Abstract
Background
Menstrual cycle irregularities are associated with cardiovascular and cardiometabolic disease. We tested associations between age at menarche and cycle irregularity in adolescence and cardiometabolic health in early adulthood in a subsample from the Pittsburgh Girls Study.
Methods and Results
Data from annual interviews were used to assess age at menarche and cycle irregularity (ie, greater or less than every 27–29 days) at age 15 years. At ages 22 to 25 years, cardiometabolic health was measured in a subsample of the Pittsburgh Girls Study (n=352; 68.2% Black), including blood pressure, waist circumference, and fasting serum insulin, glucose, and lipids. T tests were used for continuous data and odds ratios for dichotomous data to compare differences in cardiometabolic health as a function of onset and regularity of menses. Early menarche (ie, before age 11 years; n=52) was associated with waist circumference (P=0.043). Participants reporting irregular cycles (n=50) in adolescence had significantly higher levels of insulin, glucose, and triglycerides, and higher systolic and diastolic blood pressure (P values range from 0.035 to 0.005) and were more likely to have clinical indicators of cardiometabolic predisease in early adulthood compared with women who reported regular cycles (odds ratios ranged from 1.89 to 2.56).
Conclusions
Increasing rates and earlier onset of cardiovascular and metabolic disease among women, especially among Black women, highlights the need for identifying early and reliable risk indices. Menstrual cycle irregularity may serve this purpose and help elucidate the role of women's reproductive health in protecting and conferring risk for later cardiovascular and cardiometabolic diseases.
Keywords: adolescent, cardiometabolic, early adulthood, health disparities, menstruation
Subject Categories: Women, Primary Prevention, Cardiovascular Disease
Clinical Perspective.
What Is New?
In a sample comprising mostly Black American women, a single assessment of cycle irregularity in adolescence was associated with multiple measures of cardiometabolic health in early adulthood.
What Are the Clinical Implications?
Empirically derived early risk indicators that can be used to increase surveillance, engage in behavioral and lifestyle preventive interventions, and, if needed, introduce pharmacologic management may reduce the morbidity and mortality due to poor cardiovascular health that young Black women are currently facing.
Further research may help reveal causal pathways to racial disparities in Black women's cardiometabolic health.
Sex differences in multiple aspects of cardiovascular and cardiometabolic health are driving the call for research focusing on women. 1 , 2 , 3 Black American women have among the highest rates of hypertension in the world. 4 Diabetes, obesity, and inflammatory conditions, each of which increases the risk for cardiovascular disease, also are more prevalent among Black American women than White American women. 5 Furthermore, heart disease is the second leading cause of death for Black American women between the ages of 25 and 34 years. 6 Data collected between 2008 and 2017 from a nationally representative sample of the US population show a rapid decrease in the age of onset for stroke, hypertension, and hypercholesterolemia for women. 7
Behavioral health factors such as diet and weight, 8 , 9 and social determinants of health, such as financial strain and adverse experiences, 10 , 11 , 12 serve as general risks for cardiovascular and metabolic disease, but such factors have not increased the specificity of risk or prevention models for women in general, 13 , 14 or for Black women specifically. 15 Reproductive health factors, in contrast, may partially explain the observed sex differences in cardiometabolic health, and as such may serve as targets for risk assessment and preventive interventions. 3 Such factors include use of hormonal contraception, 16 miscarriage, 17 parity, 18 and menstrual cycle irregularities. 19 , 20 For example, in an Iranian population‐based sample of 18‐ to 49‐year‐old women followed for 15 years, cycle irregularity was associated with an increased risk for diabetes. 21 Cycle irregularity in Black and White US adolescents measured at ages 14 to 19 years was predictive of metabolic syndrome and type 2 diabetes at ages 20 to 28 years. 22 Menstrual cycle irregularities may be particularly useful for identifying risk early in life and before overt disease.
Among all groups of US women, Black women experience the highest rates of cardiovascular and cardiometabolic associated morbidity and mortality. 23 Rates of hypertension are highest among Black women compared with Latina and White women, and cardiovascular disease is one of the leading causes of death for Black women. 10 Racial disparities in cardiovascular disease–related mortality are now evident even among young adults. 24 The cardiovascular health crisis for Black women has persisted and worsened; research conducted for Black women is necessary to reverse the widening race gap in protecting women's heart health. 25
In the present study, we leveraged data from an ongoing community‐based longitudinal study, the Pittsburgh Girls Study, which included annual interviews of girls and their caregivers beginning in childhood. As part of an ancillary study on cardiometabolic health, cardiometabolic risk factors were measured in a subset of participants at ages 22 to 24 years. We tested prospective associations between the age of onset of menses, and irregular cycles at age 15 years and cardiometabolic health using continuous measures and dichotomous indices of pre‐disease. We focus on menstrual irregularities for 2 reasons. First, among reproductive health factors associated with cardiometabolic disease, it can be assessed early in development. Second, among many potential risk factors for cardiometabolic disease such as behavioral and social determinants of health, it can be assessed within existing clinical platforms with less subjectivity, bias, and burden.
