent of the ‘ear-of-the-lynx’ sign (Fig. 2). Additional prominent features included bilateral symmetric T2-Fluid attenuated inversion recovery (FLAIR) hyperintensity of the substantia nigra (13/18, 72.2%), enlargement of the cerebral CSF spaces (11/18, 61.1%), a short midbrain (10/18, 55.5%), white matter volume loss with an antero-posterior gradient (9/18, 50%), mild inferior vermis hypoplasia (8/18, 44.4%) and other periventricular white matter signal alterations (8/18, 44.4%). Mild cerebellar atrophy (3/18, 16.6%) was detected in a subset of patients (Supplementary Fig. 2). Clinical features are summarized in Table 1 and Fig. 1D and extensively available in Supplementary Table 1.
Figure 2.
Neuroimaging features of LNPK-related disorder. Brain MRI studies performed in Patient II:1 from Family 6 at 4 years of age (A–D) and in Patient II:2 from Family 7 at 2.5 years of age (E–H). Sagittal T1- (A) or T2-weighted (E) images demonstrate corpus callosum hypoplasia with prevalent involvement of the anterior portions (thick arrows). Coronal (B), axial (C, G) and sagittal (F) T2-weighted images reveal symmetric marked T2 hyperintensity of the substantia nigra (arrowheads). Note the ‘ears-of-the-lynx’ sign (thin arrows) on axial FLAIR images (D, H) consisting of hyperintense signal of the forceps minor bilaterally, which resembles the shape of the ears of a lynx with their characteristic apical hair tuft. Additional posterior periventricular white matter signal alterations are noted in Patient II:2 from Family 7 (dotted arrows). A short midbrain is also visible in Patient II:1 from Family 6 (empty arrow).
Table 1.
Genetic and phenotypic characteristics of patients with LNPK variants
| Family ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | II:3 | II:2 | II:1 | II:1 | II:2 | II:3 | II:1 | II:2 | II:2 | II:2 | II:3 | II:1 | II:2 | II:2 | II:1 | II:2 | A-III-1 | A-III-2 | B-III-2 | CGE_14 |
| Age, sex | 13y, F | 16y, F | 13y, M | 3y5m, M | 9y, F died | 2.5y, F | 19y, F | 15y, F | 7y, M | 7y, M | 3y, F | 12y, M | 3y, M | 2y, F | 3y,M | 3y,F | 15y, M | 7y4m, M | 16y, F died | 9y, F |
| GDD/ID | ++++ | +++ | ++++ | +++ | +++ | ++ | +++ | ++ | ++++ | +++ | ++ | +++ | ++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Nonambulate | + | + a | + | + | + | + | + | + | + | + | + | +a | + | + | + | + | + | − | − | +a |
| Nonverbal | + | − | + | + | + | + | + | + | + | + | + | + | − | − | + | − | + | − | − | + |
| Regression | + | − | + | − | + | − | + | − | + | − | + | + | − | − | − | − | + | − | + | + |
| Epilepsy | + | + | + | + | + | − | + | + | + | + | + | + | − | − | + | − | + | + | + | + |
| Seizure-AOO | 10m | 6y | 4y | 2m | 3y | 2y | 18m | 2y | 4y | 2y | 5y | 2y3m | 2y | 2y | 6y | 7y | ||||
| Seizure type | Myo, TC | Myo, TC | Myo | Focal, TC | Myo, TC | TC, atyp Abs. | NA | Myo | Focal TC | TC | Myo, TC | Myo, TC | Myo | Myo | TC | Myo, TC | ||||
