Abstract
Background
Triglyceride and glucose (TyG) index and TyG‐related indices combined with obesity‐related markers are considered important markers of insulin resistance. We aimed to examine the association between the TyG index and modified TyG indices with new‐onset hypertension and their predictive ability stratified by sex.
Methods and Results
We analyzed data from 5414 Korean Genome and Epidemiology Study participants aged 40 to 69 years. Multiple Cox proportional hazard regression analyses were conducted to estimate the hazard ratio (HR) and 95% CI for new‐onset hypertension according to sex‐specific tertile groups after confounder adjustments. To evaluate the predictive performance of these indices for new‐onset hypertension, we calculated Harrell's C‐index (95% CI). Over a 9.5‐year follow‐up period, 1014 men and 1012 women developed new‐onset hypertension. Compared with the lowest tertile (T) group, the adjusted HR and 95% CI for new‐onset hypertension in T3 for TyG, TyG‐body mass index, TyG‐waist circumference, and TyG‐waist‐to‐height ratio were 1.16 (0.95–1.40), 1.11 (0.84–1.48), 1.77 (1.38–2.27), and 1.68 (1.33–2.13) in men and 1.37 (1.13–1.66), 1.55 (1.16–2.06), 1.43 (1.15–1.79), and 1.64 (1.30–2.07) in women, respectively. The C‐indices of TyG‐waist‐to‐height ratio for new‐onset hypertension were significantly higher than those of TyG and TyG‐body mass index in both men and women.
Conclusions
TyG and TyG‐body mass index were significantly associated with new‐onset hypertension only in women. TyG‐waist circumference and TyG‐waist‐to‐height ratio were significantly associated with new‐onset hypertension in both men and women. A sex‐specific approach is required when using TyG and modified TyG indices to identify individuals at risk of incident hypertension.
Keywords: body mass index, hypertension, triglyceride and glucose index
Subject Categories: Cardiovascular Disease; Epidemiology; Risk Factors; Diabetes, Type 2; Women
Nonstandard Abbreviations and Acronyms
- HIEC
hyperinsulinemic‐euglycemic clamp
- HOMA‐IR
homeostatic model assessment of insulin resistance
- IR
insulin resistance
- TyG
triglyceride and glucose index
- WC
waist circumference
- WHtR
waist to height ratio
Clinical Perspective.
What Is New?
TyG‐WC and TyG‐waist‐to‐height ratio were significantly associated with new‐onset hypertension in both men and women.
TyG and TyG‐body mass index were significantly associated with new‐onset hypertension in women but not in men.
What Are the Clinical Implications?
Sex‐specific approaches in using TyG and modified TyG indices as potential indicators for incident hypertension are required to identify individuals who are at risk of developing hypertension.
Further research should explore the underlying mechanism of sex differences on the effectiveness of TyG indices combining body composition indicators for the prediction of incident hypertension.
Hypertension is an important public‐health challenge worldwide. 1 In 2019, the global prevalence of hypertension was over 1 billion; a number that doubled since 1990. 2 Globally, elevated blood pressure (BP) is closely associated with mortality from coronary heart disease, stroke, heart failure, and other vascular and renal diseases. 3 , 4 Numerous studies have verified the benefits of lowering BP in patients with an increased cardiovascular risk. 5
Insulin resistance (IR), which plays a key role in the development of type 2 diabetes, is involved in the pathogenesis of hypertension and atherosclerotic cardiovascular diseases. 6 Therefore, various direct and indirect markers to assess IR have been developed using the hyperinsulinemic‐euglycemic clamp test and homeostatic model assessment of IR (HOMA‐IR). 7 , 8 However, the hyperinsulinemic‐euglycemic clamp is impractical in clinical practice and epidemiological studies due to the laborious process and high cost. 7 HOMA‐IR, based on fasting glucose and insulin, has been validated and is a useful surrogate index to measure IR in clinical application. 8
The triglyceride–glucose (TyG) index, which is calculated using fasting triglyceride and blood glucose, has been proposed as a simple and available marker for IR to reveal common pathology. 9 Since TyG index was initially used by Simental‐Mendia et al in 2008, 10 many studies have recognized that TyG index is associated with an increased risk of hypertension and cardiovascular disease (CVD). 11 , 12 , 13 , 14 , 15 , 16 , 17 Recently, TyG‐related markers combining TyG and body composition indices, such as body mass index (BMI) and waist circumference (WC), have been shown to have higher predictive performance for metabolic diseases than TyG alone. 18 , 19 , 20
However, previous studies have investigated the relationship between the TyG index and hypertension in either the general population or specific high‐risk groups. 12 , 13 , 14 , 15 , 16 , 21 There has been limited research examining this relationship with regard to sex differences.
A recent study reported significant difference in the TyG index between men and women. 22 The study found the combined metabolic indices to be stronger risk indicators for incident type 2 diabetes in women. Specifically, WC and waist‐to‐height ratio (WHtR) are metrics that represent sex‐specific differences in the distribution of body fat. Therefore, we aimed to investigate the relationship between new‐onset hypertension and TyG index and modified TyG indices from a combination of easily measurable values, such as BMI, WC, and WHtR in a sex‐specific manner. Moreover, we aimed to compare the predictive performance of the TyG index and modified TyG index for new‐onset hypertension in the middle‐aged and older Korean population.
METHODS
Availability of Data and Materials
Anonymized data and the associated codebook from the KOGES (Korean Genome and Epidemiology Study), conducted by the National Institute of Health and the Korea Disease Control and Prevention Agency in the Republic of Korea, have been made publicly available (https://www.nih.go.kr/ko/main/contents.do?menuNo=300563).
Study Population
A community‐based cohort study (KOGES_Ansan_Ansung cohort) was used in the current study. The KOGES_Ansan_Ansung cohort, which consists of adults aged 40 to 69 years, was conducted biennially at the baseline (2001–2002) up to the seventh follow‐up (2015–2016). From a total of 10 030 participants at baseline, we included the participants who had at least 1 follow‐up during 2003 to 2016 (n=9118). We excluded participants who had hypertension at baseline (n=2995) or incomplete data at baseline (n=1131). Finally, we included a total of 5414 participants in this study (Figure 1). All participants provided written consent and agreed to participate in the study. This study was approved by the institutional review board of Yongin Severance Hospital (IRB No. 9‐2022‐0090).
