Abstract
The extracellular cellular matrix (ECM) maintains tissue structure and regulates signaling functions by continuous degradation and remodeling. Inflammation or other disease conditions activate proteases including matrix metalloproteinases (MMPs) that degrade ECM proteins and in particular generate fragments of collagen and elastin, some of which are biologically active ECM peptides or matrikines. Stepwise degradation of collagen by MMP 8, 9 and prolyl endopeptidase release the matrikine proline-glycine-proline (PGP) and its product acetyl-PGP (AcPGP). These peptides are considered as potential biomarkers and therapeutic targets for many disease conditions such as chronic lung disease, heart disease, and cancer. However, there is no published, validated method for the measurement of PGP and AcPGP in plasma and therefore, we developed a sensitive, selective and reliable, isotope dilution LC-multiple reaction monitoring MS method for their determination in human plasma. The chromatographic separation of PGP and AcPGP was achieved in 3 min using Jupiter column with a gradient consisting of acidified acetonitrile and water at a flow rate of 0.5 ml/min. The limit of detection (LOD) for PGP and AcPGP was 0.01 ng/ml and the limit of quantification (LOQ) was 0.05 ng/ml and 0.1 ng/ml, respectively. Precision and accuracy values for all analytes were within 20% except for the lowest QC of 0.01 ng/ml. The mean extraction recoveries of these analytes were >90% using a Phenomenex Phree cartridge and the matrix effect was <15% for all the QCs for PGP and AcPGP except the lowest QC. The stability of PGP and AcPGP was >90% in several tested conditions including autosampler use, storage at −80°C, and after 6 times freeze-thaw cycles. Using this method, we successfully extracted and determined PGP levels in human plasma from healthy and COPD subjects. Therefore, this method is suitable for quantification of these peptides in the clinical setting.
Keywords: PGP, AcPGP, LC-MS/MS, Method Development, COPD, Plasma, Matrikines
1. Introduction
Extracellular matrix (ECM) maintains the structure and signaling function of tissue and organs that helps the tissues to regulate cell shape, migration, homeostasis, development, inflammation, and wound repair [1, 2]. The primary components of ECM are fibrous structural proteins such as collagen, elastin, fibronectin, vitronectin, glycoprotein, and proteoglycans [1–3]. ECM degradation and remodeling continuously occur within tissues, and the remodeling rate is high during development and wound healing. ECM modifying proteins such as matrix metalloproteases (MMPs) are one of the agents responsible for ECM degradation and remodeling. ECM degradation by MMPs initiates generation of bioactive fragments termed “matrikines” that modulate the cell behavior [2]. The dynamics of ECM composition changes have been reported in many disease conditions such as cancer initiation, growth, progression, and tumor invasion as well as chronic disease conditions associated with abnormal systemic inflammation including chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and heart failure [4–10].
Among the various constituents of ECM proteins, collagen represents 90% of the total protein mass in mammals [2]. Collagen degradation by MMP 8 and 9 and prolyl endopeptidase (PE) generates the matrikine proline-glycine-proline (PGP); direct chemical modification on the N-terminal position of PGP results into acetylated PGP (AcPGP) [11, 12]. Initially, these matrikines were reported in corneal injury but have subsequently been studied extensively in respiratory matrices (e.g., sputum, bronchoalveolar lavage) from animal models and in humans with chronic lung diseases [13–16]. In the last few years, PGP and AcPGP were also found to be associated with acute respiratory distress syndrome (ARDS) and cardiovascular disease [9, 17]. Under inflammatory conditions, the increased activity of MMP 8, 9, and PE in tissue degrades collagen and generates PGP and AcPGP, leading to a feed-forward cycle of inflammation. As a result, these peptides have been proposed as potential biomarkers for disease. Further, PGP and AcPGP can be released from tissue into the bloodstream, increasing the utility of measuring these peptides in circulation as potential therapeutic targets or biomarkers for the presence, severity, or progression of diseases [15, 18, 19]. Therefore, it is essential to have a reliable, sensitive, and selective method of PGP detection that can be used for analyses in plasma as well as in other biofluids including sputum, bronchoalveolar lavage fluid, and urine. An earlier version of a LC-/MS method for detecting PGP and AcPGP in respiratory matrices has been reported [7]; however, a validated method for measurement in multiple matrices is still lacking. Herein, we report a sensitive and reproducible LC-multiple reaction monitoring (MRM) MS method for PGP and AcPGP measurements that can be used in clinical experiments for determination of these peptides in plasma.
2. Material and Methods
2.1. Chemicals and Reagents
Pure peptides PGP and AcPGP, and 13C10- and 15N2-labeled PGP and AcPGP (Supplementary Figure 1A) were purchased as dry powder from Bachem Americas, Inc., Torrance, CA, USA. The purity of the stable isotopes labeled peptides are nearly 98% (Supplementary Figure 1 B). HPLC-grade methanol (MeOH), acetonitrile, and formic acid were purchased by Fisher Scientific. Milli Q double distilled water (ddH2O) used in the study was purified from the EMD Millipore Milli Q Ultrapure water system. Custom-made two times (2X) charcoal-stripped pooled human plasma (blank plasma) were purchased from BioIVT, Westbury, NY, USA.