| Seizure frequency | Up to 100/day | 3–4/week | 4–5/day | 1–2/month | 20/day | 20/day | NA | 30–50/day | 1/month | 1–2/day | 3–4/day | NA | Up to 10/day | NA | Up to 10/day | Up to 20/day | ||||
| Response to ASM | − | + | − | + | − | − | NA | − | − | − | − | − | − | + | − | − | ||||
| Age at brain MRI | 8y3m | 7y | 8y | 1y | 1y1m | NA | 4y10m | 1y6m | 2y5m | 3y | 4y | 8m; 1y9m | 3y | 2y | NA | 2y | 6y | 4y | 14y | 2y7m; 9y |
| CCH | + | + | + | + | + | NA | + | + | + | + | + | + | + | + | NA | + | + | + | + | + |
| Ears of lynx sign | + | + | + | + | + | NA | + | + | + | + | + | + | + | + | NA | + | + | + | + | + |
| WMVL | − | − | +++ | − | − | NA | ++ | ++ | − | ++ | ++ | − | − | − | NA | − | + | + | + | ++ |
| Enlarged FP CSF spaces | + | − | + | + | + | NA | + | + | − | + | + | + | − | − | NA | − | − | − | + | + |
| Midbrain height | Short | Short | Normal | Short | Normal | NA | Short | Short | Normal | Short | Short | Normal | Normal | Short | NA | Normal | Normal | Normal | Short | Short |
| Substantia nigra SA | − | + | + | − | − | NA | + | + | + | + | + | − | + | + | NA | + | − | − | + | + |
| Cerebellum | Mild atrophy | Normal | Mild atrophy, IVH | Normal | Mild IVH | NA | Mild IVH | Mild IVH | Mild IVH | Normal | Normal | Mild IVH | Normal | Normal | NA | Normal | Normal | Mild IVH | Mild atrophy | Mild IVH |
| OFC (SDS) | −0.9 | −3.2 | +0.5 | −1.2 | +0.6 | +0.2 | +3.3 | +2.5 | −2.6 | +1.1 | +1 | −1.1 | −0.1 | −2.4 | NA | NA | −1.1 | −1.0 | −1.4 | +1.14 |
| Axial hypotonia | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − |
| Limbs hypertonia | − | + | − | − | + | − | − | − | − | + | + | + | − | − | − | − | + | + | − | + |
| Cerebellar signs | − | + | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | + | − | + |
| Dysmorphism | + | + | + | + | − | − | − | − | − | + | + | + | + | − | − | − | − | − | − | − |
| Eye features | − | Bil. cataracts | Nystagmus | Bil. ONA | Nystagmus esotropia | Esotropia | − | Esotropia | Nystagmus | − | − | Esotropia, Bil. cataracts | − | − | − | − | − | − | Bil. ONA | − |
+ and − denote the presence or absence of a specific feature, respectively. Families 13 and 14 have been reported by Breuss et al.9 and Türkyılmaz et al.,10 respectively. ASM, antiseizure medications; AOO, of onset; atyp, atypical; Bil., bilateral; CCH, corpus callosum hypoplasia; GDD, global developmental delay; DTR, deep tendon reflexes; F, female; FP, frontoparietal; hom, homozygous; myo, myoclonic; TC, tonic–clonic; ID, intellectual disability; IVH, inferior vermis hypoplasia; m, months; OFC, occipital frontal circumference; ONA, optic nerve atrophy; M, male; NA, not available; SA, signal alterations; SDS, standard deviations; WMVL, white matter volume loss; y, years.
Previously able to walk; unable to walk after regression.
