Abstract
Omalizumab is effective in chronic spontaneous urticaria unresponsive to antihistamines. Of the licensed dosing schedules, Korean patients prefer a low dose, of 150 mg/month, for financial reasons. However, real-world experiences of low-dose omalizumab consumption have not been reported. The aim of this retrospective study was to assess the treatment outcomes and long-term clinical course of patients with chronic spontaneous urticaria who were treated with low-dose omalizumab. The study included 179 patients aged ≥ 20 years who were treated with omalizumab 150 mg/month for ≥ 12 weeks. Baseline disease activity was mild, moderate, and severe in 54.7%, 35.2%, and 10.1% of patients, respectively. A complete response was observed in 133 patients at 12 weeks, among whom 88 patients showed early responses within 4 weeks. Overall, 158 patients finally achieved a complete response. Multivariate analyses revealed that baseline disease activity is more likely to be mild in patients who experience early and final complete responses. The absence of atopic comorbidities correlated with an early response. Smoking was associated with a final complete response. This study shows that low-dose omalizumab provides favourable treatment outcomes in antihistamine-refractory chronic spontaneous urticaria. Disease severity, atopic comorbidity, and smoking may be predictive factors for studying the response to omalizumab.
SIGNIFICANCE
This retrospective study identified treatment outcomes of low-dose omalizumab in adult patients with chronic spontaneous urticaria in South Korea. A rapid and affirmative response was obtained with low-dose omalizumab. Mild disease activity at baseline, absence of comorbid atopic disease, and current smoking may be predictors of a better response to omalizumab. This study provides important information on the clinical course of patients both during omalizumab treatment and after discontinuation of treatment due to clinical remission and provides evidence for the prescription of low-dose omalizumab for chronic spontaneous urticaria.
Key words: chronic urticaria, comorbidities, omalizumab, severity, smoking
Chronic spontaneous urticaria (CSU) is defined as recurrent pruritic wheals or angioedema lasting > 6 weeks without apparent triggers. Prevalence of CSU in the adult population is estimated to be 0.5–5% (1). Current guidelines recommend a stepwise approach that aims for complete symptom control (2). However, even if the standard dose of antihistamine used in the first step is increased 4-fold, or other antihistamines are added, 54% of patients still have insufficient responses and experience a significantly diminished quality of life (3).
Omalizumab is a humanized anti-immunoglobulin E (IgE) antibody that reduces the level of serum free IgE and the function of the high-affinity receptor for the Fc region of IgE (FcεRI) in basophils and mast cells (4). The efficacy and safety of omalizumab in treating CSU have been demonstrated in 3 pivotal phase III studies (ASTERIA I, ASTERIA II, and GLACIAL) that showed a dose-dependent response pattern (5–7).
Based on these data, Europe approved only the CSU 300 mg dose, which was efficacious even in adolescents or patients weighing > 80 kg without additional safety risks, whereas the USA approved the 150 and 300 mg doses because of the overlapping response ranges between them. The Asian population improved significantly with omalizumab 300 mg compared with 150 mg (8). However, in a meta-analysis of the Urticaria Activity Score 7 (UAS7), the difference in improvement between 2 doses was smaller than that observed in the Western population (9). Accordingly, the licensed dose of omalizumab is 300 mg in the EU and either 150 or 300 mg in the USA and Korea, and is administered through subcutaneous injection every 4 weeks (10).
In a clinical setting, most patients in Korea prefer to receive 150 mg/month of omalizumab, because the cost of omalizumab for treating CSU is not covered by the national healthcare system. However, current real-world data on the response to omalizumab are available only for Western countries and a dose of 300 mg/month (11–13). Although a Spanish working group recommended the use of doses < 300 mg/month during disease control, studies examining the long-term treatment response to low-dose omalizumab (150 mg/month) and clinical course after treatment discontinuation are lacking (14, 15). Furthermore, in retrospective cohort studies using omalizumab 300 mg/month, baseline total IgE level, disease duration, and angioedema were predictive of treatment response to omalizumab, whereas predictors of omalizumab 150 mg/month remain largely unknown (13, 16, 17).
The aims of this study are to investigate the long-term treatment response to low-dose omalizumab and the clinical course after treatment discontinuation in patients with refractory CSU. A further aim is to assess the clinical factors related to the treatment response to low-dose omalizumab in a real-world clinical setting.