2.2. Chromatographic and mass spectrometric conditions
The HPLC-MRM-MS analysis of plasma samples was performed on the Shimadzu Prominence HPLC system with a refrigerated autosampler SIL-20A (Shimadzu Scientific Instruments, Inc. Columbia, MD) coupled with 6500 Qtrap mass spectrometer (SCIEX, Framingham, MA, USA).
Chromatography was performed using Jupiter 4u Proteo column (80Å, 150 × 2.1 mm Phenomenex, Torrance, CA). The mobile phase consisted of water with 0.1% v/v formic acid [A] and acetonitrile with 0.1% FA [B] with a flow rate of 0.5 ml/min. The detailed, stepwise, gradient program is presented in Table 1. The injection volume and total run time were 30 μl and 5 min, respectively. The column temperature was set at 40°C. The operating parameters were: positive electrospray ionization mode with an electrospray voltage of 4000 V and an interface temperature of 600°C. The curtain, GS1 and GS2 gas sources were set at 45, 50 and 50 PSI, respectively. Instrument parameters for the MRM transitions used in the study are listed in Supplementary Table 1. The LC-MS/MS system was controlled by Analyst software version 1.7.2.
Table 1: Stepwise gradient and mass spectrometric conditions for the analytes PGP and AcPGP.
| Time (min) | A% | B% |
|---|---|---|
| 0.5 | 95 | 5 |
| 2.5 | 0 | 100 |
| 2.75 | 0 | 100 |
| 3.0 | 95 | 5 |
2.3. Preparation of calibration, quality controls and labeled internal standards
Stock solutions of 1 mg/ml PGP and AcPGP were prepared in ddH2O individually. Next, a mixture of PGP and AcPGP was prepared with 10 μg/ml concentration in ddH2O. Further, the 10 μg/ml stock solution was diluted in MQ water to yield standard solutions of PGP and AcPGP with the following concentrations: 50, 10, 5, 1, 0.5, 0.1, 0.05, and 0.01 ng/ml. In a similar way, a stock solution of PGP and AcPGP mix was prepared in PBS, and the calibration curve was prepared with the same concentrations as the calibration curves prepared in water. Labeled internal standard (IS) was prepared by mixing 13C10/15N2- labeled analog stock solutions and diluting in PBS to yield a 100 ng/ml labeled IS solution.
The quality control (QC) solutions were prepared by mixing stock solutions of PGP and AcPGP in 2X charcoal-stripped human plasma (blank plasma) to yield six QCs: 0.01, 0.07, 0.25, 2.5, 25, and 50 ng/ml. For the extraction of PGP and AcPGP, QCs prepared in human plasma were spiked with 10 ng/ml IS, and the mix was added to the 96-well plate Phree cartridge (Phenomenex, Torrance CA, USA).To remove phospholipids and proteins, 600 μl pre-chilled solvent mix [methanol(60), acetonitrile (40), and 1% formic acid] was added to each well and vortexed for 2 min. Next, the plate was placed on the 2 ml collection plate and on a positive pressure manifold (Presston 1000, Phenomenex, Torrance, CA, USA). The pressure was set to 20–30 psi for 10–20 min to collect the column eluate. A second 600 μl pre-chilled solvent mix was added to each well - the plate was vortexed for 2 min and the positive pressure manifold step was repeated. The combined eluates were dried under nitrogen. DdH2O (100 μl) was added to each well and centrifuged at 3,320xg for 30 min. The resulting clear supernatants were transferred to sample vials and the analytes were quantified by MRM using the method described in section 2.2.
2.4. Method validation
The analytical method was fully validated using 2X charcoal-stripped human plasma (Blank plasma) according to the US Food and Drug Administration (US FDA) Bioanalytical Method Validation Guidance [20]. In the study, blank plasma (100 μl) was used for each QC replicates and extracted using the method described in section 2.3.
2.4.1. Specificity, selectivity and carryover
The specificity and selectivity of the method was evaluated by comparing chromatograms of the MeOH blank, 3 matrix blanks, i.e., 2X charcoal-stripped human plasma, with IS, PGP, and AcPGP standards in water, and with matrix spiked with PGP and AcPGP, with and without IS.
To evaluate the carryover of the analytes, a sample representing the upper limit of quantification (ULOQ), i.e., 50 ng/ml of PGP and AcPGP mix, was used. The assay was performed by one injection of blank plasma sample followed by the injection of plasma spiked with 50 ng/ml PGP and AcPGP. After the injection of spiked plasma, blank plasma was injected twice. The analytical response of the latter should not exceed 20% of LLOQ and 5% of the average IS analytical response.
2.4.2. Limit of quantification and linearity
The limit of quantification (LOQ) for PGP and AcPGP was determined from repeated analysis (n=5) of the four lowest calibrants following the US FDA’s Bioanalytical Method Validation Guidance. The lowest calibrant meeting the following criteria was set as the LOQ: accuracy between 80–120%, precision of 80–120%, and calculated concentration that was ≥ 5 times that of blank samples.
The linearity of the method was determined by using the peak ratio (ratio of peak area of analytes and IS) of neat standards curve (n=3) and matrix-spiked standards (n=5) at 8 levels of concentrations in three different analytical runs. Linear regression without any weighting was used to calculate r2.