Discussion
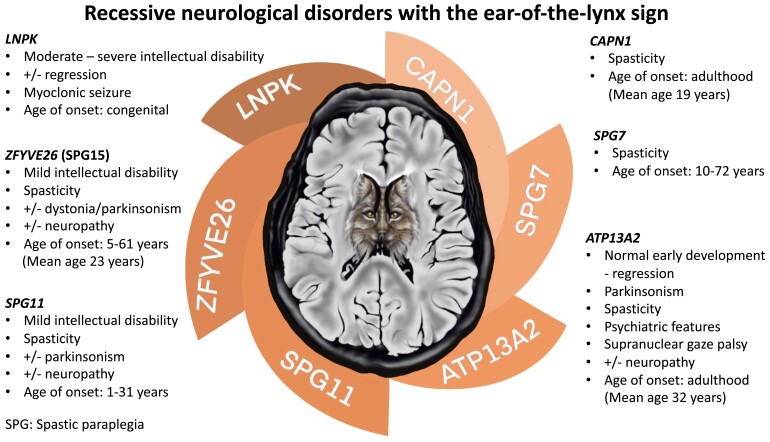
All affected individuals of our and previous cohorts9,10 harbour LoF homozygous variants in LNPK, resulting in a neurodevelopmental phenotype characterized by moderate to profound DD/ID, refractory epilepsy and a recognizable neuroradiological pattern. Interestingly, brain MRI analysis including review of previously published patients led us to identify a consistent neuroimaging phenotype characterized by callosal hypoplasia and abnormal signal of the forceps minor (‘ear-of-the-lynx’ sign), variably associated with substantia nigra signal alterations, mild brain atrophy, short midbrain and cerebellar hypoplasia/atrophy. Of note, the ‘ear-of-the-lynx’ sign has been typically described in SPG11 (MIM#604360) and SPG15 (MIM#270700),12 linked to pathogenic variants in genes encoding spatacsin (SPG11) and spastizin (ZFYVE26), respectively, which play pivotal roles in intracellular trafficking and are part of a multiprotein complex important for ER function.13-15 The presence of this sign in the LNPK-related disorder further underscores the importance of ER for axon development and function.3 Moreover, signal alterations of the forceps minor with an ‘ear-of-the-lynx’ or ‘ear-of-the-grizzly’ morphology have been recently described in AP-4-associated hereditary SPG (AP-4-SPG)16 and in the allelic disorders SPG78 (MIM#617225) and Kufor–Rakeb syndrome (MIM#606693), due to biallelic variants in ATP13A2.17 The ‘ear-of-the-lynx’ sign has been also occasionally reported in patients with variants in the SPG7 and CAPN1 genes, linked to SPG7 (MIM#607259) and SPG76 (MIM #616907), respectively.18,19 Notably, several genes associated with the ‘ear-of-the-lynx’ sign such as SPG11,15ZFYVE26,15ATP13A217 and LNPK20 have been implicated in autophagy, raising the suspicion for a possible common underlying mechanism related to ER-phagy dysfunction. Interestingly, myoclonic seizure is frequently observed in our cohort while it does not typically occur in the above disorders. This association when present may help clinicians to recognize LNPK-related disorder in the clinical setting. Main features of the NDD disorders presenting with the ‘ear-of-the-lynx’ sign and comparison with LNPK are displayed in the Supplementary Table 2.
In addition, most patients (72.2%) had additional T2-FLAIR hyperintensity of the substantia nigra. Remarkably, loss of normal susceptibility signal dropout of the substantia nigra is found in some neurodegenerative disorders such as Parkinson disease and related conditions21 in which the nigrostriatal pathway is impaired. However, signal alterations of the substantia nigra are unusual in neurodevelopmental disorders and have never been described in the group of SPGs linked to ER protein dysfunction. Notably, LNPK is abundantly expressed in the human substantia nigra (normalized protein-coding transcripts per million: 9.2 according to the Human Protein Atlas database), yet its role in the nigrostriatal dopaminergic circuit remains to be investigated. Neurological follow-up of affected individuals with LNPK pathogenic variants will be important to determine whether they may develop parkinsonism later in life like in the ATP13A2-related disorders, which could be potentially treated.
The effect of LNP deficiency on ER has previously been elucidated by knockout studies in Saccharomyces cerevisiae6 and mammalian cell lines,8 showing that its loss leads to a reduction of tubules and junctions and an increased sheet-like appearance at the cellular periphery, overall affecting the abundance of the three-way junctions. In humans, fibroblasts of patients harbouring a homozygous truncating variant in LNPK exhibited aberrant ER shape and increased luminal mass density.9 Likewise, we expect that the homozygous LoF variants identified in our patients result in a loss of protein function with consequent perturbation of ER morphology and homeostasis. However, the mechanism underlying impact on central nervous system development, resulting in cognitive impairment, epilepsy and brain malformations, is yet to be elucidated. The typical biphasic disease course with a neurodegenerative phase occurring on the background of a neurodevelopmental impairment may support at least in part a pathomechanism related to autophagy dysfunction as seen in other congenital disorders of autophagy.22 Of note, autophagosomes form at the ER in mammals, and ER membrane contacts are known to play a central role in regulating autophagosome formation.23 Although we may speculate that LNP deficiency impairs ER homeostasis and function with consequent perturbation of autophagy, a direct functional linkage between LNP and autophagosomes remains elusive and related signalling pathways yet unknown.
