Abstract
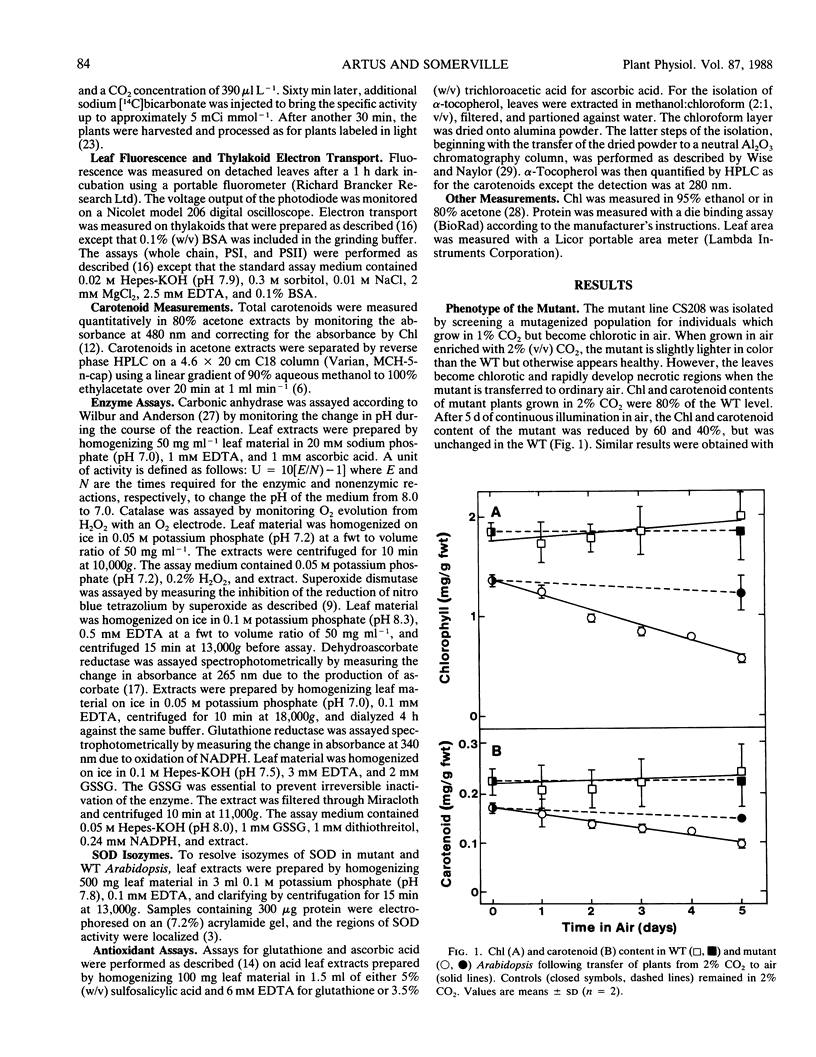
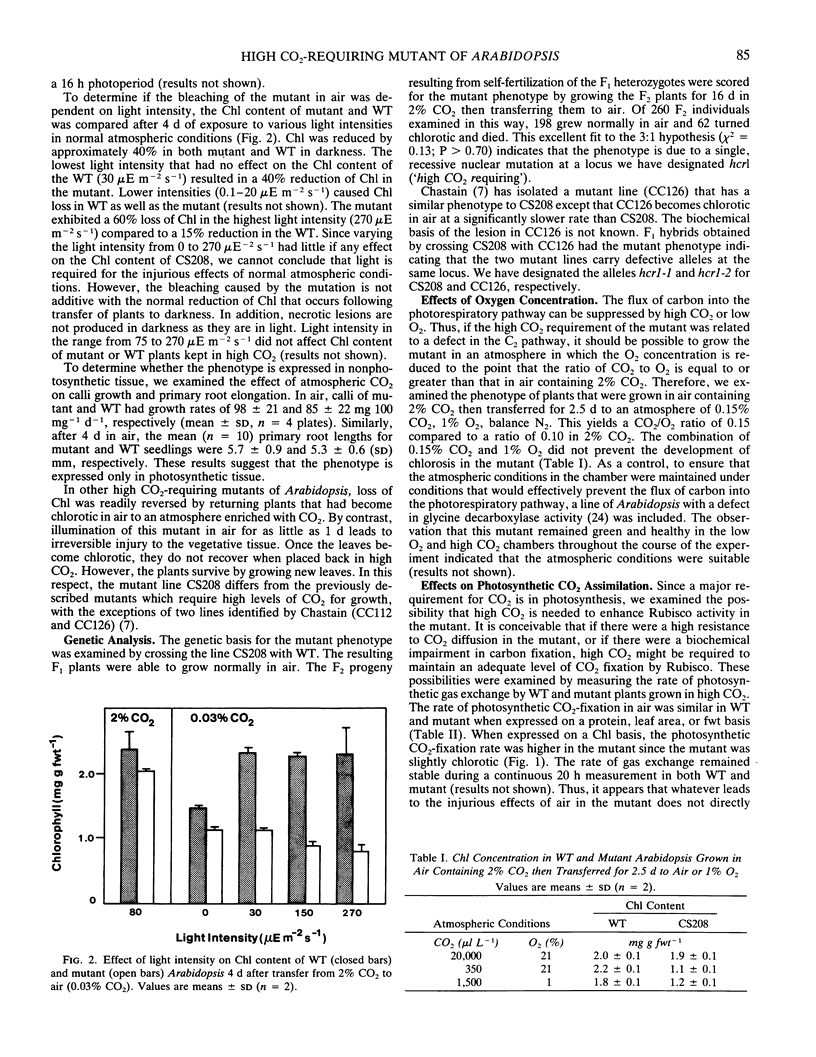
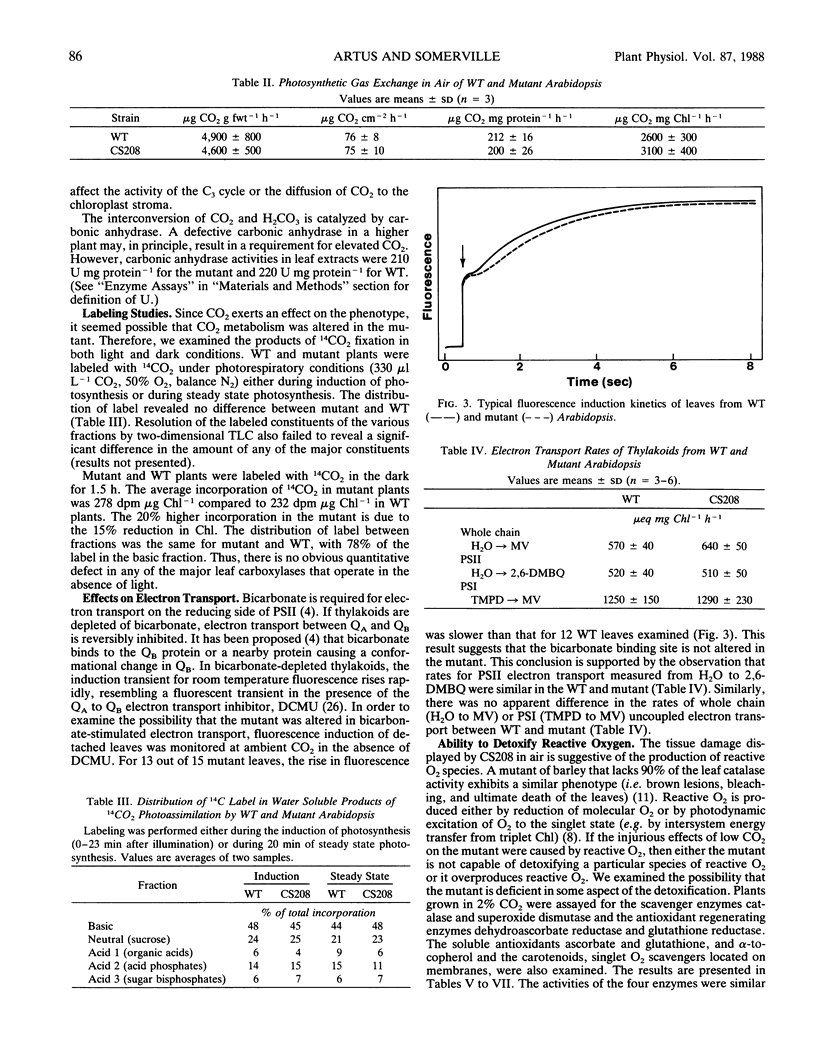
A mutant of Arabidopsis thaliana (L.) Heynh. which requires a high concentration (2% by volume) of atmospheric CO2 for growth has been isolated. Unlike previous mutants of this type, this line does not have any apparent defect in photosynthetic CO2-fixation, photorespiration, or photosynthetic electron transport. The mutant is abnormally susceptible to pigment bleaching in air but not in 2% CO2. The presence of normal or above-normal levels of antioxidants, carotenoids, and enzymes involved in reactive oxygen detoxification suggests that the mutant is equipped to detoxify activated oxygen species. Although it was not possible to establish a biochemical basis for the lesion, the properties of the mutant suggest the existence of a previously unidentified role for CO2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Blubaugh D. J., Govindjee Bicarbonate, not CO2, is the species required for the stimulation of Photosystem II electron transport. Biochim Biophys Acta. 1986 Jan 28;848(1):147–151. doi: 10.1016/0005-2728(86)90170-2. [DOI] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannopolitis C. N., Ries S. K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977 Feb;59(2):309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J. T., Allen R. L. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun. 1965 Dec 21;21(6):523–530. doi: 10.1016/0006-291x(65)90516-4. [DOI] [PubMed] [Google Scholar]
- Law M. Y., Charles S. A., Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem J. 1983 Mar 15;210(3):899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCourt P., Kunst L., Browse J., Somerville C. R. The effects of reduced amounts of lipid unsaturation on chloroplast ultrastructure and photosynthesis in a mutant of Arabidopsis. Plant Physiol. 1987 Jun;84(2):353–360. doi: 10.1104/pp.84.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C. R., Ogren W. L. Mutants of the cruciferous plant Arabidopsis thaliana lacking glycine decarboxylase activity. Biochem J. 1982 Feb 15;202(2):373–380. doi: 10.1042/bj2020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C. R., Portis A. R., Ogren W. L. A Mutant of Arabidopsis thaliana Which Lacks Activation of RuBP Carboxylase In Vivo. Plant Physiol. 1982 Aug;70(2):381–387. doi: 10.1104/pp.70.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Wise R. R., Naylor A. W. Chilling-enhanced photooxidation : evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol. 1987 Feb;83(2):278–282. doi: 10.1104/pp.83.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]