2.4.3. Intra-day and inter-day accuracy and precision
The accuracies were presented as percent bias [Bias (%) = (Grand mean of observed concentration-Actual concentration/ Actual concentration)*100]. For inter-day and intra-day precision, the coefficient of variation was calculated [(CV (%) = Deviation/mean)*100]. The accuracy and precision of the method were assessed by analyzing the quality control samples (n=6) for consecutive 3 days.
2.4.4. Extraction recovery and matrix effect
To analyze the recovery of AcPGP and PGP, quality control samples were pre-spiked with analytes to blank plasma prior to extraction and compared the response to the blank plasma of those that were spiked post-extraction. The extraction efficiency was calculated from the area ratio obtained from the analyte to IS response using the formula: Extraction efficiency (%) = (Pre-spike QC/Post-spike QC) *100.
For the matrix effect (ME), the area of peak obtained for post-spiked blank plasma (blank plasma spiked with analytes after extraction) was compared with the area ratio of the peaks of analytes prepared in water at QC concentrations. The matrix effect was calculated from the area ratio obtained from the analyte to IS response using the formula ME (%) = [1-(Mean peak area of post spiked plasma/Mean peak area of analytes in water)]*100.
2.4.5. Stability
QC samples were evaluated for post-extraction stability in the autosampler for 24 and 48 h, freezer storage (−80°C) for long-term storage, and multiple freeze-thaw cycles. For autosampler stability, extracted QCs replicates (n=6) were kept in the autosampler and re-injected using 0, 24, and 48 h time intervals. For long-term storage, extracted QCs (n=6) were stored at −80°C and injected into a mass spectrometer after 7 and 30 days and compared the response for sample measurement immediately prior to storage (i.e., 0 h). To test the stability of PGP in response to multiple freeze-thaw cycles, we measured PGP and AcPGP in extracted QCs (n=6) before freezing at −80°C and after 3 and 6 freeze-thaw cycles.
2.4.6. Method application
Plasma samples (100 μl) of healthy and COPD patients were obtained from the Prospectve Repository for Coupling EVEnts to Novel paThways in COPD and Asthma (PREVENT) study, an observational cohort conducted at the UAB Lung Health Center. Study participants provided information on demographic, medical comorbidity, lung function, tobacco and environmental exposures, and quality of life data as well as underwent biospecimen (blood and sputum) collection. Plasma specimens were collected using standard phlebotomy into K2EDTA tubes and stored at −80°C until analysis. We used specimens from 20 individuals (n=10 with COPD or control) and extracted and analyzed for PGP and AcPGP using the method described in section 2.3. The University of Alabama at Birmingham Institutional Review Board approved this study (IRB-170301002).
3. Results
3.1. Liquid chromatography and mass spectrometry
The LC-MS/MS method operated in MRM in the positive ion mode was developed to analyze PGP and AcPGP. The chromatographic conditions successfully separated PGP and AcPGP with retention times (RT) at 1.1 min and 2.6 min for PGP and AcPGP, respectively. For MRM analysis, the mass transitions m/z 270/173 and m/z 270/70 were used for PGP and m/z 312/112 and m/z 312/140 were used for AcPGP. The representative chromatograms of standard solutions of PGP and AcPGP are presented in Figure 1. Among these mass transitions, m/z 270/70 for PGP has an interfering peak from plasma and m/z 312/112 for AcPGP were less sensitive compared to m/z 312/140 for AcPGP. Therefore, the mass transitions for PGP m/z 270/70 and AcPGP m/z 312/140 were not used to quantify PGP and AcPGP in the analysis. The mass transitions for the 13C10/15N2-labeled versions of PGP and AcPGP were m/z 282/179 and m/z 324/146, respectively.
Figure 1:

Representative overlay of chromatogram for PGP (m/z 270/70 and 270/173) RT 1.2 min [A] and AcPGP (m/z 312/112 and 312/140) RT 2.6 min [B]
3.2. Method validation
3.2.1. Specificity, selectivity and carryover
Specificity and selectivity were evaluated by comparing blank plasma, standard PGP, and AcPGP in water, PGP and AcPGP in spiked blank plasma, and internal standard spiked in blank plasma. As shown in figures 2A, with the mass transition m/z 270/173 for PGP, the blank plasma sample did not show any interfering peak at RT 1.2 min. Similarly, standard PGP was eluted at RT 1.2 min (Figure 2B). We did not detect any shift or interference of matrix on retention times in PGP, IS in spiked plasma, and IS in spiked blank plasma, respectively (Figures 2C and D). Similarly, no interfering peak was observed for PGP at RT 2.6 min in blank plasma (Figure 3A) with the mass transition m/z 312/140 for AcPGP. Standard AcPGP, spiked AcPGP and spiked IS were eluted at 2.6 min (Figures 3B-D).
