Abstract
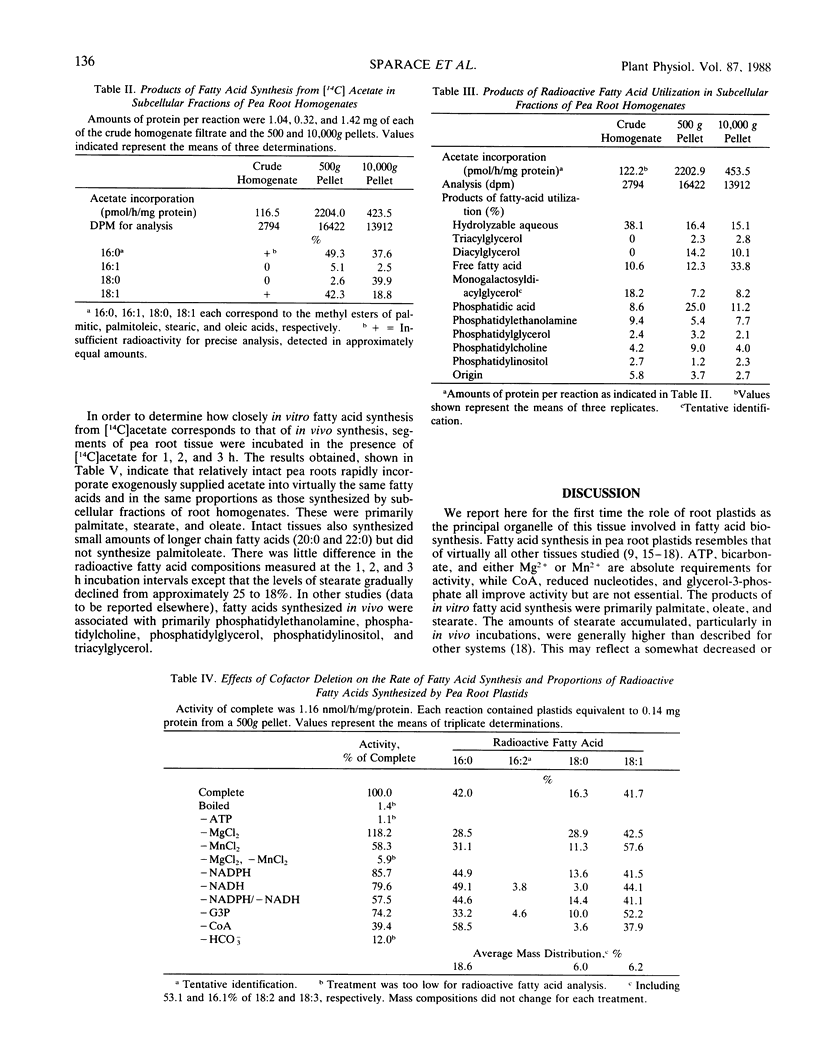
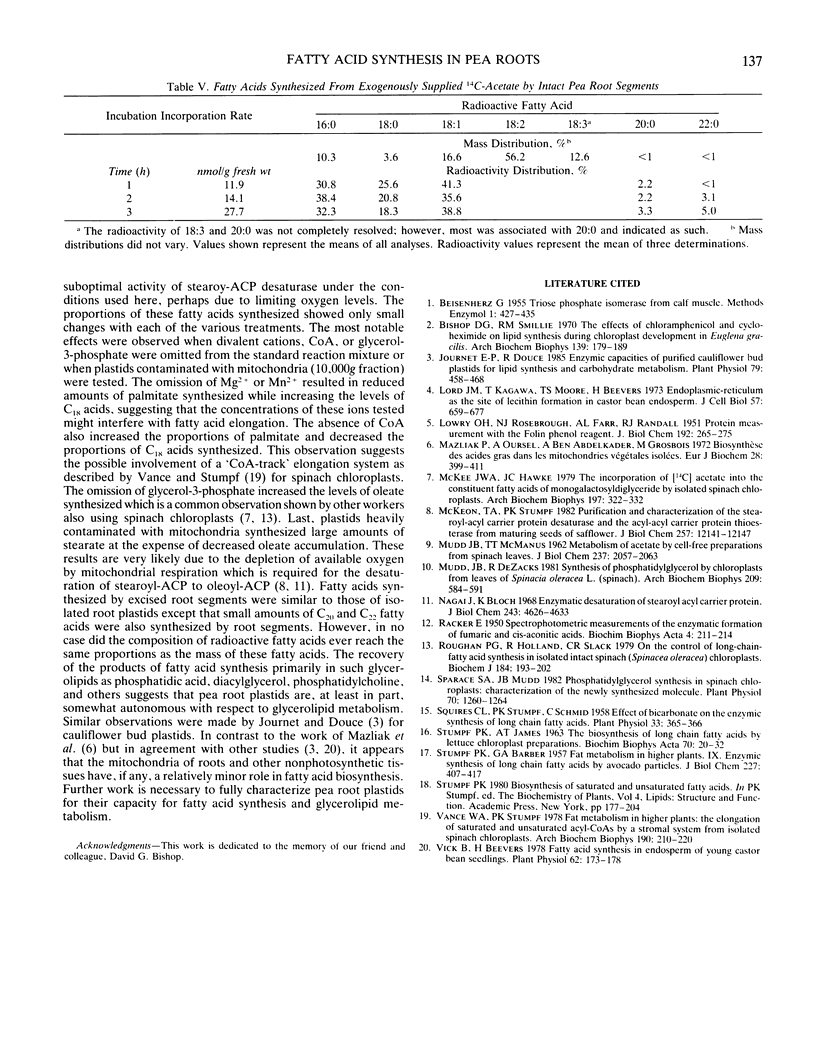
Subcellular fractions from pea (Pisum sativum L.) roots have been prepared by differential centrifugation techniques. Greater than 50% of the recovered plastids can be isolated by centrifugation at 500g for 5 minutes. Plastids of this fraction are largely free from mitochondrial and microsomal contamination as judged by marker enzyme analysis. De novo fatty acid biosynthesis in pea roots occurs in the plastids. Isolated pea root plastids are capable of fatty acid synthesis from acetate at rates up to 4.3 nanomoles per hour per milligram protein. ATP, bicarbonate, and either Mg2+ or Mn2+ are all absolutely required for activity. Coenzyme A at 0.5 millimolar improved activity by 60%. Reduced nucleotides were not essential but activity was greatest in the presence of 0.5 millimolar of both NADH and NADPH. The addition of 0.5 millimolar glycerol-3-phosphate increased activity by 25%. The in vitro and in vivo products of fatty acid synthesis from acetate were primarily palmitate, stearate, and oleate, the proportions of which were dependent on experimental treatments. Fatty acids synthesized by pea root plastids were recovered in primarily phosphatidic acid and diacylglycerol or as water soluble derivatives and the free acids. Lesser amounts were found in phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, and monogalactosyldiacylglycerol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. G., Smillie R. M. The effect of chloramphenicol and cycloheximide on lipid synthesis during chloroplast development in Euglena gracilis. Arch Biochem Biophys. 1970 Jul;139(1):179–189. doi: 10.1016/0003-9861(70)90059-7. [DOI] [PubMed] [Google Scholar]
- Journet E. P., Douce R. Enzymic capacities of purified cauliflower bud plastids for lipid synthesis and carbohydrate metabolism. Plant Physiol. 1985 Oct;79(2):458–467. doi: 10.1104/pp.79.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUDD J. B., McMANUS T. T. Metabolism of acetate by cellfree preparations from spinach leaves. J Biol Chem. 1962 Jul;237:2057–2063. [PubMed] [Google Scholar]
- Mazliak P., Oursel A., Abdelkader A. B., Grosbois M. Biosynthèse des acides gras dans les mitochondries végétales isolées. Eur J Biochem. 1972 Jul 24;28(3):399–411. doi: 10.1111/j.1432-1033.1972.tb01926.x. [DOI] [PubMed] [Google Scholar]