Methods
Sample and Procedures
Details of the Pittsburgh Girls Study were previously published. 26 Briefly, the Pittsburgh Girls Study was initiated in 1999–2000 to study the development of behavioral and emotional problems among girls. All homes in neighborhoods in the City of Pittsburgh in which at least 25% of the families were living at or below the poverty level were contacted to determine whether the household contained an eligible girl (between the ages of 5 and 8 years), and a random selection of 50% of households in all other city neighborhoods were contacted. A total of 103 238 households were enumerated. Among 2992 eligible families, 85% agreed to participate, resulting in a total sample size of 2450.
A subsample of Pittsburgh Girls Study participants (n=352) were recruited for an ancillary study on cardiometabolic health in early adulthood (ages 22–25 years). Laboratory study visits typically occurred in the afternoon, during which blood pressure and waist circumference were measured. Within 2 weeks of the laboratory study visit, a fasting blood draw was completed during an in‐home study visit. Serum risk factors included a lipid panel, insulin, and glucose level. The study period did overlap with the COVID‐19 pandemic, and as a result study data are not complete for all participants: 87% have blood pressure readings, and 84% have measured waist circumference. Institutional Review Board approval of study procedures and measures was obtained. Written informed consent processes were conducted with caregivers and then directly with participants once they reached the age of 18 years. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Methods and Measures
Menarche Onset and Menstrual Cycle Irregularities
Menarche was assessed each year starting in the first year of the study, when girls were ages 5 to 8 years. Girls and their caregivers were asked if they had started having regular periods (3 consecutive monthly periods) and if so, the month and year that they reached menarche. Age at menarche was calculated using the reported data and the girls’ date of birth. Caregiver report was used if the girl's report was not available. Age of onset was treated as a continuous variable and was also dichotomized to denote early onset as before age 11 years. By age 15 years, 99% of the sample had begun menstruating. At age 15 years, girls self‐reported on regularity of cycle by indicating whether they had 27 to 29 days between each cycle (yes or no).
Blood Pressure and Waist Circumference
Resting blood pressure and waist circumference were measured in the laboratory. Three blood pressure readings were taken ≈15 minutes after arrival to the laboratory at 5‐minute intervals during quiet rest: these 3 readings of systolic and diastolic blood pressure were averaged. Waist circumference was measured at the level of the umbilicus by trained research assistants.
Blood Biomarkers
Morning fasting venous blood samples were collected in the home by trained field phlebotomists. Samples were returned to the laboratory and collection tubes were spun, aliquoted, and stored at −80°C until assayed. Frozen plasma samples were shipped on dry ice to the Clinical Laboratory Improvement Amendments‐certified Central Ligand Assay Satellite Services Laboratory at the University of Michigan. Glucose was determined by electroimmunoassay with a coefficient of variation of 2.1%. Total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, and triglycerides were determined by conventional enzymatic methods (coefficient of variation, 1.3%–2.1%). Insulin was measured by radioimmunoassay procedure (coefficient of variation, 2.6%).
Blood biomarker data were transformed using log10 to reduce skewness. Associations between age at menarche and cardiometabolic health indices were tested using Kendall's tau‐b, and t tests were used to compare differences in cardiometabolic health between participants as a function of early‐onset menarche and irregular menses. Levene's test was used to determine whether the assumption of equality of variances was met.
Cardiometabolic risk indices were based on the following cutoffs: systolic blood pressure >120 mm Hg, diastolic blood pressure >80 mm Hg, total cholesterol >200 mg/dL, high‐density lipoprotein cholesterol <50 mg/dL, low‐density lipoprotein cholesterol >100 mg/dL, triglycerides >150 mg/dL, glucose >99 mg/dL, and insulin >25 mIU/L. Cumulative cardiometabolic risk was assessed with a count of the total number of risk indices. Odds ratios were computed from univariate tests of differences between the dichotomous risk indices, and ordinal logistic regression was used to test whether cumulative number of risk indices differed as a function of early‐onset menarche and irregular menses; the proportional odds assumption was verified and satisfied.
Results
Descriptive Statistics
Among 352 participants in the ancillary study, 68.2% self‐identified as Black or Black and another race, 31.8% as White, and 2.3% as Latina or Hispanic. Close to one‐third of the families in the ancillary study (32%) had received public assistance when girls were between ages 8 and 15 years. Receipt of public assistance was associated with race: of the 106 families who received public assistance, 89 (84%) were families of girls who self‐identified as Black (χ2 [1]=19.83, P<0.001).