Figure 2:
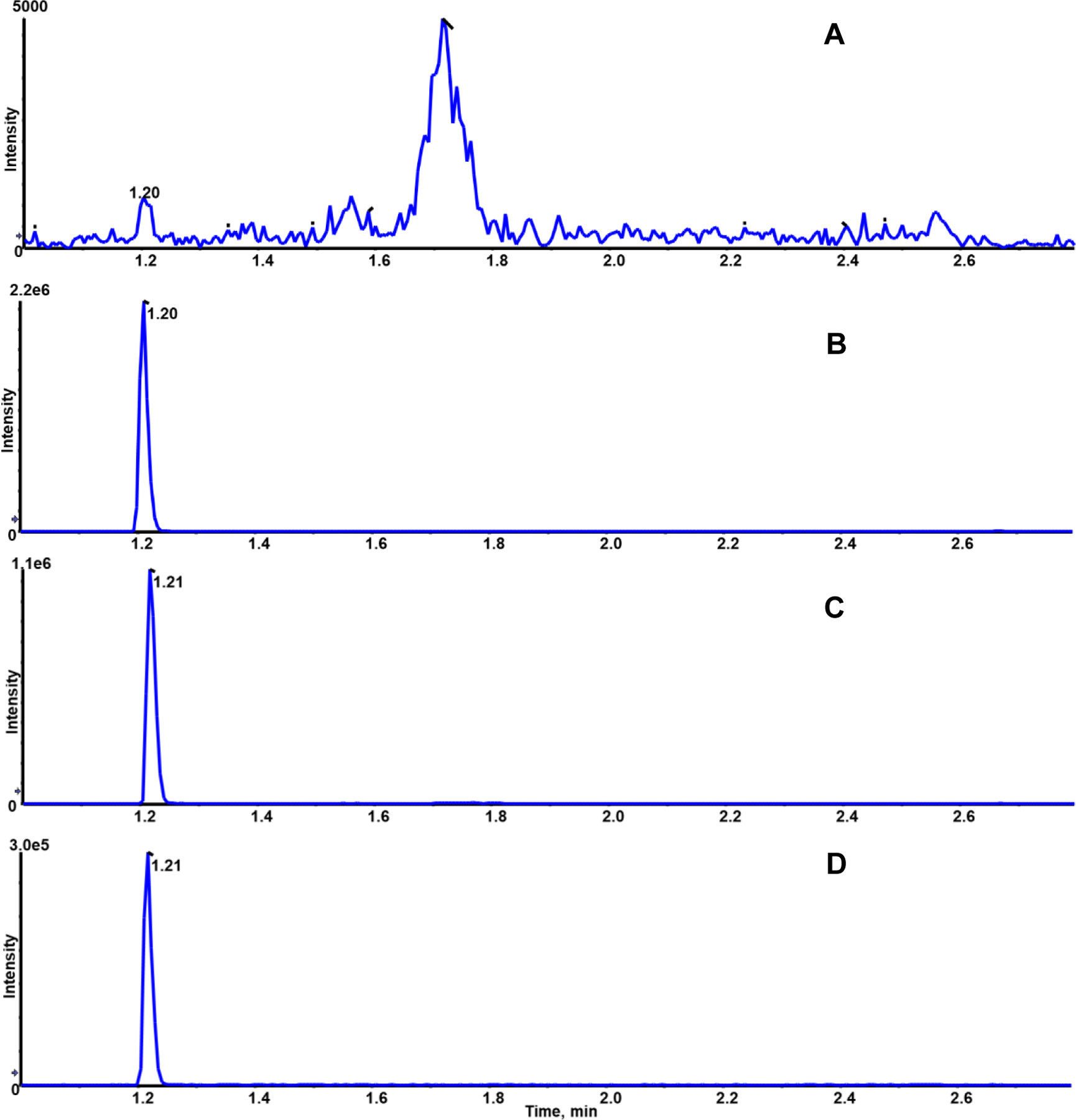
Representative SRM Chromatogram for PGP (m/z 270/173) blank plasma [A], neat standard 10 ng/ml [B], spiked blank plasma with 10 ng/ml [C] and IS-spiked blank plasma [D]
Figure 3:

Representative SRM Chromatogram for AcPGP (m/z 312/140) blank plasma [A], neat standard 10 ng/ml [B], spiked blank plasma with 10 ng/ml [C] and IS-spiked blank plasma [D]
We did not detect carryover for AcPGP in blank plasma injected after 50 ng/ml (ULOQ) spiked plasma. For PGP, a very small carryover peak (0.2±0.09%) was detected in blank plasma injected following the 50 ng/ml spiked plasma.
3.2.2. Limit of quantification and linearity
The linearity of the curve was evaluated by using neat standard solution (n=3) in water, PBS and matrix-spiked standard solution (n=5) in the range of 0.01–50 ng/ml (0.01, 0.05, 0.1, 0.5, 1, 5,10 and 50 ng/ml) for PGP and AcPGP. The method was linear over 1000-fold concentration range in water, PBS, and plasma (Supplementary Figure 2). The value of r2 for each of the curves is >0.99.
A summary of standards prepared in the matrix is presented in Table 2. As shown in Table 2, the CV (%) for PGP using m/z 270/173 was in a range of 2–11%, with the exception of 0.01 ng/ml. Based on the CVs, the LOD is 0.01 ng/ml and the LOQ is 0.05 ng/ml for PGP in plasma. In the case of AcPGP using m/z 312/140, the CV (%) was in the range of 2–14%, except for the 0.01 and 0.05 ng/ml samples. Based on the CVs, the LOD is 0.01 ng/ml, and the limit of quantification is 0.1 ng/ml for AcPGP in plasma.