Figure 1. Flow chart of the study population.
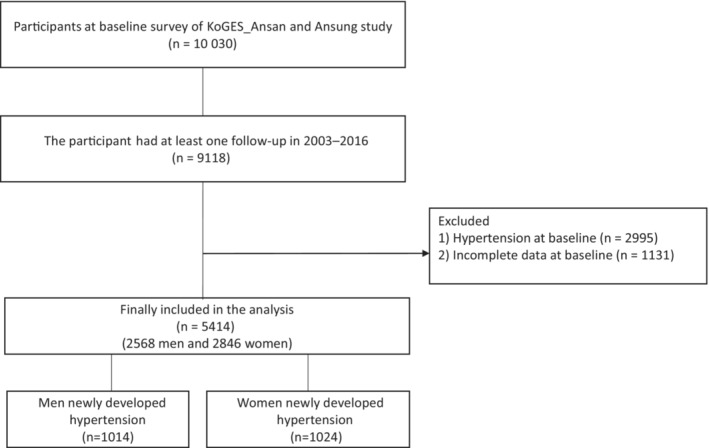
KOGES indicates Korean Genome and Epidemiology Study.
Data Collection
Trained medical staff measured the anthropometric variables including height, weight, and WC. Height and weight were measured to the nearest 0.1 cm and 0.1 kg, respectively. BMI was calculated by dividing weight (kg) by height (m) squared. WC was measured with inelastic tape at the midpoint between the iliac crest and lowest rib at the end of normal expiration. WHtR was calculated by dividing WC (cm) by height (cm).
At rest in a seated position, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured at least twice at 1‐minute intervals. Mercury sphygmomanometer was used for the measurement of BP according to standardized protocol.
Each participant's blood samples were collected after >8 hours of fasting. Fasting plasma glucose (FPG), serum insulin, total cholesterol (TC), triglyceride (TG), high‐density lipoprotein cholesterol (HDL‐C), and CRP (C‐reactive protein) levels were analyzed using the ADVIA 1650 Auto Analyzer (Siemens, Alpharetta, GA) and ADVIA 1800 Auto Analyzer (Siemens). Self‐reported questionnaires contained information about smoking status, alcohol consumption, physical activity, drug history, and disease history. Physical activity was measured as metabolic equivalent of task (MET)‐hours per week (MET‐h/day) using an International Physical Activity Questionnaire. 23 According to the level of physical activity, participants were categorized into 3 groups: (low: <7.5 MET‐h/day, moderate: 7.5 to 30 MET‐h/day, and high: >30 MET‐h/day). Detailed protocol used in KOGES is available at the following website: https://nih.go.kr/ko/main/contents.do?menuNo=300583.
Assessment of Triglyceride Glucose Index and Modified Triglyceride Glucose Indices
TyG was modified by multiplying with BMI, WC, and WHtR to produce TyG‐BMI, 24 TyG‐WC, 19 and TyG‐WHtR, 20 respectively, as follows:
TyG index and modified TyG indices were categorized into tertiles (T) as follows:
Definition of Hypertension
Hypertension was defined when at least 1 of the following criteria was met: (1) SBP ≥140 mm Hg, (2) DBP ≥90 mm Hg, (3) treatment with anti‐hypertensive medications, or (4) diagnosis by a physician. 25 New‐onset hypertension was defined as the occurrence of a hypertension diagnosis during a follow‐up period for individuals who previously had normal blood pressure levels. Right censoring was defined as lost to follow‐up or not having developed hypertension by the time of last follow‐up. In addition, we defined hypertension according to the 2017 American Heart Association/American College of Cardiology guidelines (SBP ≥130 mm Hg or DBP ≥80 mm Hg). 26
Statistical Analysis
All data are presented as mean±SD for continuous variables or number (percentage) for categorical variables. Independent 2‐sample t test or Mann–Whitney U test were used to compare the differences in continuous variables between men and women, including age, BMI, WC, WHtR, SBP, DBP, FPG, TC, TG, HDL‐C, CRP, and METs. Chi‐square test was used to compare the differences in categorical variables between men and women. After testing the interaction between TyG and sex for new‐onset hypertension, sex‐specific analyses were conducted (P for interaction=0.003).
The cumulative incidence rate of new‐onset hypertension based on tertiles of TyG and modified TyG indices are presented as Kaplan–Meier curves. Log‐rank tests were used to determine whether the cumulative incidence rate of hypertension differed among the tertile groups. Univariable and multivariable Cox proportional hazard regression analyses were performed to calculate the hazard ratio (HR) with a 95% CI for incident hypertension. In Model 1, we adjusted for age. In Model 2, we adjusted for the Model 1 variable plus BMI, physical activity, smoking status, and alcohol consumption. In Model 3, we adjusted for Model 2 variables plus total cholesterol, HDL cholesterol, FPG, CRP, antidiabetic drugs, and antidyslipidemia drugs.
Cox proportional hazard model was also used to determine the dose–response relationship between each TyG, modified and TyG indices and new‐onset hypertension. Subgroup analysis for presence of diabetes was conducted to explore if there was an effect modification on the association between TyG, modified TyG, and new‐onset hypertension. We also confirmed that all interaction terms for TyG and modified TyG indices and diabetes were significant at a significance level of 0.05. We calculated the Harrell's C‐index (95% CI) to evaluate the predictive performance of TyG, TyG‐BMI, TyG‐WC, and TyG‐WHtR for new‐onset hypertension. We calculated P values for significance of the difference between 2 Harrell's C‐indices. 27 All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R software (version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria). The significance level was set at P<0.05.
RESULTS
Clinical Characteristics of the Study Population
A total of 5414 participants (2568 men and 2846 women) were included in this study. The mean follow‐up period was 9.5 years. Baseline characteristics of the study population based on the presence of new‐onset hypertension are presented in Table 1. Among 5414 participants, 2026 (1014 men and 1012 women) individuals developed new‐onset hypertension. Participants who developed new‐onset hypertension were more likely to be older and men. The values of BMI, WC, WHtR, SBP, DBP, FPG, TC, TG, CRP, and HDL‐C differed between participants who developed new‐onset hypertension and those who did not. Participants who developed new‐onset hypertension had higher levels of TyG, TyG‐BMI, TyG‐WC, and TyG‐WHtR than those who did not develop new‐onset hypertension. The proportions of daily smokers and current alcohol consumers were not significantly different between groups. There was a significant interaction between TyG and sex, with a P value of 0.003.