- McKee J. W., Hawke J. C. The incorporation of [14C]acetate into the constituent fatty acids of monogalactosyldiglyceride by isolated spinach chloroplasts. Arch Biochem Biophys. 1979 Oct 1;197(1):322–332. doi: 10.1016/0003-9861(79)90252-2. [DOI] [PubMed] [Google Scholar]
- McKeon T. A., Stumpf P. K. Purification and characterization of the stearoyl-acyl carrier protein desaturase and the acyl-acyl carrier protein thioesterase from maturing seeds of safflower. J Biol Chem. 1982 Oct 25;257(20):12141–12147. [PubMed] [Google Scholar]
- Mudd J. B., Dezacks R. Synthesis of phosphatidylglycerol by chloroplasts from leaves of Spinacia oleracea L. (spinach). Arch Biochem Biophys. 1981 Jul;209(2):584–591. doi: 10.1016/0003-9861(81)90316-7. [DOI] [PubMed] [Google Scholar]
- Nagai J., Bloch K. Enzymatic desaturation of stearyl acyl carrier protein. J Biol Chem. 1968 Sep 10;243(17):4626–4633. [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. On the control of long-chain-fatty acid synthesis in isolated intact spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Nov 15;184(2):193–202. doi: 10.1042/bj1840193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUMPF P. K., BARBER G. A. Fat metabolism in higher plants. IX. Enzymic synthesis of long chain fatty acids by avocado particles. J Biol Chem. 1957 Jul;227(1):407–417. [PubMed] [Google Scholar]
- Sparace S. A., Mudd J. B. Phosphatidylglycerol synthesis in spinach chloroplasts: characterization of the newly synthesized molecule. Plant Physiol. 1982 Nov;70(5):1260–1264. doi: 10.1104/pp.70.5.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. L., Stumpf P. K., Schmid C. Effect of Bicarbonate on the Enzymic Synthesis of Long Chain Fatty Acids. Plant Physiol. 1958 Sep;33(5):365–366. doi: 10.1104/pp.33.5.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance W. A., Stumpf P. K. Fat metabolism in higher plants. The elongation of saturated and unsaturated acyl-CoAs by a stromal system from isolated spinach chloroplasts. Arch Biochem Biophys. 1978 Sep;190(1):210–220. doi: 10.1016/0003-9861(78)90270-9. [DOI] [PubMed] [Google Scholar]
- Vick B., Beevers H. Fatty Acid synthesis in endosperm of young castor bean seedlings. Plant Physiol. 1978 Aug;62(2):173–178. doi: 10.1104/pp.62.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]