Table 2: Summary of calibration curves n=5.
| Compound | Nominal Concentration (ng/ml) | Measured Concentration (ng/ml) ± SD | %CV |
|---|---|---|---|
| PGP | 0.01 | 0.01 ± 0.01 | 59.71 |
| 0.05 | 0.05 ± 0.00 | 9.50 | |
| 0.1 | 0.10 ± 0.01 | 11.15 | |
| 0.5 | 0.47 ± 0.02 | 4.68 | |
| 1 | 0.93 ± 0.03 | 2.71 | |
| 5 | 5.38 ± 0.51 | 9.44 | |
| 10 | 10.40 ±0.88 | 8.06 | |
| 50 | 51.70 ± 5.61 | 10.20 | |
| AcPGP | 0.01 | 0.01 ± 0.0 | 27.70 |
| 0.05 | 0.05 ± 0.01 | 24.63 | |
| 0.1 | 0.11 ± 0.01 | 14.29 | |
| 0.5 | 0.48 ± 0.03 | 7.71 | |
| 1 | 1.04 ± 0.03 | 6.96 | |
| 5 | 4.85 ± 0.20 | 2.58 | |
| 10 | 9.81 ± 0.26 | 3.95 | |
| 50 | 47.75 ± 2.60 | 2.24 |
3.2.3. Accuracy and precision
The accuracy and precision of the method were evaluated using 0.01 (LOD), 0.07, 0.25, 2.5, 25, and 50 (ULOQ) ng/ml QCs. For accuracy (bias %), 6 replicates of QCs were injected within the same run for 3 consecutive days, and results are presented in Table 3. The intra-day accuracy was maintained at <20% except for the lowest QC, i.e., 0.01 ng/ml for PGP. Similarly, the intra-day accuracy was maintained at <20% in the case of AcPGP except for the lowest concentration of QC.
Table 3: Precision and accuracy of quality control samples (n=6).
| Analyte | Nominal concentration (ng/ml) | Accuracy (Bias (%)) | Precision (CV (%)) | |||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | Inter-day (n=18) | ||
| PGP | 0.01 | 158.10 | −18.57 | 35.10 | 11.76 | 41.40 | 27.14 | 64.11 |
| 0.07 | 17.89 | −2.73 | 7.03 | 7.91 | 16.75 | 18.68 | 19.89 | |
| 0.25 | −22.68 | −17.92 | −16.76 | 8.07 | 8.05 | 14.61 | 16.15 | |
| 2.5 | 1.64 | 12.32 | −0.60 | 5.39 | 12.39 | 10.87 | 12.26 | |
| 25 | 6.20 | 13.12 | 3.00 | 9.59 | 8.98 | 10.67 | 11.39 | |
| 50 | 13.42 | 14.10 | 0.32 | 6.47 | 8.66 | 10.81 | 10.53 | |
| AcPGP | 0.01 | −15.41 | 1.50 | 51.30 | 84.20 | 111.32 | 48.56 | 61.67 |
| 0.07 | 15.77 | −7.96 | 12.54 | 18.19 | 18.09 | 16.97 | 19.54 | |
| 0.25 | 6.00 | −1.72 | −2.20 | 6.80 | 11.58 | 6.96 | 12.27 | |
| 2.5 | 8.00 | −3.56 | −1.56 | 5.39 | 7.96 | 1.98 | 5.45 | |
| 25 | 10.36 | −3.36 | −5.64 | 2.00 | 4.61 | 4.76 | 6.56 | |
| 50 | 9.64 | −4.38 | −4.90 | 3.46 | 1.76 | 6.21 | 4.64 | |
The precision of the method was represented as CV (%). As shown in Table 3, CV (%) for intra-day and inter-day was maintained <20% for PGP and AcPGP except for the lowest concentration of QC, i.e., 0.01 ng/ml.
3.2.4. Recovery and matrix effect
The recovery of PGP and AcPGP was determined by comparing pre-spiked and post-spiked area ratios of QCs with the concentration of 0.01, 0.07, 0.25, 2.5, 25, and 50 ng/ml (n=6). As presented in Table 4, recovery was achieved in the range between 90–100% for all QCs for PGP. For AcPGP, recovery was achieved in the range of 90–105%.
Table 4: Recovery and matrix effect of quality control samples (n=6).
| Compound | Nominal Concentration (ng/ml) | Mean Recovery (%) ± SD | Mean matrix effect (%) |
|---|---|---|---|
| PGP (270/173) | 0.01 | 98.63 ± 7.17 | 10 |
| 0.07 | 92.66 ± 2.87 | −12.7 | |
| 0.25 | 100.43 ± 17.23 | −14.16 | |
| 2.5 | 96.62 ± 5.25 | −1.7 | |
| 25 | 100.47 ± 3.09 | 3.6 | |
| 50 | 96.61 ± 8.70 | −6.6 | |
| AcPGP (312/140) | 0.01 | 91.26 ± 17.14 | 27.18 |
| 0.07 | 98.57 ± 5.44 | 5.44 | |
| 0.25 | 104.85 ± 14.53 | −1.85 | |
| 2.5 | 97.59 ± 10.75 | −4.91 | |
| 25 | 101.09 ± 14.52 | 0.97 | |
| 50 | 98.08 ± 2.93 | 3.5 |
The matrix effect (ME) was evaluated by comparing peak response between post-spiked and neat solutions at QCs 0.01, 0.07, 0.25, 2.5, 25, and 50 ng/ml in six replicates for each concentration. As presented in Table 4, ME was <15% for PGP and in the range between −1 to 10%. For AcPGP, ME ranged between −1 to 28% and 27.18% for the lowest concentration (0.01 ng/ml).