Table 1.
Baseline Characteristics of the Study Population According to New‐Onset Hypertension
| Variable | New‐onset hypertension | P value | |
|---|---|---|---|
| Yes | No | ||
| No. | 2026 | 3388 | |
| Age, y | 52.49±8.74 | 48.74±7.85 | <0.001 |
| Sex, male* | 1014 (50.0%) | 1554 (45.9%) | 0.003 |
| BMI, kg/m2 | 24.74±3.10 | 23.81±2.88 | <0.001 |
| Waist circumference, cm | 83.43±8.35 | 79.38±8.17 | <0.001 |
| Waist to height ratio | 0.52±0.06 | 0.49±0.05 | <0.001 |
| Systolic BP, mm Hg | 118.54±10.11 | 108.51±10.53 | <0.001 |
| Diastolic BP, mm Hg | 78.67±6.78 | 72.76±7.84 | <0.001 |
| Fasting plasma glucose, mg/dL | 87.42±22.16 | 85.00±18.41 | <0.001 |
| Total cholesterol, mg/dL | 191.31±35.17 | 188.40±33.69 | 0.003 |
| Triglyceride, mg/dL | 136 (101–192) | 120 (91–165) | <0.001 |
| High‐density lipoprotein‐cholesterol, mg/dL | 44.13±9.63 | 45.57±10.16 | <0.001 |
| CRP | 0.14 (0.07–0.25) | 0.12 (0.05–0.21) | <0.001 |
| TyG | 8.72±0.55 | 8.57±0.51 | <0.001 |
| TyG‐BMI | 216.09±33.40 | 204.49±30.56 | <0.001 |
| TyG‐waist circumference | 728.73±97.95 | 682.10±93.92 | <0.001 |
| TyG‐waist‐to‐height ratio | 4.56±0.62 | 4.25±0.58 | <0.001 |
| Smoking status* | 0.074 | ||
| Never smoker | 1144 (56.5%) | 2032 (60.0%) | |
| Former smoker | 311 (15.4%) | 498 (14.7%) | |
| Intermittent smoker | 64 (3.2%) | 96 (2.8%) | |
| Everyday smoker | 507 (25.0%) | 762 (22.5%) | |
| Alcohol use status | 0.449 | ||
| Never | 896 (44.2%) | 1558 (46.0%) | |
| Former | 123 (6.1%) | 202 (6.0%) | |
| Current | 1007 (49.7%) | 1628 (48.1%) | |
| Metabolic equivalent of task, h/d | 25.03±15.26 | 22.10±13.55 | <0.001 |
BMI indicates body mass index; BP, blood pressure; CRP, C‐reactive protein; and TyG, triglyceride and glucose index. P values were calculated using the independent 2‐sample t test for continuous variables. P values also were calculated using Mann–Whitney U test for triglycerides and CRP.
Categorical variable represent as number (percent).
Table S1 shows the baseline characteristics of the study population. Mean±SD age was 50.2±8.4 years in men and 50.1±8.4 years in women. The values of WC, WHR, BMI SBP, DBP, FPG, TC, TG, HDL‐C, CRP, and METs were significantly different between men and women. The proportions of daily smokers, current alcohol consumers, and those taking antidiabetic drugs were higher in men than in women. The mean levels of TyG, TyG‐BMI, TyG‐WC, and TyG‐WHtR were different between men and women.
Table S2 shows the incidence rate of hypertension according to the follow‐up period. The incidence rate of new hypertension was analyzed for men and women biennially. The rates of newly diagnosed hypertension for men and women were the highest in the first 2 years at 11.8% and 9.9%, respectively. Subsequently, after the first 2 years, the incidence rate decreased to 4.6% in men and 4.9% in women.
Longitudinal Relationships Between Triglyceride Glucose Index and Modified Triglyceride Glucose Indices and New‐Onset Hypertension
The dose–response relationships between TyG, TyG‐BMI, TyG‐WC, TyG‐WHtR, and new‐onset hypertension in both men and women are shown in Figure 2 using the Cox proportional hazard model. A linear relationship between TyG, TyG‐BMI, TyG‐WC, TyG‐WHtR, and new‐onset hypertension was observed in men (Figure 2A through 2D) and women (Figure 2E through 2H).
Figure 2. Cox proportional hazards curves showing changes in hazard ratios for incident hypertension with TyG, TyG‐BMI, TyG‐WC, and TyG‐WHtR values.
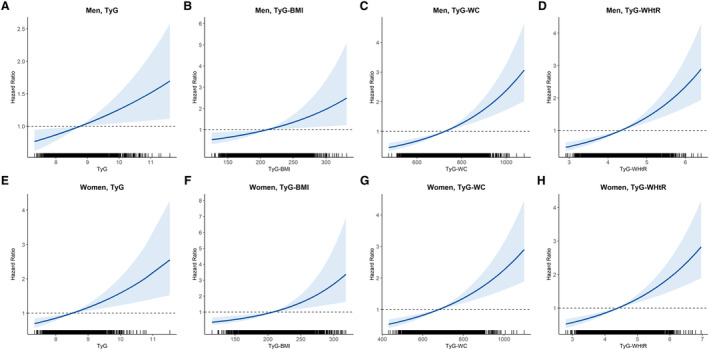
Relationship between incident hypertension in men and TyG tertile (A), TyG‐BMI (B), TyG‐WC (C), and TyG‐WHtR (D). Relationship between incident hypertension in women and TyG tertile (E), TyG‐BMI (F), TyG‐WC (G), and TyG‐WHtR (H). The x axis is a value of TyG or modified TyG, and y axis is the hazard ratio corresponding to the x axis value relative to the mean value of TyG or modified TyG. In each panel, the blue line denotes the estimated hazard ratio, and gray shading indicates the 95% confidence intervals. BMI indicates body mass index; TyG, triglyceride‐glucose index; TyG‐BMI, TyG index×BMI; TyG‐WC, TyG index×WC; TyG‐WHtR, TyG index×WHtR; WC, waist circumference; and WHtR, waist‐to‐height ratio.