The average age at menarche was 12.05 years (SD=1.29). Fifty‐three participants (15.2%) reported early menarche (4 individuals were missing data for this variable). Black participants had an earlier age of onset of menses compared with White participants (11.75 versus 12.68 years, F [1, 320]=40.18, P<0.001) and were more likely to have early‐onset menarche (χ2 [1]=6.59, P<0.010). Receipt of public assistance was not associated with age at menarche or with early‐onset menarche.
At age 15 years, 298 participants provided data on cycle irregularity (28 were not interviewed that year, 17 did not know if their cycle was regular, and 9 did not answer the question); 48 (16.1%) participants reported irregular menstrual cycles. Age of menarche did not differ for participants with or without cycle irregularity (mean=12.20, SD=1.13; and mean=12.00, SD=1.12, respectively, P=0.178). There were no differences between Black and White participants in the rate of irregular menstrual cycles (χ2 [1]=2.26, P=0.136). Receipt of public assistance was not associated with cycle irregularity.
At ages 22 to 24 years, triglyceride levels were lower among Black versus White women (96.60 versus 115.42, P<0.001) and waist circumference was higher in Black compared with White women (95.07 cm versus 87.72 cm, P=0.003); no other comparisons were statistically significant.
Associations Between Age at Menarche and Cardiometabolic Health
When age of menarche was treated as a continuous variable, there was a positive association with high‐density lipoprotein cholesterol (Kendall's τ‐b=0.115, P<0.01) and a negative association with waist circumference (Kendall's τ‐b=0.175, P<0.001). No other significant associations were observed. Tests of early onset (age <11 years) compared with average or later onset of menarche (age ≥11 years) and cardiometabolic health using t tests yielded a single statistically significant difference: waist circumference was higher among girls with earlier onset (mean=38.62, SD=1.16) compared with girls with average to later onset (mean=36.05, SD=0.49) (t=2.03, P=0.022).
Associations Between Irregular Menses and Cardiometabolic Health
A more consistent pattern of results was observed when comparing indices of cardiometabolic health for women with and without menstrual cycle irregularity in adolescence. As shown in Table 1, most comparisons were statistically significant and indicated that irregular menstrual cycle was associated with higher levels of insulin (mean=31.36 mIU/L versus 21.04 mIU/L, P=0.015), glucose (110.79 versus 101.11, P=0.035), and triglycerides (115.92 versus 98.98, P=0.028), and higher resting systolic (114.19 mm Hg versus 110.29 mm Hg, P=0.026) and diastolic (75.49 mm Hg versus 72.01 mm Hg, P=0.005) blood pressure. A follow‐up multivariate analysis of variance including the 5 cardiometabolic indices that were significant in the univariate tests (insulin, glucose, triglycerides, and systolic and diastolic blood pressure) was consistent in demonstrating significant difference in all 5 indices as a function of menstrual cycle irregularity (Wilk's Λ=0.95, F [5, 252]=2.83, P=0.017, η=0.23).
Table 1.
Comparison of Cardiometabolic Health Indices in Early Adulthood (Ages 22–24 Years) by Menstrual Regularity at Age 15 Years
| Health index | Irregular menses | Regular menses | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | P level | |
| Insulin, mIU/L | 31.36 | 39.28 | 21.04 | 17.14 | 2.45 | 0.015* |
| Glucose, mg/dL | 110.79 | 47.55 | 101.11 | 26.64 | 2.12 | 0.035* |
| Triglycerides, mg/dL | 115.92 | 63.32 | 98.98 | 70.75 | 2.21 | 0.028* |
| Total cholesterol, mg/dL | 185.08 | 32.33 | 176.71 | 34.42 | 1.69 | 0.092 |
| HDL‐C, mg/dL | 67.77 | 17.05 | 71.43 | 17.90 | −1.38 | 0.169 |
| LDL‐C, mg/dL | 94.13 | 28.91 | 85.48 | 29.37 | 1.95 | 0.052 |
| Waist circumference, cm | 97.03 | 19.32 | 91.42 | 20.78 | 1.66 | 0.097 |
| Systolic blood pressure, mm Hg | 114.19 | 12.90 | 110.29 | 10.23 | 2.24 | 0.026* |
| Diastolic blood pressure, mm Hg | 75.49 | 9.27 | 72.01 | 7.16 | 2.84 | 0.005* |
HDL‐C indicates high‐density lipoprotein cholesterol; and LDL‐C, low‐density lipoprotein cholesterol. t tests were conducted with log10 transformations of biomarkers; for ease of interpretation, nontransformed values are reported in the Table.
