The Case
A 43-year-old woman with asthma presented with chest tightness and dyspnea despite albuterol use. She was alert, normotensive, and tachypneic. Arterial blood gas measurement with the patient breathing air demonstrated a pH of 7.32; a carbon dioxide tension/partial pressure measurement, PCO2, of 35 mm Hg; an oxygen tension/partial pressure measurement, Po2, of 76 mm Hg; HCO3− at 18 mEq/L; and lactate at 2.6 mmol/L. After continuous nebulized albuterol and 3 L crystalloid, her serum lactate increased to 6.3 mmol/L. A point-of-care ultrasound demonstrated normal cardiac function, and she had normal urine output.
The Synopsis
Lactate accumulates because of an imbalance between production and clearance. Although hyperlactatemia in critically ill patients often results from tissue hypoxia caused by reduced oxygen delivery, there are other potential causes. Alternative mechanisms should be considered in patients who demonstrate adequate organ perfusion.
How Is Lactate Produced and Cleared?
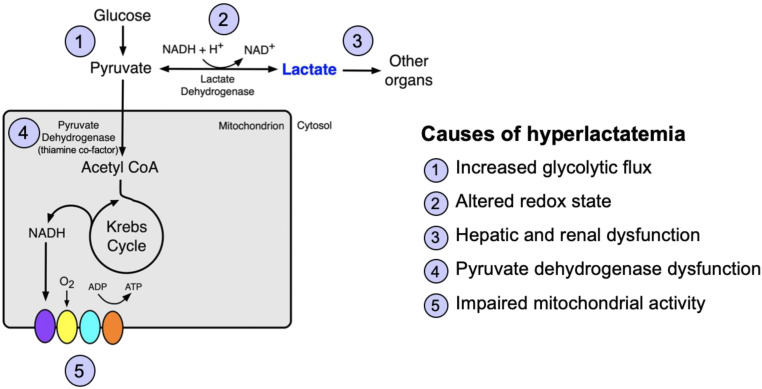
The metabolic pathways for lactate and the causes of hyperlactatemia are shown in Figure 1.
Figure 1.
Lactate metabolism and causes of hyperlactatemia. Glycolysis yields pyruvate, which is reduced to lactate by lactate dehydrogenase. Lactate is either converted back to pyruvate or cleared by other organ systems, including the liver and the kidneys. Alternatively, pyruvate is converted to acetyl CoA by pyruvate dehydrogenase, which requires the cofactor thiamine and enters the Krebs cycle. Alterations at any point along these pathways can result in hyperlactatemia. ADP = adenosine diphosphate; ATP = adenosine triphosphate; CoA = coenzyme A; NAD+ = nicotinamide adenine dinucleotide; NADH + H+ = nicotinamide adenine dinucleotide plus hydrogen.
What Accounts for Elevations in Serum Lactate Concentration?
Alterations in the steps in Figure 1 can result in hyperlactatemia:
-
1.
Increased glycolytic flux: Pyruvate production is increased by endogenous or exogenous catecholamines (e.g., epinephrine) or excess β-adrenergic agonist administration by means of the β2 receptor and cyclic AMP–mediated activation of Na+/K+-ATPase pump activity increasing the conversion of glucose to pyruvate (1).
-
2.
Altered redox state: An increased NADH/NAD+ ratio favors the conversion of pyruvate to lactate, as seen with severe alcohol intoxication (2).
-
3.
Impaired lactate clearance: Lactate is cleared predominantly by the liver (60%) and the kidneys (30%). Dysfunction of either organ can cause hyperlactatemia (2).
-
4.
Pyruvate dehydrogenase (PDH) dysfunction: Because PDH requires the cofactor thiamine, thiamine deficiency from inadequate intake or malabsorption impairs the conversion of pyruvate to acetyl coenzyme A (CoA).
-
5.
Mitochondrial dysfunction: In addition to impaired mitochondrial oxygen supply (e.g., shock), certain medications (e.g., nucleoside reverse transcriptase inhibitors) or toxins (e.g., cyanide) impair mitochondrial function, slowing the conversion of pyruvate to acetyl CoA and favoring conversion to lactate (3).
Why Does Acidosis Occur with Only Some Causes of Hyperlactatemia?
Hyperlactatemia is not always accompanied by metabolic acidosis, as lactate production itself does not cause acidosis; protons produced when glucose is converted to pyruvate are consumed when pyruvate is reduced to lactate. Acidosis results from mitochondrial dysfunction. Adenosine triphosphate (ATP) hydrolysis elaborates protons in the cytosol and releases energy used in oxidative phosphorylation to create the electrochemical proton gradient necessary for ATP synthesis. With impaired mitochondrial activity, ATP hydrolysis produces protons that cannot be scavenged by mitochondria or buffered, thereby increasing the hydrogen ion concentration and causing metabolic acidosis (3, 4).
Hyperlactatemia is commonly seen in hospitalized patients. Increased glycolytic flux caused by continuous β-adrenergic agonist administration was the source of hyperlactatemia in this patient with asthma. When the lactate concentration is elevated in patients without shock, consideration of the metabolic pathways that affect lactate concentration, and the presence or absence of acidosis, can help to identify the cause of hyperlactatemia.
References
- 1. Levy B, Desebbe O, Montemont C, Gibot S. Increased aerobic glycolysis through beta2 stimulation is a common mechanism involved in lactate formation during shock states. Shock . 2008;30:417–421. doi: 10.1097/SHK.0b013e318167378f. [DOI] [PubMed] [Google Scholar]
- 2. Rabinowitz JD, Enerbäck S. Lactate: the ugly duckling of energy metabolism. Nature metabolism . 2020;2:566–571. doi: 10.1038/s42255-020-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adeva-Andany M, López-Ojén M, Funcasta-Calderón R, Ameneiros-Rodríguez E, Donapetry-García C, Vila-Altesor M, et al. Comprehensive review on lactate metabolism in human health. Mitochondrion . 2014;17:76–100. doi: 10.1016/j.mito.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 4. Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol . 2004;287:R502–R516. doi: 10.1152/ajpregu.00114.2004. [DOI] [PubMed] [Google Scholar]