Figure S1 shows the cumulative incidence rate of hypertension in relation to the tertiles of TyG, TyG‐BMI, TyG‐WC, and TyG‐WHtR using Kaplan–Meier curves. T3 of TyG, TyG‐BMI, TyG‐WC, and TyG‐WHtR were associated with a significantly higher incidence of hypertension than the first reference tertile (T1) during the follow‐up period in men (log‐rank test of TyG, P<0.001; log‐rank test of TyG‐BMI, P<0.001; log‐rank test of TyG‐WC, P<0.001; log‐rank test of TyG‐WHtR, P<0.001; Figure S1A through S1D). T3 of TyG, TyG‐BMI, TyG‐WC, and TyG‐WHtR were also associated with a significantly higher incidence of hypertension than reference T1 during the follow‐up period in women (log‐rank test of TyG, P<0.001; log‐rank test of TyG‐BMI, P<0.001; log‐rank test of TyG‐WC, P<0.001; log‐rank test of TyG‐WHtR, P<0.001; Figure S1E and S1F).
Table 2 presents the Cox proportional hazard regression model for incident hypertension in relation to the tertiles of each TyG and modified TyG indices in both men and women. In the unadjusted model, the HR (95% CI) for incident hypertension of T3 TyG, when compared with T1, was 1.42 (1.21–1.68) in men and 2.07 (1.78–2.41) in women. TyG was significantly associated with a higher risk of incident hypertension only in women after adjusting for age, BMI, physical activity, smoking, alcohol consumption, TC, HDL‐C, FPG, CRP, antidiabetic drugs, and antidyslipidemia drugs (T3 versus T1; HR, 1.37 [95% CI, 1.13–1.66], P=0.003 in women; and HR, 1.16 [95% CI, 0.95–1.40], P=0.324 in men).
Table 2.
Multiple Cox Proportional Hazards Regression Analysis for Incident Hypertension of Tertiles of TyG and Modified TyG Indices
| Hazard ratio with 95% CI | ||||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | |||||||
| T1 | T2 | T3 | P value | T1 | T2 | T3 | P value | |
| TyG | ||||||||
| Unadjusted | 1 | 1.19 (1.00–1.42) | 1.42 (1.21–1.68) | <0.001 | 1 | 1.37 (1.18–1.59) | 2.07 (1.78–2.41) | <0.001 |
| Model 1 | 1 | 1.21 (1.02–1.44) | 1.49 (1.27–1.76) | <0.001 | 1 | 1.13 (0.97–1.32) | 1.61 (1.38–1.88) | <0.001 |
| Model 2 | 1 | 1.09 (0.92–1.30) | 1.20 (1.01–1.43) | 0.103 | 1 | 1.06 (0.91–1.24) | 1.36 (1.16–1.60) | <0.001 |
| Model 3 | 1 | 1.08 (0.91–1.30) | 1.16 (0.95–1.40) | 0.324 | 1 | 1.07 (0.91–1.25) | 1.37 (1.13–1.66) | 0.003 |
| TyG‐BMI | ||||||||
| Unadjusted | 1 | 1.29 (1.10–1.52) | 1.69 (1.45–1.97) | <0.001 | 1 | 1.47 (1.25–1.73) | 2.34 (2.00–2.74) | <0.001 |
| Model 1 | 1 | 1.37 (1.16–1.61) | 1.89 (1.61–2.21) | <0.001 | 1 | 1.38 (1.18–1.63) | 2.08 (1.78–2.43) | <0.001 |
| Model 2 | 1 | 1.10 (0.90–1.33) | 1.20 (0.92–1.57) | 0.416 | 1 | 1.25 (1.03–1.52) | 1.61 (1.23–2.10) | 0.002 |
| Model 3 | 1 | 1.06 (0.86–1.30) | 1.11 (0.84–1.48) | 0.759 | 1 | 1.23 (1.01–1.50) | 1.55 (1.16–2.06) | 0.011 |
| TyG‐waist circumference | ||||||||
| Unadjusted | 1 | 1.41 (1.16–1.70) | 2.16 (1.81–2.59) | <0.001 | 1 | 1.83 (1.57–2.14) | 2.83 (2.43–3.30) | <0.001 |
| Model 1 | 1 | 1.46 (1.20–1.76) | 2.27 (1.89–2.71) | <0.001 | 1 | 1.48 (1.27–1.74) | 2.03 (1.73–2.38) | <0.001 |
| Model 2 | 1 | 1.28 (1.04–1.57) | 1.76 (1.39–2.22) | <0.001 | 1 | 1.26 (1.06–1.49) | 1.49 (1.21–1.84) | <0.001 |
| Model 3 | 1 | 1.29 (1.04–1.59) | 1.77 (1.38–2.27) | <0.001 | 1 | 1.23 (1.03–1.47) | 1.43 (1.15–1.79) | 0.006 |
| TyG‐waist‐to‐height ratio | ||||||||
| Unadjusted | 1 | 1.48 (1.26–1.74) | 2.17 (1.85–2.54) | <0.001 | 1 | 2.21 (1.85–2.65) | 3.34 (2.82–3.96) | <0.001 |
| Model 1 | 1 | 1.50 (1.27–1.76) | 2.18 (1.86–2.56) | <0.001 | 1 | 1.80 (1.49–2.16) | 2.27 (1.90–2.72) | <0.001 |
| Model 2 | 1 | 1.30 (1.09–1.56) | 1.69 (1.36–2.10) | <0.001 | 1 | 1.54 (1.27–1.87) | 1.69 (1.35–2.11) | <0.001 |
| Model 3 | 1 | 1.31 (1.08–1.58) | 1.68 (1.33–2.13) | <0.001 | 1 | 1.53 (1.25–1.86) | 1.64 (1.30–2.07) | <0.001 |
BMI indicates body mass index; and TyG, triglyceride and glucose index. Significance was set at P<0.05. Model 1: adjusted for age. Model 2: adjusted for variables used in Model 1 plus BMI, physical activity, smoking status, and current drinker. Model 3: adjusted for variables used in Model 2 plus total cholesterol, high‐density lipoprotein cholesterol, fasting plasma glucose, C‐reactive protein, antidiabetes drugs, and antidyslipidemic drugs.