Furthermore, it is unknown why spasticity is not a major finding in individuals with LNP deficiency in contrast to the SPG phenotype of individuals with pathogenic variants in other ER genes. Finally, deletion of the LNPK homologue (lnp-1) in Caenorhabditis elegans causes mislocalization of presynaptic proteins, suggesting a role of Lnp-1 in synaptogenesis through regulation of vesicular transport or localization.24 This finding is in line with the clinical presentation of refractory epilepsy in our cohort, pointing to a possible synaptic dysfunction due to LNP deficiency.
In summary, we outline the clinical features of the LNPK-related NDD, mainly characterized by moderate to profound ID, epilepsy and recognizable brain anomalies. Specifically, the ‘ear-of-the-lynx’ sign associated with corpus callosum hypoplasia and substantia nigra signal alterations are the key feature that could guide clinicians toward an early clinical diagnosis. Further studies are needed to elucidate the LNP’s role in ER of developing neurons and the exact pathomechanism leading to LNP deficiency.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
We are grateful for the essential support from patients and families and for grateful funding from The Wellcome Trust, The MRC, The MSA Trust, The National Institute for Health Research University College London Hospitals Biomedical Research Centre, The Michael J Fox Foundation (MJFF), BBSRC, The Fidelity Trust, Rosetrees Trust, Ataxia UK, Brain Research UK, Sparks GOSH Charity, Alzheimer’s Research UK (ARUK) and CureDRPLA. We are grateful to Valentina Turchetti for her contribution to the graphical abstract. We thank the support from Egypt Science and Technology Development Fund (STDF) project 26040, Academy of Science Research and Technology, Egypt (Grant number: 33650). This research was supported by the NIHR Manchester Biomedical Research Centre (NIHR203308). A.J. and S.B. acknowledge the support of Solve-RD. The Solve-RD project has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement no. 779257. The authors would like to thank the patients’ families for their cooperation. We thank Dr. Haluk Yavuz for his support and assistance in data collection. This research was made possible through access to the data and findings generated by the 100,000 Genomes Project. The 100,000 Genomes Project is managed by Genomics England Limited (a wholly owned company of the Department of Health and Social Care). Sequencing for Family 6 was provided by the Broad Institute of MIT and Harvard Center for Mendelian Genomics (Broad CMG). We are grateful to Ms. Carolyn Le (from Department of Neurology, University of California, San Francisco, USA) for gathering clinical information of Familiy 6.
Contributor Information
Andrea Accogli, Division of Medical Genetics, Department of Specialized Medicine, McGill University, Montreal H3G 1A4, Canada; Department of Human Genetics, McGill University, Montreal, QC H3A 0C7, Canada.
Maha S Zaki, Clinical Genetics Department, Human Genetics and Genome Research Institute, National Research Centre, Cairo 12622, Egypt.
Mohammed Al-Owain, Department of Medical Genomics, Center for Genomics Medicine, King Faisal Specialist Hospital and Research Center, Riyadh 11211, Saudi Arabia.
Mansour Y Otaif, Department of Pediatric, Neurology Section, Abha Maternity and Childern Hospital, Abha 62521, Saudi Arabia.
Adam Jackson, Division of Evolution, Infection and Genomics, School of Biological Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester M13 9PT, UK; Manchester Centre for Genomic Medicine, University of Manchester, St Mary’s Hospital, Manchester Academic Health Science Centre, Manchester M13 9WL, UK.
Emanuela Argilli, Department of Neurology, University of California, San Francisco, San Francisco, CA 94143, USA.
Kate E Chandler, Division of Evolution, Infection and Genomics, School of Biological Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester M13 9PT, UK; Manchester Centre for Genomic Medicine, University of Manchester, St Mary’s Hospital, Manchester Academic Health Science Centre, Manchester M13 9WL, UK.