MATERIALS AND METHODS
Subjects and data collection
A single-centre retrospective review of the electronic medical records of patients with CSU who were administered omalizumab at the Department of Dermatology and Division of Allergy and Clinical Immunology, Department of Internal Medicine, Seoul National University Bundang Hospital (Seongnam, Korea) between January 2014 and February 2021, was performed. The study was approved by the institutional review board of Seoul National University Bundang Hospital (IRB No. B-2105-685-102) and was performed in accordance with the Declaration of Helsinki. Informed consent was waived because of the retrospective nature of the study.
The inclusion criteria were: (i) patients aged ≥ 20 years and with symptomatic CSU despite increase of the non-sedating H1-antihistamine dose to up to 4 times the approved dose or combination with other antihistamines; and (ii) patients receiving omalizumab at a starting dose of 150 mg/month for ≥ 12 weeks. Patients who had chronic inducible urticaria without CSU were excluded.
The study followed CSU management guidelines by adding omalizumab to H1-antihistamines. If the effect was insufficient or the symptoms worsened, rescue medications that were reimbursable in Korea, such as antihistamines, cyclosporine, or oral corticosteroids, were administered. Since antihistamines in excess of the standard dose are not reimbursable in Korea, a combination of different H1-antihistamines were conditionally used for patients with CSU that was refractory to the standard dose.
Patient demographics, clinical information, and laboratory results were also collected. Smoking status was assessed before the start of omalizumab, and the participants were divided into non-smokers (never smoked or had not smoked in the last 28 days or more) or current smokers. Thyroid stimulating hormone (TSH) abnormalities were regarded as levels outside the normal range (0.3–4.0 mIU/L). Owing to reimbursement eligibility, the anti-thyroid peroxidase (TPO) antibody test (normal range < 60 U/mL) was conducted only in cases of thyroid hormone or TSH abnormalities.
Assessment of treatment response
Baseline disease activity of CSU was evaluated according to UAS7 and categorized as follows: 0–6, well controlled; 7–15, mild; 16–27, moderate; 28–42, severe (18, 19). The treatment response to omalizumab was measured in accordance with the modified physician’s global assessment, which can be applied more quickly and conveniently in daily practice. The evaluation was as described previously (20): complete or almost complete response, ≥ 90% reduction of symptoms; partial response, reduction in symptoms of 30–89%; and no response or limited response, reduction in symptoms of < 30%.
Patients with complete or almost complete response were defined as responders. In addition, depending on the timing of the response to omalizumab, treatment response was classified as early when noted within the first month of treatment, and late when noted after 3 months, as proposed previously (21). Therefore, the patients in this study were subdivided into 5 groups according to their response to omalizumab at 12 weeks: early responders (complete response at 4 weeks), responders (complete response at 12 weeks), partial responders (partial response at 12 weeks), no responders (no response at 12 weeks), and final responders (complete response before the end of the omalizumab treatment period) (13, 22).
The half-life of omalizumab is 24–26 days and the washout period of a drug is generally known to be 4–5 times its half-life. Therefore, discontinuation of omalizumab treatment was defined as an injection interval of ≥ 16 weeks (23, 24).
Statistical analysis
Descriptive statistics, including means (standard deviation; SD) for continuous variables and relative frequencies (%) for categorical variables, are reported. The χ2 test, independent t-test, or Mann–Whitney U test was used to compare variables between subgroups. A linear regression model was used to identify the clinical factors associated with an early complete response. Cox proportional hazard analysis was performed to identify the clinical factors associated with a final complete response. Those factors in the univariate analysis with p < 0.1 were entered into multivariable analysis. The rates of complete response to omalizumab were calculated using the Kaplan–Meier method and displayed as cumulative incidence curves for overall complete response. Data were constructed and analysed using R software (version 4.1.0). Statistical significance was assumed at p < 0.05. Data are reported as mean ± SD unless otherwise stated.
RESULTS
Patient demographics, clinical data, and laboratory results
A total of 179 patients (age 44.83 ± 15.40 years; 55.9% female) were included in this study (Table SI). The duration of patients having CSU before starting omalizumab treatment was 41.31 ± 53.85 months. At baseline, UAS7 was 15.64 ± 6.68, and disease activity, as categorized according to the UAS7, was mild (54.7%), moderate (35.2%), or severe (10.1%). The most common comorbidities were atopic comorbidities (31.8%), including atopic dermatitis (5.6%), asthma (11.2%), allergic rhinitis (19.0%), and food allergy (5.6%), followed by hypertension (14.5%), and diabetes (8.9%). Of these, 17.3% of patients had concomitant angioedema, 21.2% had dermographism, and 4.5% had cholinergic urticaria.