Compared with T1, T3 of TyG‐BMI was associated with a higher risk of incident hypertension in both men and women in unadjusted model (HR, 1.69 [95% CI, 1.45–1.97], P<0.001 in men; HR, 2.34 [95% CI, 2.00–2.74], P<0.001 in women). TyG‐BMI was not associated with incident hypertension in men (HR, 1.11 [95% CI, 0.84–1.48]; P=0.759) but was significantly associated with incident hypertension in women (HR, 1.55 [95% CI, 1.16–2.06]; P=0.011) after adjusting for the same confounders.
Compared with T1, T3 of TyG‐WC was associated with a higher risk of incident hypertension in both men and women (HR, 2.16 [95% CI, 1.81–2.59], P<0.001 in men; HR, 2.83 [95% CI, 2.43–3.30], P<0.001 in women). A significant relationship between TyG‐WC and incident hypertension persisted in both men and women after adjusting for confounding variables.
Compared with T1, T3 of TyG‐WHtR was associated with a higher risk of incident hypertension in both men and women (HR, 2.17 [95% CI, 1.85–2.54], P<0.001 in men; HR, 3.34 [95% CI, 2.82–3.96], P<0.001 in women). A significant relationship between TyG‐WHtR and incident hypertension persisted in both men and women after adjusting for confounders.
In addition, we also examined the association between HOMA‐IR, HOMA‐beta (homeostasis model assessment of β‐cell function), and incident hypertension (Table S3). In the unadjusted model, compared with T1, the HR for incident hypertension of T3 HOMA‐IR was 1.34 (95% CI, 1.15–1.55) in men and 1.26 (95% CI, 1.08–1.47) in women. The significant associations between HOMA‐IR and incident hypertension persisted in Models 1 and 2. After adjusting for age, BMI, physical activity, smoking, alcohol consumption, TC, HDL‐C, FPG, CRP, antidiabetic drugs, and antidyslipidemia drugs, theses significant associations were attenuated (T3 versus T1; HR, 1.12 [95% CI, 0.95–1.33] in men; HR, 1.97 [95% CI, 0.91–1.26] in women). There was no significant association between HOMA‐beta and incident hypertension.
We additionally showed the results from Cox proportional hazards regression analysis and Harrell's C‐index for incident hypertension in relation to the tertiles of TyG and modified TyG indices, using the 2017 American Heart Association/American College of Cardiology definition of hypertension (Tables S4 and S5). The association between TyG and modified TyG indices and new‐onset hypertension remained similar even after applying the 2017 definition of hypertension.
Figure S2 shows the subgroup analysis in relation to the presence of diabetes. There were 484 (285 men and 199 women) participants with diabetes and 4930 (2283 men and 2647 women) without diabetes. TyG, TyG‐BMI, TyG‐WC, and TyG‐WHtR were not significantly associated with incident hypertension in both men and women with diabetes. In the group without diabetes, TyG and TyG‐BMI were significantly associated with new‐onset hypertension only in women without diabetes. TyG‐WC and TyG‐WHtR were significantly associated with new‐onset hypertension in both men and women without diabetes.
Comparison of the Triglyceride Glucose Index and Modified Triglyceride Glucose Indices to Predict New‐Onset Hypertension
We identified the Harrell's C‐indices of TyG, TyG‐BMI, TyG‐WC, and TyG‐WHtR that were significantly related to incident hypertension in both men and women (Table 3). The Harrell's C‐indices in men were 0.549 (95% CI, 0.530–0.567) in TyG, 0.576 (95% CI, 0.557–0.594) in TyG‐BMI, 0.595 (95% CI, 0.576–0.613) in TyG‐WC, and 0.599 (95% CI, 0.580–0.617) in TyG‐WHtR. The Harrell's C‐index (95% CI) of TyG‐WC was significantly higher than that of TyG (P<0.001) and TyG‐BMI (P<0.001) in men. The Harrell's C‐index (95% CI) of TyG‐WHtR was also significantly higher than that of TyG (P<0.001) and TyG‐BMI (P<0.001) in men. However, there was no significant difference in the C‐index between TyG‐WC and TyG‐WHtR. Harrell's C‐indices in women were 0.588 (95% CI, 0.570–0.606) in TyG, 0.599 (95% CI, 0.580–0.618) in TyG‐BMI, 0.639 (95% CI, 0.622–0.657) in TyG‐WC, and 0.650 (95% CI, 0.633–0.668) in TyG‐WHtR in women. The Harrell's C‐index (95% CI) of TyG‐WC and TyG‐WHtR was significantly higher than that of TyG (P<0.001 for TyG‐WC and P<0.001 for TyG‐WHtR) and TyG‐BMI (P<0.001 for TyG‐WC and P<0.001 for TyG‐WHtR) in women, respectively. Out of the 4 indices, the Harrell's C‐index of TyG‐WHtR was significantly higher than the other indices in women.
Table 3.
Harrell's Concordance Index and 95% CIs for Predicting Hypertension Incidence of the TyG, TyG‐BMI, TyG‐WC, and TyG‐WHtR
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C‐index | 95% CI | P1 | P2 | P3 | C‐index | 95% CI | P1 | P2 | P3 | |
| TyG | 0.549 | 0.530–0.567 | Ref | 0.588 | 0.570–0.606 | Ref | ||||
| TyG‐BMI | 0.576 | 0.557–0.594 | <0.001 | Ref | 0.599 | 0.580–0.618 | 0.229 | Ref | ||
| TyG‐WC | 0.595 | 0.576–0.613 | <0.001 | <0.001 | Ref | 0.639 | 0.622–0.657 | <0.001 | <0.001 | Ref |
| TyG‐WHtR | 0.599 | 0.580–0.617 | <0.001 | <0.001 | 0.100 | 0.650 | 0.633–0.668 | <0.001 | <0.001 | <0.001 |
BMI indicates body mass index; TyG, triglyceride and glucose index; WC, waist circumference; and WHtR, waist‐to‐height ratio.