Christian G E L De Goede, Department of Paediatric Neurology, Clinical Research Facility, Lancashire Teaching Hospital NHS Trust, Preston PR2 9HT, UK.
Tülün Cora, Department of Medical Genetics, Selcuk University School of Medicine, Konya 42100, Turkey.
Javeria Raza Alvi, Department of Pediatric Neurology, Institute of Child Health, Children's Hospital, Lahore 54590, Pakistan.
Atieh Eslahi, Department of Medical Genetics, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad 917794-8564, Iran; Student Research Committee, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad 9137-86177, Iran.
Mahsa Sadat Asl Mohajeri, Department of Medical Genetics, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad 917794-8564, Iran.
Setareh Ashtiani, Alberta Children’s Hospital Research Institute, Department of Medical Genetics, University of Calgary, Alberta T2N 4Z6, Canada.
P Y Billie Au, Alberta Children’s Hospital Research Institute, Department of Medical Genetics, University of Calgary, Alberta T2N 4Z6, Canada.
Alicia Scocchia, Blueprint Genetics Inc, Marlborough, MA 01752, USA.
Kirsi Alakurtti, Blueprint Genetics Inc, Marlborough, MA 01752, USA.
Alistair T Pagnamenta, NIHR Biomedical Research Centre, Wellcome Centre for Human Genetics, University of Oxford, Oxford OX3 7BN, UK.
Mehran Beiraghi Toosi, Pediatric Neurology Department, Mashhad University of Medical Sciences, Mashhad 913791-6847, Iran; Neuroscience Research Center, Mashhad University of Medical Sciences, Mashhad 91375-33116, Iran.
Ehsan Ghayoor Karimiani, Molecular and Clinical Sciences Institute, St. George’s, University of London, Cranmer Terrace, London SW17 0RE, UK; Department of Medical Genetics, Next Generation Genetic Polyclinic, Mashhad 91869-51591, Iran.
Majid Mojarrad, Department of Medical Genetics, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad 917794-8564, Iran; Student Research Committee, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad 9137-86177, Iran; Genetic Center of Khorasan Razavi, Mashhad 91877-53831, Iran.
Fatemeh Arab, Department of Medical Genetics, Faculty of Medicine, Tehran University of Medical Sciences, Tehran 1411713135, Iran.
Fahrettin Duymuş, Department of Medical Genetics, Selcuk University School of Medicine, Konya 42100, Turkey; Department of Medical Genetics, Konya City Hospital, Konya 42020, Turkey.
Morris H Scantlebury, Departments of Pediatrics and Clinical Neuroscience, University of Calgary; Alberta Children’s Hospital Research Institute, Hotchkiss Brain Institute & Owerko Center, University of Calgary, Alberta T2N 4N1, Canada.
Gözde Yeşil, Department of Medical Genetics, Istanbul Medical Faculty, Istanbul University, Istanbul 34093, Turkey.
Jill Anne Rosenfeld, Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX 77030, USA; Baylor Genetics Laboratories, Houston, TX 77021, USA.
Ayberk Türkyılmaz, Department of Medical Genetics, Karadeniz Technical University Faculty of Medicine, Trabzon 61080, Turkey.
Safiye Güneş Sağer, Clinics of Pediatric Neurology, Kartal Dr. Lütfi Kırdar City Hospital, İstanbul 34890, Turkey.
Tipu Sultan, Department of Pediatric Neurology, Institute of Child Health, Children's Hospital, Lahore 54590, Pakistan.
Farah Ashrafzadeh, Pediatric Neurology Department, Mashhad University of Medical Sciences, Mashhad 913791-6847, Iran.
Tatheer Zahra, Department of Developmental-Behavioral Pediatrics, University of Child Health Sciences, The Children’s Hospital, Lahore 54590, Pakistan.
Fatima Rahman, Department of Developmental-Behavioral Pediatrics, University of Child Health Sciences, The Children’s Hospital, Lahore 54590, Pakistan.
Shazia Maqbool, Department of Developmental-Behavioral Pediatrics, University of Child Health Sciences, The Children’s Hospital, Lahore 54590, Pakistan.