For patients whose laboratory data were available, the total IgE at baseline was 387.58 ± 762.38 kUA/L, 32.7% were anti-nuclear antibody (ANA)-positive, and 15.7% had TSH abnormalities. Eight and 13 patients had high and low TSH levels, respectively. Five of 12 patients tested positive for anti-TPO antibodies. Regarding previous treatment, 47.5% of patients typically received concurrent treatment with ≥ 3 antihistamines. The proportion of patients who received cyclosporine or low-dose corticosteroids for > 3 months was 14.5% and 14%, respectively.
Treatment response during low-dose omalizumab and clinical course after treatment discontinuation
The treatment data for patients receiving 150 mg/month of omalizumab are shown in Table SII. During the follow-up period of 22.18 ± 21.10 months, the duration of treatment with omalizumab was 15.44 months (range 12–604 weeks), and the total number of injections was 13.96 (range 3–116). In clinical practice, rescue medications during omalizumab treatment were allowed for disease control, and 83.2%, 7.3% and 26.3% of the patients simultaneously used antihistamines, cyclosporine and oral corticosteroids, respectively, for a short period (< 1 month).
Regarding the treatment response of 179 patients at 12 weeks, 133 patients (74.3%) achieved complete remission of their symptoms, 88 of whom (66.2%) were early responders. Of the partial or no responders at 12 weeks, 25 patients additionally achieved complete remission after 12 weeks (late responders). Overall, 158 patients (88.3%) achieved a complete response by the last injection, and 21 patients had either no or partial improvement. Among the 158 final responders, most (81.6%) maintained treatment with 150 mg/month omalizumab throughout the treatment period to sustain symptomatic remission, while 1 patient temporarily reduced the injection interval, and 28 patients were changed to 300 mg/month omalizumab owing to symptomatic deterioration. After achieving clinical remission, 10 patients were down-titrated to the original dose and maintained complete responses, whereas 18 patients maintained a high dose until the final injection, to prevent relapse.
Sixty-two patients maintained omalizumab treatment until the final follow-up, 38 patients were lost to follow-up, and 79 patients discontinued omalizumab treatment during the follow-up period. The most common reason for treatment discontinuation was complete remission (54.4%), followed by insufficient response (24.1%), adverse effects (10.1%), pregnancy (6.1%), and financial burden (5.1%). Among 43 patients who discontinued omalizumab due to complete remission, 11 patients had no recurrence during a follow-up period of 18.31 ± 15.12 weeks, whereas 32 patients relapsed after 23.94 ± 23.13 weeks. Eight of the relapsed patients received retreatment with omalizumab for 33.73 ± 15.87 weeks, among whom 6 patients obtained complete remission at 11.14 ± 6.75 weeks.
Clinical factors associated with treatment response to low-dose omalizumab
The clinical factors that were significantly associated with an early complete response were baseline disease activity and atopic comorbidities, whereas those associated with a final complete response by the final injection were baseline activity and smoking (Tables SIII and SIV). In patients with moderate baseline activity, the proportion of early and final complete responses decreased by 50% and 64.1%, respectively, compared with those with mild activity (p = 0.042 and 0.045, respectively). Compared with patients without atopic comorbidities, the early complete response was reduced by 35.4% and the final complete response by 67.7% in those with atopic comorbidities (p = 0.003 and 0.065, respectively). Specifically, atopic dermatitis and allergic rhinitis were associated with an early complete response (p = 0.034 and 0.032, respectively, data not shown). It is notable that current smokers had a 1.57-fold increased likelihood of a final complete response compared with non-smokers (p = 0.039).