DISCUSSION
In the current study, we found that TyG and modified TyG indices (TyG‐BMI, TyG‐WC, and TyG‐WHtR) are significantly associated with new‐onset hypertension in women, whereas only TyG‐WC and TyG‐WHtR are significantly associated with new‐onset hypertension in men after adjusting for confounders. The predictive performance of TyG‐WHtR for new‐onset hypertension was significantly higher than other indices in women, whereas there was no significant difference in the predictive power between TyG‐WHtR and TyG‐WC in men. The same results were observed even after applying the 2017 American Heart Association/American College of Cardiology definition of hypertension.
TyG index comprising fasting TG and glucose has been identified as a reliable alternative biomarker of IR. 9 , 11 , 12 Several epidemiological studies have shown that a higher TyG index is significantly associated with an increased risk of hypertension. 13 , 14 , 15 , 16 , 17 However, unlike previous studies, we found that the TyG for new‐onset hypertension showed poor predictive performance in men (C‐index, 0.549 [95% CI, 0.530–0.567]).
Although the reasons for the differential relationship between the TyG index and new‐onset hypertension in men and women are not entirely clear, there are some possible explanations. A greater impact of visceral adipose tissue on CVD risk among women has been reported in previous studies. 22 , 28 Contribution of visceral lipolysis in the delivery of nonesterified fatty acids to the liver is more significant in women. 21 A previous study also reported more robust correlation between visceral adipose tissue and serum TG in women than in men. 29
We additionally conducted subgroup analyses to confirm that the results were independent of diabetes status. Modified TyG index was not associated with new‐onset hypertension in either men or women with diabetes. There have been inconsistent findings regarding the association between TyG index and CVD. A previous study 30 showed that the cumulative TyG index was significantly associated with the incidence of major adverse cardiovascular events in patient with type 2 diabetes. However, several recent studies did not show significant association between TyG index and CVD and CVD death in patients with diabetes. 31 , 32 , 33 Although the underlying mechanism is unclear, the use of hypoglycemic drugs or insulin could affect the actual blood glucose and blood insulin level. Moreover, diabetes itself may have a greater impact on development of hypertension as an important risk factor. 33 Alizargar et al also suggested that using the TyG index in individuals with CVD could be biased by diabetes and hyperlipidemia. 34 Therefore, the application of TyG as a marker of hypertension should be investigated further while taking sex and underlying glucose status into consideration.
There are several reasons why the TyG and TyG‐related indices are associated with incident hypertension. IR serves as the link between the TyG and TyG‐related indices and hypertension. Dysregulation in glucose metabolism and disruptions in lipid metabolism are prominent features of IR. Hyperglycemia induces oxidative stress and systemic inflammation, which lead to hypertension by causing endothelial cell dysfuntion. 35 Increased glycosylated products and free radicals lead to decreased nitric oxide production. 35 Reduced bioavailability of nitrous oxide can cause arterial stiffness, which can ultimately result in hypertension. 36 Furthermore, lipid triad under IR status, which is characterized by elevated TG, lowered HDL‐C, and appearance of small dense low‐density lipoprotein may contribute to the initiation of atherosclerosis. 37
In the current study, the predictive performances of TyG‐WC and TyG‐WHtR were greater than those of TyG and TyG‐BMI, which was in line with previous studies. One study showed that TyG‐WC outperformed the prediction of diabetes compared with that of TyG and TyG‐BMI. 19 WC is a useful assessment parameter for abdominal obesity, which is closely associated with disturbances in glucose and lipid metabolism. 38 Visceral adipose tissue secretes proinflammatory cytokines and adipokines, which lead to IR. 39 WC is also significantly associated with increased mortality risk with or without adjusting for BMI. 40 These studies imply that WC may be more effective than BMI for predicting IR and metabolic disturbance. WHtR is also a useful tool for assessing central obesity. 41 WHtR is more sensitive than WC in various ethnic populations and in both sexes, because it adjusts for different statures. 41 A cohort study reported that WHtR is a better predictor for CVD in patients with hypertension than BMI and WC. 42 A 15‐year prospective study proved that TyG‐related markers that combine obesity markers with TyG index, especially TyG‐WHtR, are superior to other parameters in identifying metabolic syndrome in both sexes in an urban Chinese population. 43
When we further examined the association between HOMA‐IR, HOMA‐beta, and incident hypertension, the link between HOMA‐IR and the incident hypertension weakened after adjusting for confounding factors, and no notable association was found between HOMA‐beta and the incident hypertension. Although the precise mechanisms that explain why the TyG index and modified TyG indices outperform HOMA‐IR in predicting the new‐onset hypertension are not fully understood, IR conditions are believed to not only be confined to impairments in glucose metabolism but also involve abnormalities in fatty acid metabolism resulting from the excessive storage of triglycerides in skeletal muscle. 44 The TyG index appears to indicate IR in both the liver and muscles. 45 Increased levels of TG in the bloodstream can disrupt glucose metabolism in muscle, which is the primary organ responsible for insulin regulation. TG originating from visceral fat stimulate the production of an excessive amount of free fatty acids within the liver, thereby resulting in IR. 46
A Korean study using the KOGES data set also showed that TyG has superior performance than HOMA‐IR in predicting metabolic syndrome. 47 In Son's study, the area under receiver operating characteristic for incident MetS was 0.654 (95% CI, 0.644–0.664) for TyG and 0.556 (95% CI, 0.531–0.581) for HOMA‐IR. 47 Wang et al 21 reported that TyG index showed superiority for indicating major adverse cardiovascular events in patients with type 2 diabetes regardless of the use of insulin‐related medication.
Our study had several limitations. First, because biochemistry (serum TG and fasting glucose) and body composition indicators were measured in a baseline survey, changes in TyG and modified TyG indices during the follow‐up period could not be considered. Second, this study was conducted using a middle‐aged and elderly population in Korea; therefore, its results cannot be generalized to other populations. Third, the incidence of hypertension decreased dramatically after the second follow‐up. Although the exact reason for this result is unclear, individuals with new‐onset hypertension may have been included in the group lost to follow‐up. Also, study participants may also have become more aware of their BP and monitored and managed it through lifestyle modification, which could have led to decreased incidence of hypertension. Despite these limitations, this is the first study to examine the association between TyG and modified TyG index and new‐onset hypertension with a large sample size and long‐term follow‐up period. Additionally, we compared the predictive performances of TyG and modified the TyG index for new‐onset hypertension in a sex‐specific manner.