3.2.5. Stability
The stability of QCs was evaluated under different conditions (autosampler, long-term storage in the freezer, and freeze-thaw cycle). Autosampler stability of QCs was evaluated for 6 replicates of each concentration. The mean measured concentrations were stable for at least 48 h on the autosampler for PGP and AcPGP (Table 4). Long-term storage stability at −80°C of QCs was evaluated for 7 and 30 days. All the QCs for PGP and AcPGP were >90% stable at −80°C for 30 days (Table 5).
Table 5: Stability (%) of quality control samples after extraction.
| Analyte | Nominal Concentration (ng/ml) | Autosampler stability (%) | −80°C (%) | Freeze thaw (%) | |||
|---|---|---|---|---|---|---|---|
| PGP | 24h | 48h | 7 days | 30 days | 3X | 6X | |
| 0.01 | 116.75 ± 25.35 | 123.77 ± 31.44 | 88.52 ± 14.10 | 100.91 ± 24.44 | 112.13 ± 34.45 | 111.98 ± 31.37 | |
| 0.07 | 112.01 ± 21.79 | 117.91 ± 18.50 | 92.63 ± 10.81 | 106.90 ± 4.90 | 99.32 ± 13.19 | 83.78 ± 18.61 | |
| 0.25 | 106.95 ± 16.52 | 104.28 ± 12.74 | 94.37 ± 7.93 | 96.78 ± 6.80 | 89.64 ± 7.49 | 90.11 ± 9.84 | |
| 2.5 | 114.04 ± 10.84 | 113.20 ± 16.89 | 104.44 ± 8.13 | 95.11 ± 6.50 | 92.29 ± 8.43 | 91.24 ± 5.07 | |
| 25 | 109.34 ± 10.20 | 113.31 ± 14.88 | 100.53 ± 7.85 | 94.15 ± 5.04 | 94.97 ± 8.98 | 91.75 ± 7.77 | |
| 50 | 102.51 ± 12.98 | 101.75 ± 13.03 | 91.93 ± 8.52 | 91.24 ± 8.62 | 90.12 ± 8.75 | 90.96 ± 5.42 | |
| AcPGP | 0.01 | 103.40 30.95 | 106.97 ± 21.40 | 103.02 ± 15.16 | 133.01 ± 32.37 | 130.35 ± 55.52 | 112.23 ± 33.31 |
| 0.07 | 89.90 ± 8.70 | 97.18 ± 7.52 | 106.99 ± 15.16 | 111.56 ± 7.65 | 110.77 ± 12.97 | 94.57 ± 24.05 | |
| 0.25 | 95.87 ± 6.79 | 99.41 ± 2.06 | 112.33 ± 10.97 | 98.10 ± 4.04 | 102.37 ± 18.05 | 93.23 ± 12.51 | |
| 2.5 | 94.93 ± 4.59 | 103.37 ± 3.90 | 114.21 ± 11.35 | 98.36 ± 5.81 | 92.60 ± 3.42 | 96.80 ± 2.79 | |
| 25 | 97.17 ± 2.49 | 103.87 ± 2.86 | 111.92 ± 3.34 | 93.01 ± 3.87 | 90.55 ± 4.33 | 92.05 ± 3.88 | |
| 50 | 94.86 ± 2.47 | 104.33 ± 5.19 | 113.78 ± 2.87 | 93.87 ± 2.42 | 93.62 ± 2.49 | 94.95 ± 2.73 | |
Repeated freeze-thaw, i.e., 3 and 6, times were evaluated for all the QCs. As presented in Table 5, the stability of PGP and AcPGP in QCs were not affected by freeze-thawing up to 6 times.
3.2.6. Application of method in clinical samples
PGP and AcPGP were measured in plasma from study participants (n=10 COPD; n=10 control) as proof of concept and representative chromatograms are presented as supplemental figures 3 and 4. PGP was measured in the range of detection in all but one subject. As presented in Figure 4, the mean value of PGP in COPD subjects is higher compared to healthy controls (p value= 0.1903). We did not detect AcPGP in plasma, even though AcPGP IS was detected in the extracted plasma samples (Supplemental Figure 4).
Figure 4:

Clinical application: Average PGP concentration in healthy controls and COPD subjects (n=10 each group). Whiskers indicate minimum/maximum values, center-line indicates median, n=10 subjects.
4. Discussion
We have successfully developed a sensitive, selective, and robust LC-MS/MS method for extraction and quantification of PGP and AcPGP from human plasma. To the best of our knowledge, this is the first report describing a detailed sample preparation and LC-MRM-MS method for PGP and AcPGP according to the FDA guidelines [20] and this method may be used for their analysis in other human biofluids. PGP and AcPGP are generated by the degradation of collagen by MMPs and PE and can be used as potential biomarkers and therapeutic targets for diseases that involve ECM degradation and remodeling. Previous studies have reported PGP and AcPGP concentrations in various matrices such as plasma, sputum, bronchoalveolar lavage, and serum; however, a detailed validated method on sample preparation and analysis is not currently available [6, 7, 9, 15, 17].