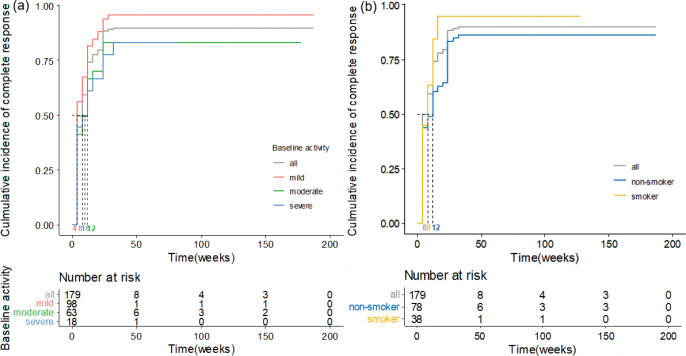
The cumulative incidence of the final complete response in patients subdivided either by baseline disease activity or smoking is shown in Fig. 1. The Kaplan–Meier curves demonstrate that patients with mild baseline activity are more likely to achieve a final complete response (median 4 weeks) than those with moderate or severe activity (median 12 and 10 weeks, respectively). Smokers also were significantly more likely to reach a final complete response and had a shorter median time to reach a final complete response (median 8 weeks) than non-smokers (median 12 weeks).
Fig. 1.
Cumulative incidence curves for final complete response until the end of the treatment period in the patient groups categorized by either (a) baseline disease activity or (b) smoking.
DISCUSSION
This retrospective study reflects the Korean real-world experience of low-dose omalizumab in the management of recalcitrant CSU and confirms that this dosing schedule provides rapid and effective relief from CSU symptoms. The majority (88.3%) of patients in the study population experienced complete response during the treatment period; 49.2% within the first 4 weeks and 74.3% within 12 weeks after omalizumab administration.
A previous real-world retrospective study in 470 Italian patients treated with 300 mg/month omalizumab reported early complete response (UAS7 of 0) in 44.9% of patients within 1 week of commencing treatment and late complete response in 70.2% within 12 weeks (13). Similarly, a multicentre study involving 235 Belgian patients revealed that most patients (93.6%) were treated with omalizumab 300 mg/month, and the disease activity was well-controlled (UAS7 ≤ 6) in 67.2% of patients during the observation period (25). In particular, 42.6% of patients experienced urticaria-free state (UAS7 of 0) after 4 months. A post-marketing surveillance study in Japan reported that most patients (97.9%) were treated with omalizumab 300 mg/month; of whom 68.7% and 54.2% achieved a UAS7 ≤ 6 or UAS7 = 0 by 12 weeks, respectively (19).
Despite the heterogeneous definition of outcome measurements, the current study has similar outcomes to those of previous studies that involved 300 mg/month omalizumab (13, 19, 25). This is probably because all or more than half of the patients included in prior studies had UAS7 ≥ 16 at baseline due to the criteria of funding programmes or national reimbursement. In the current study, more than half of the patients had mild CSU at baseline. In Korea, where reimbursement for omalizumab treatment is not available, a high baseline UAS7 is not mandatory for treatment initiation. When disease control is insufficient (UAS7 > 6) despite up-dosing or combination of antihistamines, a considerable number of patients with mildly severe CSU choose omalizumab treatment, if affordable. The differences in national background, healthcare system, and clinical practice, as well as race, might have affected the response rates to omalizumab.
Autoimmunity is one of the most common causes of CSU (26). For type I autoimmunity, serum IgE targeting autoantigens, such as TPO and dsDNA, bind to FcεRI on mast cells and basophils, resulting in the release of histamine. Whereas, in type IIb autoimmunity, IgG autoantibodies targeting IgE and FcεRI are responsible for the activation of mast cells. By binding to free IgE, omalizumab prevents the degranulation of mast cells and basophils, which explains the rapid (≤ 1 week) response to treatment. Thereafter, omalizumab reduces FcεRI expression on effector cells, making patients with type IIb autoimmunity less susceptible to activation by IgG autoantibodies (27).
To date, many studies have reported the predictors of a favourable response to omalizumab as follows: high total IgE, negative autologous serum skin test, and short disease duration (13, 16, 24, 26). These predictors generally show the features of IgE-mediated autoimmunity. However, in the current study, total IgE level was not associated either with an early or final response to omalizumab. In fact, recent studies have reported an overlap between the 2 types of autoimmune responses (28, 29). This means that not all patients can be strictly grouped into either to IgE- or IgG-mediated autoimmunity (30). This finding suggests that the presence of overlapping immune responses renders the response to omalizumab reliant on the balance between the 2 types. Also, the current study found that patients showing early or final complete response had lower disease severity, in accordance with prior studies reporting that baseline disease activity is significantly higher in non-responders or partial responders than in complete responders (12, 17). Patients with severe baseline disease activity also had slightly poorer therapeutic outcomes than those with mild baseline disease activity, but of statistical insignificance. This may be attributed to the small proportion (10.1%) of patients with severe CSU in this study.