Conclusion
The current study revealed a significant association between TyG and TyG‐BMI and the incidence of hypertension, especially among women. TyG‐WC and TyG‐WHtR were significantly associated with new‐onset hypertension in both men and women. Notably, the combined utilization of TyG and body composition indices such as WC and WHtR exhibited superior predictive performance compared to TyG alone in both men and women. Further research is warranted when employing TyG and modified TyG indices for identifying individuals at risk of developing incident hypertension, while considering sex differences.
Sources of Funding
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry through the High Value‐added Food Technology Development Program funded by the Ministry of Agriculture, Food and Rural Affairs (321030051HD030). This research was financially supported by Ministry of SMEs and Startups and Korea Technology and Promotion Agency for SMEs through the Regional Specialized Industry Development Plus Program (Grant Number: S3370378).
Disclosures
None.
Supporting information
Tables S1–S5
Figures S1–S2
Acknowledgments
This study was conducted using data from the Korean Genome and Epidemiology Study. Author contributions: Joo Hyung Lee, Seok‐Jae Heo, and Yu‐Jin Kwon contributed to the conception or design of the work. Joo Hyung Lee, Seok‐Jae Heo, and Yu‐Jin Kwon contributed to the acquisition, analysis, or interpretation of the data, as well as drafting the article. All authors have critically revised the article, provided final approval, and agree to be accountable for all aspects of the work, ensuring integrity and accuracy.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030022
This article was sent to Tochukwu M. Okwuosa, DO, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Seok‐Jae Heo, Email: sjheo@yuhs.ac.
Yu‐Jin Kwon, Email: digda3@yuhs.ac.
References
- 1. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–237. doi: 10.1038/s41581-019-0244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. NCD Risk Factor Collaboration (NCD‐RisC) . Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population‐representative studies with 104 million participants. Lancet. 2021;398:957–980. doi: 10.1016/s0140-6736(21)01330-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van den Hoogen PC, Feskens EJ, Nagelkerke NJ, Menotti A, Nissinen A, Kromhout D. The relation between blood pressure and mortality due to coronary heart disease among men in different parts of the world. Seven Countries Study Research Group. N Engl J Med. 2000;342:1–8. doi: 10.1056/nejm200001063420101 [DOI] [PubMed] [Google Scholar]
- 4. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285–292. doi: 10.1161/hypertensionaha.119.14240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387:957–967. doi: 10.1016/s0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 6. Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride‐glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta‐analysis of cohort studies. Cardiovasc Diabetol. 2021;20:76. doi: 10.1186/s12933-021-01268-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pisprasert V, Ingram KH, Lopez‐Davila MF, Munoz AJ, Garvey WT. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care. 2013;36:845–853. doi: 10.2337/dc12-0840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487 [DOI] [PubMed] [Google Scholar]
- 9. Khan SH, Sobia F, Niazi NK, Manzoor SM, Fazal N, Ahmad F. Metabolic clustering of risk factors: evaluation of triglyceride‐glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;10:74. doi: 10.1186/s13098-018-0376-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simental‐Mendía LE, Rodríguez‐Morán M, Guerrero‐Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034 [DOI] [PubMed] [Google Scholar]
- 11. Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, Tang C. High triglyceride‐glucose index is associated with poor prognosis in patients with acute ST‐elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. 2019;18:150. doi: 10.1186/s12933-019-0957-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma X, Dong L, Shao Q, Cheng Y, Lv S, Sun Y, Shen H, Wang Z, Zhou Y, Liu X. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc Diabetol. 2020;19:31. doi: 10.1186/s12933-020-01006-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang K, He G, Zhang Y, Yin J, Yan Y, Zhang Y, Wang K. Association of triglyceride‐glucose index and its interaction with obesity on hypertension risk in Chinese: a population‐based study. J Hum Hypertens. 2021;35:232–239. doi: 10.1038/s41371-020-0326-4 [DOI] [PubMed] [Google Scholar]
- 14. Jian S, Su‐Mei N, Xue C, Jie Z, Xue‐Sen W. Association and interaction between triglyceride‐glucose index and obesity on risk of hypertension in middle‐aged and elderly adults. Clin Exp Hypertens. 2017;39:732–739. doi: 10.1080/10641963.2017.1324477 [DOI] [PubMed] [Google Scholar]
- 15. Zheng R, Mao Y. Triglyceride and glucose (TyG) index as a predictor of incident hypertension: a 9‐year longitudinal population‐based study. Lipids Health Dis. 2017;16:175. doi: 10.1186/s12944-017-0562-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sánchez‐Íñigo L, Navarro‐González D, Pastrana‐Delgado J, Fernández‐Montero A, Martínez JA. Association of triglycerides and new lipid markers with the incidence of hypertension in a Spanish cohort. J Hypertens. 2016;34:1257–1265. doi: 10.1097/hjh.0000000000000941 [DOI] [PubMed] [Google Scholar]
- 17. Zhu B, Wang J, Chen K, Yan W, Wang A, Wang W, Gao Z, Tang X, Yan L, Wan Q, et al. A high triglyceride glucose index is more closely associated with hypertension than lipid or glycemic parameters in elderly individuals: a cross‐sectional survey from the reaction study. Cardiovasc Diabetol. 2020;19:112. doi: 10.1186/s12933-020-01077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, Ko YL. Triglyceride glucose‐body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS One. 2016;11:e0149731. doi: 10.1371/journal.pone.0149731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng S, Shi S, Ren X, Han T, Li Y, Chen Y, Liu W, Hou PC, Hu Y. Triglyceride glucose‐waist circumference, a novel and effective predictor of diabetes in first‐degree relatives of type 2 diabetes patients: cross‐sectional and prospective cohort study. J Transl Med. 2016;14:260. doi: 10.1186/s12967-016-1020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malek M, Khamseh ME, Chehrehgosha H, Nobarani S, Alaei‐Shahmiri F. Triglyceride glucose‐waist to height ratio: a novel and effective marker for identifying hepatic steatosis in individuals with type 2 diabetes mellitus. Endocrine. 2021;74:538–545. doi: 10.1007/s12020-021-02815-w [DOI] [PubMed] [Google Scholar]