In the present method, we assessed the sensitivity of measuring PGP and AcPGP in water due to concerns that salts present in PBS suppress the ionization of PGP, affecting the peak area as compared to PGP solubilized in water. It is well established that the presence of salt suppresses the ionization of the molecule during mass spectrometry analysis [21]. Indeed, we found that PGP and AcPGP quantification were not affected by the nature of the aqueous solvent. Using water, the RT for PGP is 1.2 min, which occurs immediately after salt elution. Our method showed linearity in the range of 0.01–50 ng/ml in water, PBS, and plasma for PGP and AcPGP (Supplementary Figure 2). The LOQ for PGP is 0.05 ng/ml in water and 0.1 ng/ml in PBS as neat standards. For AcPGP, the LOQ was not affected by the solvents and is 0.1 ng/ml in water and PBS.
The current method of quantification is reliable and reproducible as inter-day bias (%) and intra-day CV (%) were less than 20% for PGP and AcPGP except at the lowest concentration of QC (0.01 ng/ml) (Table 3). These findings suggest that the current method cannot be used to quantify at or below the 0.01 ng/ml concentration threshold of PGP and AcPGP. However, this method accurately quantifies 0.05 ng/ml and higher concentrations of PGP and 0.1 ng/ml and higher for AcPGP.
The matrix effect was <15% and most of the concentrations of QCs showed negative values for PGP and AcPGP indicating that there is no significant suppression in the signal/ionization in presence of the matrix. The lowest concentration of QC (0.01 ng/ml) showed signal suppression by plasma for PGP and AcPGP. With the current method, we achieved >90% recovery for all QCs that confirmed the extraction method is efficient.
Stability data shows that the detection of PGP and AcPGP were not affected for 48 h when they are used for analysis of long runs. Long-term storage and freeze-thaw stability data suggested that the samples are stable up to 30 days and the samples can be freeze-thawed at least 6X for the analysis without any significant degradation. Thus, the current method is suitable for long-term storage and repeated freeze-thaw cycles.
We used this method in determining PGP and AcPGP levels in healthy and COPD patients. LC-MS/MS analysis of extracted plasma samples showed slightly higher PGP in the COPD group compared to healthy controls; however, the difference was not significant. It’s likely our sample size was too small to detect between-group differences and future studies with larger sample sizes should be conducted. AcPGP was not detected in plasma samples of healthy or COPD patients. However, it is well-established that cigarette smoke-induces collagen degradation from prior studies of PGP and AcPGP in sputum and BAL [19, 22].
5. Conclusion
We have developed a rapid, sensitive, selective and reliable LC-MS/MS method for PGP and AcPGP extraction and determination in plasma. Further work is underway to to establish a similar method for other matrices such as BAL, sputum, and urine.
Supplementary Material
Funding
The study was supported by the American Lung Association (ALA-ACRC pilot award); NIH (R01 HL148215, UH3 TR002450). Funds for the mass spectrometer used in these studies came from a UAB Health Service Foundation General Endowment Fund (SB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT Author’s contributions statement
Ekta Tiwary: Conceptualization, planning, methodology, investigation, validation, data curation, visualization, writing original draft, analysis, project administration. Taylor Berryhill: Planning, methodology, analysis, review and editing. Landon Wilson: Planning, methodology, analysis, review and editing. Stephen Barnes: Project administration, Supervision, review and editing, resources. Jeevan Prasain: Supervision, review and editing. James Michael Wells: Funding acquisition, supervision, review and editing, resources, project administration.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
References
- [1].Daley WP, Peters SB, Larsen M, Extracellular matrix dynamics in development and regenerative medicine, J Cell Sci, 121 (2008) 255–264. 10.1242/jcs.006064 [DOI] [PubMed] [Google Scholar]
- [2].Patel DF, Snelgrove RJ, The multifaceted roles of the matrikine Pro-Gly-Pro in pulmonary health and disease, Eur Respir Rev, 27 (2018). 10.1183/16000617.0017-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Silva AC, Pereira C, Fonseca A, Pinto-do OP, Nascimento DS, Bearing My Heart: The Role of Extracellular Matrix on Cardiac Development, Homeostasis, and Injury Response, Front Cell Dev Biol, 8 (2020) 621644. 10.3389/fcell.2020.621644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lu P, Takai K, Weaver VM, Werb Z, Extracellular matrix degradation and remodeling in development and disease, Cold Spring Harb Perspect Biol, 3 (2011). 10.1101/cshperspect.a005058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Najafi M, Farhood B, Mortezaee K, Extracellular matrix (ECM) stiffness and degradation as cancer drivers, Journal of cellular biochemistry, 120 (2019) 2782–2790. 10.1002/jcb.27681 [DOI] [PubMed] [Google Scholar]