Comorbid atopic diseases were negatively associated with the rapid response to omalizumab. This suggests that the pathogenesis of CSU and the mechanism of omalizumab action are otherwise complex, and autoimmunity does not necessarily play a decisive role in pathogenicity (31). The aetiologies of atopic diseases and CSU involve not only IgE-mediated hypersensitivity, but also innate and adaptive immunity (32, 33). An abnormal immune milieu in patients with atopic comorbidities might have compromised the response to omalizumab by creating a positive feedback loop between allergen-specific T helper type 2 cells and mast cells, and amplifying the immune response (32, 33). There are a few data on the association between atopy and poor therapeutic response in patients with CSU, and a higher dosage of omalizumab is recommended for patients with CSU and comorbid atopic diseases (31, 34). In the current study, early response was evaluated at 4 weeks due to the nature of the visit interval. Therefore, further studies are needed to ascertain whether comparable results are obtained at 1 week, when differences in the rapidity of the response are more clearly identified.
Although tobacco exposure can induce contact urticaria, an immediate hypersensitivity reaction, in the current study smoking was a predictor of a final complete response to omalizumab (35). A previous study involving 117 Danish patients was in line with the current results, in that smokers showed greater improvement in UAS7 from baseline to 3 months after omalizumab initiation compared with non-smokers (18). In an in vitro study, cigarette smoke suppressed IgE-mediated degranulation and cytokine release by mouse mast cells (36). Furthermore, Linneberg et al. reported that sustained smoking was negatively associated with allergic sensitization to aeroallergens despite elevated IgE levels (37, 38). Smoking may suppress the immune responses associated with CSU pathogenesis, making smokers more responsive to omalizumab treatment (33, 39, 40). Further studies are warranted to elucidate the causal direction of the associations reported in the current study and the mechanisms by which smoking exerts a suppressive effect on the immune system.
This study holds a key limitation. Data are retrospective and retrieved from a single centre. Therefore, the results may not represent the wider Asian population of patients with CSU because of variations in health plan coverage policies for omalizumab, provider prescribing patterns, and other subjective issues that may influence treatment outcomes.
To the best of our knowledge, this is the largest cohort study to evaluate treatment outcomes following the omalizumab 150 mg/month dosing schedule. The results suggest that low-dose omalizumab may effectively treat CSU phenotype, particularly in patients with mild baseline disease activity, with no atopic comorbidities, or who are current smokers. This could reduce costs and possible adverse effects, even in countries in which the 300 mg/month dose is reimbursable. To achieve improved therapeutic development for CSU and better predict the response to treatments, further studies that explore the complex patho-mechanism of CSU and employ a prospective design to evaluate treatment responses are needed.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the staff of the Medical Research Collaborating Center at the Seoul National University Bundang Hospital for performing the statistical analyses.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Kim YS, Park SH, Han K, Bang CH, Lee JH, Park YM. Prevalence and incidence of chronic spontaneous urticaria in the entire Korean adult population. Br J Dermatol 2018; 178: 976–977. [DOI] [PubMed] [Google Scholar]
- 2.Zuberbier T, Abdul Latiff AH, Abuzakouk M, Aquilina S, Asero R, Baker D, et al. The international EAACI/GA(2)LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022; 77: 734–766. [DOI] [PubMed] [Google Scholar]
- 3.van den Elzen MT, van Os-Medendorp H, van den Brink I, van den Hurk K, Kouznetsova OI, Lokin A, et al. Effectiveness and safety of antihistamines up to fourfold or higher in treatment of chronic spontaneous urticaria. Clin Transl Allergy 2017; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. J Allergy Clin Immunol 2004; 114: 527–530. [DOI] [PubMed] [Google Scholar]