- 21. Wang L, Cong HL, Zhang JX, Hu YC, Wei A, Zhang YY, Yang H, Ren LB, Qi W, Li WY, et al. Triglyceride‐glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19:80. doi: 10.1186/s12933-020-01054-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brahimaj A, Rivadeneira F, Muka T, Sijbrands EJG, Franco OH, Dehghan A, Kavousi M. Novel metabolic indices and incident type 2 diabetes among women and men: the Rotterdam study. Diabetologia. 2019;62:1581–1590. doi: 10.1007/s00125-019-4921-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oh JY, Yang YJ, Kim BS, Kang JH. Validity and reliability of Korean version of International Physical Activity Questionnaire (IPAQ) short form. J Korean Acad Fam Med. 2007;28:532–541. [Google Scholar]
- 24. Khamseh ME, Malek M, Abbasi R, Taheri H, Lahouti M, Alaei‐Shahmiri F. Triglyceride glucose index and related parameters (triglyceride glucose‐body mass index and triglyceride glucose‐waist circumference) identify nonalcoholic fatty liver and liver fibrosis in individuals with overweight/obesity. Metab Syndr Relat Disord. 2021;19:167–173. doi: 10.1089/met.2020.0109 [DOI] [PubMed] [Google Scholar]
- 25. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al; Authors/Task Force Members . 2018 ESC/ESH guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. doi: 10.1097/hjh.0000000000001940 [DOI] [PubMed] [Google Scholar]
- 26. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and Management of High Blood Pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/hyp.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 27. Kang L, Chen W, Petrick NA, Gallas BD. Comparing two correlated C indices with right‐censored survival outcome: a one‐shot nonparametric approach. Stat Med. 2015;34:685–703. doi: 10.1002/sim.6370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanaya AM, Harris T, Goodpaster BH, Tylavsky F, Cummings SR. Adipocytokines attenuate the association between visceral adiposity and diabetes in older adults. Diabetes Care. 2004;27:1375–1380. doi: 10.2337/diacare.27.6.1375 [DOI] [PubMed] [Google Scholar]
- 29. Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, Volafova J, Bray GA. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–435. doi: 10.1053/meta.2001.21693 [DOI] [PubMed] [Google Scholar]
- 30. Tai S, Fu L, Zhang N, Yang R, Zhou Y, Xing Z, Wang Y, Zhou S. Association of the cumulative triglyceride‐glucose index with major adverse cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2022;21:161. doi: 10.1186/s12933-022-01599-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang R, Wang Z, Chen J, Bao X, Xu N, Guo S, Gu R, Wang W, Wei Z, Wang L. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated heart failure. Cardiovasc Diabetol. 2022;21:88. doi: 10.1186/s12933-022-01507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu Q, Si F, Liu Z, Wu Y, Yu J. Association between triglyceride‐glucose index and risk of cardiovascular disease among postmenopausal women. Cardiovasc Diabetol. 2023;22:21. doi: 10.1186/s12933-023-01753-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu L, Wu Z, Zhuang Y, Zhang Y, Cui H, Lu F, Peng J, Yang J. Association of triglyceride‐glucose index and traditional risk factors with cardiovascular disease among non‐diabetic population: a 10‐year prospective cohort study. Cardiovasc Diabetol. 2022;21:256. doi: 10.1186/s12933-022-01694-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alizargar J, Bai CH, Hsieh NC, Wu SV. Use of the triglyceride‐glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. 2020;19:8. doi: 10.1186/s12933-019-0982-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride‐glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21:68. doi: 10.1186/s12933-022-01511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hermann M, Flammer A, Lüscher TF. Nitric oxide in hypertension. J Clin Hypertens (Greenwich). 2006;8:17–29. doi: 10.1111/j.1524-6175.2006.06032.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:122. doi: 10.1186/s12933-018-0762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177–189. doi: 10.1038/s41574-019-0310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/jci10842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cerhan JR, Moore SC, Jacobs EJ, Kitahara CM, Rosenberg PS, Adami HO, Ebbert JO, English DR, Gapstur SM, Giles GG, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89:335–345. doi: 10.1016/j.mayocp.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ashwell M, Hsieh SD. Six reasons why the waist‐to‐height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. 2005;56:303–307. doi: 10.1080/09637480500195066 [DOI] [PubMed] [Google Scholar]
- 42. Zhang S, Fu X, Du Z, Guo X, Li Z, Sun G, Zhou Y, Yang H, Yu S, Zheng L, et al. Is waist‐to‐height ratio the best predictive indicator of cardiovascular disease incidence in hypertensive adults? A cohort study. BMC Cardiovasc Disord. 2022;22:214. doi: 10.1186/s12872-022-02646-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang X, Zhang T, He S, Jia S, Zhang Z, Ye R, Yang X, Chen X. Association of metabolic syndrome with TyG index and TyG‐related parameters in an urban Chinese population: a 15‐year prospective study. Diabetol Metab Syndr. 2022;14:84. doi: 10.1186/s13098-022-00855-4 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44. Kelley DE, Goodpaster BH. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care. 2001;24:933–941. doi: 10.2337/diacare.24.5.933 [DOI] [PubMed] [Google Scholar]
- 45. Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, Kim MJ, Kim MK, Park JS. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17:41. doi: 10.1186/s12933-018-0692-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015;14:121. doi: 10.1186/s12944-015-0123-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Son DH, Lee HS, Lee YJ, Lee JH, Han JH. Comparison of triglyceride‐glucose index and HOMA‐IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2022;32:596–604. doi: 10.1016/j.numecd.2021.11.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figures S1–S2
Data Availability Statement
Anonymized data and the associated codebook from the KOGES (Korean Genome and Epidemiology Study), conducted by the National Institute of Health and the Korea Disease Control and Prevention Agency in the Republic of Korea, have been made publicly available (https://www.nih.go.kr/ko/main/contents.do?menuNo=300563).