Mohamed S Abdel-Hamid, Medical Molecular Genetics Department, Human Genetics and Genome Research Institute, National Research Centre, Cairo 12622, Egypt.
Mahmoud Y Issa, Clinical Genetics Department, Human Genetics and Genome Research Institute, National Research Centre, Cairo 12622, Egypt.
Stephanie Efthymiou, Department of Neuromuscular Diseases, UCL Queen Square Institute of Neurology, Queen Square, London WC1N 3BG, UK.
Peter Bauer, CENTOGENE, Rostock 18057, Germany.
Giovanni Zifarelli, CENTOGENE, Rostock 18057, Germany.
Vincenzo Salpietro, Department of Neuromuscular Diseases, UCL Queen Square Institute of Neurology, Queen Square, London WC1N 3BG, UK; Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila, L’Aquila 67100, Italy.
Zuhair Al-Hassnan, Department of Medical Genomics, Center for Genomics Medicine, King Faisal Specialist Hospital and Research Center, Riyadh 11211, Saudi Arabia; College of Medicine, Alfaisal University, Riyadh 11533, Saudi Arabia.
Siddharth Banka, Division of Evolution, Infection and Genomics, School of Biological Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester M13 9PT, UK; Manchester Centre for Genomic Medicine, University of Manchester, St Mary’s Hospital, Manchester Academic Health Science Centre, Manchester M13 9WL, UK.
Elliot H Sherr, Department of Neurology, University of California, San Francisco, San Francisco, CA 94143, USA.
Joseph G Gleeson, Department of Neurosciences, University of California, San Diego, La Jolla 92093, USA; Rady Children’s Institute for Genomic Medicine, San Diego 92123, USA.
Pasquale Striano, Department of Neurosciences Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health (DiNOGMI), University of Genoa, Genoa 16132, Italy; Pediatric Neurology and Muscular Diseases Unit, IRCCS Istituto ‘Giannina Gaslini’, Genoa 16147, Italy.
Henry Houlden, Department of Neuromuscular Diseases, UCL Queen Square Institute of Neurology, Queen Square, London WC1N 3BG, UK.
Mariasavina Severino, Neuroradiology Unit, IRCCS Istituto Giannina Gaslini, Genoa 16146, Italy.
Reza Maroofian, Department of Neuromuscular Diseases, UCL Queen Square Institute of Neurology, Queen Square, London WC1N 3BG, UK.
Funding
This study was funded by the Medical Research Council (MR/S01165X/1, MR/S005021/1, and G0601943) and by the Wellcome Trust (FC001187) and the NIHR Oxford Biomedical Research Centre. The 100,000 Genomes Project is funded by the National Institute for Health Research and National Health Service of England. The Wellcome Trust, Cancer Research UK and the Medical Research Council have also funded research infrastructure. The 100,000 Genomes Project uses data provided by patients and collected by the National Health Service as part of their care and support. This study has been also supported by NIH grant R01NS058721.
Competing interests
The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing completed at Baylor Genetics Laboratories.
Data availability
All variants have been deposited into the LOVD database: https://databases.lovd.nl/shared/variants/KIAA1715/unique.