- 5.Maurer M, Rosen K, Hsieh HJ, Saini S, Grattan C, Gimenez-Arnau A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med 2013; 368: 924–935. [DOI] [PubMed] [Google Scholar]
- 6.Saini SS, Bindslev-Jensen C, Maurer M, Grob JJ, Bulbul Baskan E, Bradley MS, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo-controlled study. J Invest Dermatol 2015; 135: 925. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan A, Ledford D, Ashby M, Canvin J, Zazzali JL, Conner E, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol 2013; 132: 101–109. [DOI] [PubMed] [Google Scholar]
- 8.Hide M, Park HS, Igarashi A, Ye YM, Kim TB, Yagami A, et al. Efficacy and safety of omalizumab in Japanese and Korean patients with refractory chronic spontaneous urticaria. J Dermatol Sci 2017; 87: 70–78. [DOI] [PubMed] [Google Scholar]
- 9.Choi JH, Lee DH, Song WJ, Choi M, Kwon JW, Kim GW, et al. The KAAACI/KDA Evidence-Based Practice Guidelines for chronic spontaneous urticaria in Korean adults and children: part 2. management of H1-antihistamine-refractory chronic urticaria. Allergy Asthma Immunol Res 2020; 12: 750–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metz M, Vadasz Z, Kocaturk E, Gimenez-Arnau AM. Omalizumab updosing in chronic spontaneous urticaria: an overview of real-world evidence. Clin Rev Allergy Immunol 2020; 59: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salman A, Ergun T, Gimenez-Arnau AM. Real-life data on the effectiveness and safety of omalizumab in monotherapy or combined for chronic spontaneous urticaria: a retrospective cohort study. J Dermatolog Treat 2020; 31: 204–209. [DOI] [PubMed] [Google Scholar]
- 12.Vadasz Z, Tal Y, Rotem M, Shichter-Confino V, Mahlab-Guri K, Graif Y, et al. Omalizumab for severe chronic spontaneous urticaria: real-life experiences of 280 patients. J Allergy Clin Immunol Pract 2017; 5: 1743–1745. [DOI] [PubMed] [Google Scholar]
- 13.Marzano AV, Genovese G, Casazza G, Fierro MT, Dapavo P, Crimi N, et al. Predictors of response to omalizumab and relapse in chronic spontaneous urticaria: a study of 470 patients. J Eur Acad Dermatol Venereol 2019; 33: 918–924. [DOI] [PubMed] [Google Scholar]
- 14.Berry R, Hollingsworth P, Lucas M. Successful treatment of idiopathic mast cell activation syndrome with low-dose Omalizumab. Clin Transl Immunol 2019; 8: e01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spertino J, Curto Barredo L, Rozas Munoz E, Figueras Nart I, Gimenez Arnau A, Serra Baldrich E, et al. Algorithm for treatment of chronic spontaneous urticaria with omalizumab. Actas Dermosifiliogr (Engl Ed) 2018; 109: 771–776. [DOI] [PubMed] [Google Scholar]
- 16.Gimenez Arnau AM, Valero Santiago A, Bartra Tomas J, Jauregui Presa I, Labrador Horrillo M, Miquel Miquel FJ, et al. Therapeutic strategy according to differences in response to omalizumab in patients with chronic spontaneous urticaria. J Investig Allergol Clin Immunol 2019; 29: 338–348. [DOI] [PubMed] [Google Scholar]
- 17.Ertas R, Ozyurt K, Atasoy M, Hawro T, Maurer M. The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy 2018; 73: 705–712. [DOI] [PubMed] [Google Scholar]
- 18.Ghazanfar MN, Holm JG, Thomsen SF. Effectiveness of omalizumab in chronic spontaneous urticaria assessed with patient-reported outcomes: a prospective study. J Eur Acad Dermatol Venereol 2018; 32: 1761–1767. [DOI] [PubMed] [Google Scholar]
- 19.Hide M, Fukunaga A, Suzuki T, Nakamura N, Kimura M, Sasajima T, et al. Real-world safety and effectiveness of omalizumab in Japanese patients with chronic spontaneous urticaria: a post-marketing surveillance study. Allergol Int 2023; 72: 286–296. [DOI] [PubMed] [Google Scholar]
- 20.Ghazanfar MN, Sand C, Thomsen SF. Effectiveness and safety of omalizumab in chronic spontaneous or inducible urticaria: evaluation of 154 patients. Br J Dermatol 2016; 175: 404–406. [DOI] [PubMed] [Google Scholar]