- [6].Turnbull AR, Pyle CJ, Patel DF, Jackson PL, Hilliard TN, Regamey N, Tan HL, Brown S, Thursfield R, Short C, Mc Fie M, Alton E, Gaggar A, Blalock JE, Lloyd CM, Bush A, Davies JC, Snelgrove RJ, Abnormal pro-gly-pro pathway and airway neutrophilia in pediatric cystic fibrosis, J Cyst Fibros, 19 (2020) 40–48. 10.1016/j.jcf.2019.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE, A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation, Nat Med, 12 (2006) 317–323. 10.1161/CIRCRESAHA.119.311148 [DOI] [PubMed] [Google Scholar]
- [8].Frangogiannis NG, The Extracellular Matrix in Ischemic and Nonischemic Heart Failure, Circ Res, 125 (2019) 117–146. 10.1161/CIRCRESAHA.119.311148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Payne GA, Li J, Xu X, Jackson P, Qin H, Pollock DM, Wells JM, Oparil S, Leesar M, Patel RP, Blalock JE, Gaggar A, The Matrikine Acetylated Proline-Glycine-Proline Couples Vascular Inflammation and Acute Cardiac Rejection, Sci Rep, 7 (2017) 7563. 10.1038/s41598-017-07610-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ito JT, Lourenço JD, Righetti RF, Tibério IF, Prado CM, Lopes FD, Extracellular matrix component remodeling in respiratory diseases: what has been found in clinical and experimental studies?, Cells, 8 (2019) 342. 10.3390/cells8040342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hahn CS, Scott DW, Xu X, Roda MA, Payne GA, Wells JM, Viera L, Winstead CJ, Bratcher P, Sparidans RW, Redegeld FA, Jackson PL, Folkerts G, Blalock JE, Patel RP, Gaggar A, The matrikine N-alpha-PGP couples extracellular matrix fragmentation to endothelial permeability, Sci Adv, 1 (2015). DOI: 10.1126/sciadv.1500175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gaggar A, Jackson PL, Noerager BD, O’Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE, A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation, J Immunol, 180 (2008) 5662–5669. 10.4049/jimmunol.180.8.5662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Koelink PJ, Overbeek SA, Braber S, Morgan ME, Henricks PA, Abdul Roda M, Verspaget HW, Wolfkamp SC, te Velde AA, Jones CW, Jackson PL, Blalock JE, Sparidans RW, Kruijtzer JA, Garssen J, Folkerts G, Kraneveld AD, Collagen degradation and neutrophilic infiltration: a vicious circle in inflammatory bowel disease, Gut, 63 (2014) 578–587. 10.1136/gutjnl-2012-303252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hardison MT, Galin FS, Calderon CE, Djekic UV, Parker SB, Wille KM, Jackson PL, Oster RA, Young KR, Blalock JE, Gaggar A, The presence of a matrix-derived neutrophil chemoattractant in bronchiolitis obliterans syndrome after lung transplantation, J Immunol, 182 (2009) 4423–4431. 10.4049/jimmunol.0802457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].O’Reilly P, Jackson PL, Noerager B, Parker S, Dransfield M, Gaggar A, Blalock JE, N-alpha-PGP and PGP, potential biomarkers and therapeutic targets for COPD, Respir Res, 10 (2009) 38. 10.1186/1465-9921-10-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wells JM, O’Reilly PJ, Szul T, Sullivan DI, Handley G, Garrett C, McNicholas CM, Roda MA, Miller BE, Tal-Singer R, Gaggar A, Rennard SI, Jackson PL, Blalock JE, An aberrant leukotriene A4 hydrolase-proline-glycine-proline pathway in the pathogenesis of chronic obstructive pulmonary disease, Am J Respir Crit Care Med, 190 (2014) 51–61. 10.1164/rccm.201401-0145OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sharma NS, Lal CV, Li J.-d., Lou X.-y., Viera L, Abdallah T, King RW, Sethi J, Kanagarajah P, Restrepo-Jaramillo R, The neutrophil chemoattractant peptide proline-glycine-proline is associated with acute respiratory distress syndrome, American Journal of Physiology-Lung Cellular and Molecular Physiology, 315 (2018) L653–L661. 10.1152/ajplung.00308.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Andriani F, Landoni E, Mensah M, Facchinetti F, Miceli R, Tagliabue E, Giussani M, Callari M, De Cecco L, Colombo MP, Roz L, Pastorino U, Sozzi G, Diagnostic role of circulating extracellular matrix-related proteins in non-small cell lung cancer, BMC Cancer, 18 (2018) 899. 10.1186/s12885-018-4772-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Roda MA, Xu X, Abdalla TH, Sadik M, Szul T, Bratcher PE, Viera L, Solomon GM, Wells JM, McNicholas CM, Redegeld FA, Folkerts G, Blalock JE, Gaggar A, Proline-Glycine-Proline Peptides Are Critical in the Development of Smoke-induced Emphysema, Am J Respir Cell Mol Biol, 61 (2019) 560–566. 10.1165/rcmb.2018-0216OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].US Food and Drug Administration, Bioanalytical Method Validation Guidance for Industry 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed 10 March 2023) [Google Scholar]
- [21].Annesley TM, Ion suppression in mass spectrometry, Clin Chem, 49 (2003) 1041–1044. 10.1373/49.7.1041 [DOI] [PubMed] [Google Scholar]
- [22].Overbeek SA, Braber S, Koelink PJ, Henricks PA, Mortaz E, LoTam Loi AT, Jackson PL, Garssen J, Wagenaar GT, Timens W, Koenderman L, Blalock JE, Kraneveld AD, Folkerts G, Cigarette smoke-induced collagen destruction; key to chronic neutrophilic airway inflammation?, PLoS One, 8 (2013) e55612. 10.1371/journal.pone.0055612 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