References
- 1. Schwarz DS, Blower MD. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell Mol Life Sci. 2016;73(1):79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perkins HT, Allan V. Intertwined and finely balanced: Endoplasmic reticulum morphology, dynamics, function, and diseases. Cells. 2021;10(9):2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fowler PC, Garcia-Pardo ME, Simpson JC, et al. NeurodegenERation: The central role for ER contacts in neuronal function and axonopathy, lessons from hereditary spastic paraplegias and related diseases. Front Neurosci. 2019;13:1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colla E. Linking the endoplasmic reticulum to Parkinson’s disease and alpha-synucleinopathy. Front Neurosci. 2019;13:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen S, Desai T, McNew JA, et al. Lunapark stabilizes nascent three-way junctions in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2015;112(2):418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shemesh T, Klemm RW, Romano FB, et al. A model for the generation and interconversion of ER morphologies. Proc Natl Acad Sci U S A. 2014;111:E5243–E5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anggrandariyanny PC, Kajiho H, Yamamoto Y, Sakisaka T. Lunapark ubiquitinates atlastin-2 for the tubular network formation of the endoplasmic reticulum. Biochem. 2022;172(4):245–257. [DOI] [PubMed] [Google Scholar]
- 8. Wang S, Tukachinsky H, Romano FB, Rapoport TA. Cooperation of the ER-shaping proteins atlastin, lunapark, and reticulons to generate a tubular membrane network. Elife. 2016;5:e18605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Breuss MW, Nguyen A, Song Q, et al. Mutations in LNPK, encoding the endoplasmic reticulum junction stabilizer lunapark, cause a recessive neurodevelopmental syndrome. Am J Hum Genet. 2018;103(2):296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Türkyılmaz A, Sağer SG, Günbey HP, Akın Y. Novel LNPK variant causes progressive cerebral atrophy: Expanding the clinical phenotype. Clin Genet. 2022;102:218–222. [DOI] [PubMed] [Google Scholar]
- 11. Jaganathan K, Panagiotopoulou SK, McRae JF, et al. Predicting splicing from primary sequence with deep learning. Cell. 2019;176(3):535–548.e24. [DOI] [PubMed] [Google Scholar]
- 12. Pascual B, de Bot ST, Daniels MR, et al. “Ears of the lynx” MRI sign is associated with SPG11 and SPG15 hereditary spastic paraplegia. AJNR Am J Neuroradiol. 2019;40(1):199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murmu RP, Martin E, Rastetter A, et al. Cellular distribution and subcellular localization of spatacsin and spastizin, two proteins involved in hereditary spastic paraplegia. Mol Cell Neurosci. 2011;47(3):191–202. [DOI] [PubMed] [Google Scholar]
- 14. Boutry M, Pierga A, Matusiak R, et al. Loss of spatacsin impairs cholesterol trafficking and calcium homeostasis. Commun Biol. 2019;2:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vantaggiato C, Panzeri E, Castelli M, et al. ZFYVE26/SPASTIZIN and SPG11/SPATACSIN mutations in hereditary spastic paraplegia types AR-SPG15 and AR-SPG11 have different effects on autophagy and endocytosis. Autophagy. 2019;15:34–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ebrahimi-Fakhari D, Alecu JE, Ziegler M, et al. Systematic analysis of brain MRI findings in adaptor protein complex 4-associated hereditary spastic paraplegia. Neurology. 2021;97(19):e1942–e1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Estrada-Cuzcano A, Martin S, Chamova T, et al. Loss-of-function mutations in the ATP13A2/PARK9 gene cause complicated hereditary spastic paraplegia (SPG78). Brain. 2017;140(2):287–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sáenz-Farret M, Lang AE, Kalia L, et al. Spastic paraplegia type 7 and movement disorders: Beyond the spastic paraplegia. Mov Disord Clin Pract. 2022;9(4):522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agarwal A, Oinam R, Goel V, et al. “Ear of the lynx” sign in hereditary spastic paraparesis (HSP) 76. Mov Disord Clin Pract. 2022;10:120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parashar S, Ferro-Novick S. Architecture of the endoplasmic reticulum plays a role in proteostasis. Autophagy. 2022;18(4):937–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bae YJ, Kim JM, Sohn CH, et al. Imaging the substantia nigra in Parkinson disease and other Parkinsonian syndromes. Radiology. 2021;300(2):260–278. [DOI] [PubMed] [Google Scholar]
- 22. Deneubourg C, Ramm M, Smith LJ, et al. The spectrum of neurodevelopmental, neuromuscular and neurodegenerative disorders due to defective autophagy. Autophagy. 2022;18(3):496–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamamoto YH, Noda T. Autophagosome formation in relation to the endoplasmic reticulum. J Biomed Sci. 2020;27(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghila L, Gomez M. The evolutionarily conserved gene LNP-1 is required for synaptic vesicle trafficking and synaptic transmission. Eur J Neurosci. 2008;27:621–630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All variants have been deposited into the LOVD database: https://databases.lovd.nl/shared/variants/KIAA1715/unique.