- 21.Syrigos N, Grapsa D, Zande M, Tziotou M, Syrigou E. Treatment response to omalizumab in patients with refractory chronic spontaneous urticaria. Int J Dermatol 2018; 57: 417–422. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan A, Ferrer M, Bernstein JA, Antonova E, Trzaskoma B, Raimundo K, et al. Timing and duration of omalizumab response in patients with chronic idiopathic/spontaneous urticaria. J Allergy Clin Immunol 2016; 137: 474–481. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman PL, Umetsu DT, Carrigan GJ, Rahmaoui A. Anaphylactic reactions associated with omalizumab administration: analysis of a case-control study. J Allergy Clin Immunol 2016; 138: 913–915.e2. [DOI] [PubMed] [Google Scholar]
- 24.Baker DL, Nakamura GR, Lowman HB, Fischer SK. Evaluation of IgE antibodies to omalizumab (Xolair(R)) and their potential correlation to anaphylaxis. AAPS J 2016; 18: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lapeere H, Baeck M, Stockman A, Sabato V, Grosber M, Moutschen M, et al. A retrospective analysis omalizumab treatment patterns in patients with chronic spontaneous urticaria: a real-world study in Belgium. J Eur Acad Dermatol Venereol 2020; 34: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurer M, Eyerich K, Eyerich S, Ferrer M, Gutermuth J, Hartmann K, et al. Urticaria: Collegium Internationale Allergologicum (CIA) Update 2020. Int Arch Allergy Immunol 2020; 181: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kulthanan K, Tuchinda P, Likitwattananurak C, Weerasubpong P, Chularojanamontri L. Does omalizumab modify a course of recalcitrant chronic spontaneous urticaria? A retrospective study in Asian patients. J Dermatol 2018; 45: 17–23. [DOI] [PubMed] [Google Scholar]
- 28.Altrichter S, Peter HJ, Pisarevskaja D, Metz M, Martus P, Maurer M. IgE mediated autoallergy against thyroid peroxidase – a novel pathomechanism of chronic spontaneous urticaria? PLoS One 2011; 6: e14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asero R, Marzano AV, Ferrucci S, Lorini M, Carbonelli V, Cugno M. Co-occurrence of IgE and IgG autoantibodies in patients with chronic spontaneous urticaria. Clin Exp Immunol 2020; 200: 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolkhir P, Munoz M, Asero R, Ferrer M, Kocaturk E, Metz M, et al. Autoimmune chronic spontaneous urticaria. J Allergy Clin Immunol 2022; 149: 1819–1831. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Z, Cai T, Chen H, Chen L, Chen Y, Gao X, et al. Expert consensus on the use of omalizumab in chronic urticaria in China. World Allergy Organ J 2021; 14: 100610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McPherson T. Current understanding in pathogenesis of atopic dermatitis. Ind J Dermatol 2016; 61: 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou B, Li J, Liu R, Zhu L, Peng C. The role of crosstalk of immune cells in pathogenesis of chronic spontaneous urticaria. Front Immunol 2022; 13: 879754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HC, Hong JB, Chu CY. Chronic idiopathic urticaria in Taiwan: a clinical study of demographics, aggravating factors, laboratory findings, serum autoreactivity and treatment response. J Formos Med Assoc 2011; 110: 175–182. [DOI] [PubMed] [Google Scholar]
- 35.Stockli SS, Bircher AJ. Generalized pruritus in a patient sensitized to tobacco and cannabis. J Dtsch Dermatol Ges 2007; 5: 303–304. [DOI] [PubMed] [Google Scholar]
- 36.Mortaz E, Folkerts G, Engels F, Nijkamp FP, Redegeld FA. Cigarette smoke suppresses in vitro allergic activation of mouse mast cells. Clin Exp Allergy 2009; 39: 679–687. [DOI] [PubMed] [Google Scholar]
- 37.Kim YS, Kim HY, Ahn HS, Sohn TS, Song JY, Lee YB, et al. The association between tobacco smoke and serum immunoglobulin E levels in Korean adults. Intern Med 2017; 56: 2571–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linneberg A, Nielsen NH, Madsen F, Frolund L, Dirksen A, Jorgensen T. Smoking and the development of allergic sensitization to aeroallergens in adults: a prospective population-based study. The Copenhagen Allergy Study. Allergy 2001; 56: 328–332. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan AP, Gimenez-Arnau AM, Saini SS. Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy 2017; 72: 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol 2002; 2: 372–377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.