P values are statistically significant at P<0.05; equality of variances was established using Levene's test.
Clinical indicators of cardiometabolic risk (eg, systolic blood pressure >120 mm Hg) were more likely to be present among women who reported irregular menses than women who had regular menses at age 15 years, including clinically high levels of triglycerides (odds ratio [OR], 2.56 [95% CI, 1.167–5.622], P=0.019), insulin (OR, 1.89 [95% CI, 1.002–3.548], P=0.032), and diastolic blood pressure (OR, 2.32 [95% CI, 1.075–5.004], P=0.032) (Table 2 and Figure 1).
Table 2.
Association of Menstrual Regularity at Age 15 Years and Clinical Indicators of Risk for Cardiometabolic Diseases in Early Adulthood (Ages 22–24 Years)
| Irregular | Regular | ||||||
|---|---|---|---|---|---|---|---|
| menses | menses | ||||||
| Health index | N | % | N | % | OR | 95% CI | P level |
| Insulin >25 mIU/L | 21 | 43.8 | 73 | 29.2 | 1.89 | 1.01–3.55 | 0.049* |
| Glucose >99 mg/dL | 29 | 60.4 | 117 | 46.8 | 1.74 | 0.92–3.26 | 0.086 |
| Triglycerides >150 mg/dL | 11 | 22.9 | 26 | 10.4 | 2.56 | 1.17–5.62 | 0.019* |
| Total cholesterol >200 mg/dL | 14 | 29.2 | 51 | 20.4 | 1.61 | 0.80–3.22 | 0.181 |
| HDL‐C <50 mg/dL | 7 | 14.6 | 22 | 8.8 | 1.90 | 0.99–3.65 | 0.054 |
| LDL‐C >100 mg/dL | 18 | 37.5 | 60 | 24.0 | 1.77 | 0.71–4.41 | 0.221 |
| Waist circumference >84 cm | 29 | 64.4 | 117 | 55.2 | 1.47 | 0.76–2.87 | 0.257 |
| Systolic BP >120 mm Hg | 11 | 23.9 | 33 | 15.1 | 1.76 | 0.81–3.81 | 0.150 |
| Diastolic BP >80 mm Hg | 12 | 26.1 | 29 | 13.3 | 2.30 | 1.07–4.95 | 0.033* |
Odds ratios (ORs) are from univariate tests. BP indicates blood pressure; HDL‐C, high‐density lipoprotein cholesterol; and LDL‐C, low‐density lipoprotein cholesterol.
P values are statistically significant at P<0.05.
Figure 1. Association between regularity of menstrual cycle at age 15 years and clinical indictors of risk for cardiometabolic disease.
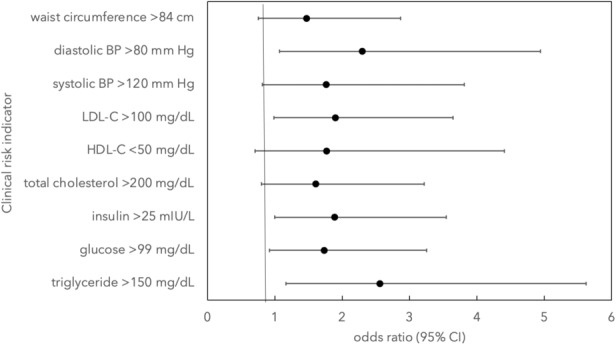
Odds ratios (OR) are from univariate tests. Comparison of women with and without regular menses at age 15 years yielded significant risk for clinically high levels of triglycerides (OR, 2.56 [95% CI, 1.167–5.622], P=0.019), insulin (OR, 1.89 [95% CI, 1.002–3.548], P=0.032), and diastolic blood pressure (OR, 2.32 [95% CI, 1.075–5.004], P=0.032). BP indicates blood pressure; HDL‐C, high‐density lipoprotein cholesterol; and LDL‐C, low‐density lipoprotein cholesterol.
A test of differences in the distributions of cumulative risk indices (0 through 5 or more) using ordinal logistic regression revealed that women who reported irregular menses in adolescence evidenced a significantly greater number of abnormal cardiometabolic risk factors in early adulthood (OR, 2.82 [96% CI, 1.62–4.91], P<0.001): This model met the proportional odds assumption. As shown in Figure 2, although having 1 or more risk factors is common for the sample, the burden of risk falls largely on the women reporting cycle irregularity in adolescence. For example, >40% of women reporting cycle regularity had 0 to 1 risk indicators in early adulthood, whereas <20% of women who reported cycle irregularity had 0 to 1 risk indicators.
Figure 2. Association between regularity of menstrual cycle at age 15 years and number of cardiometabolic risk indices.
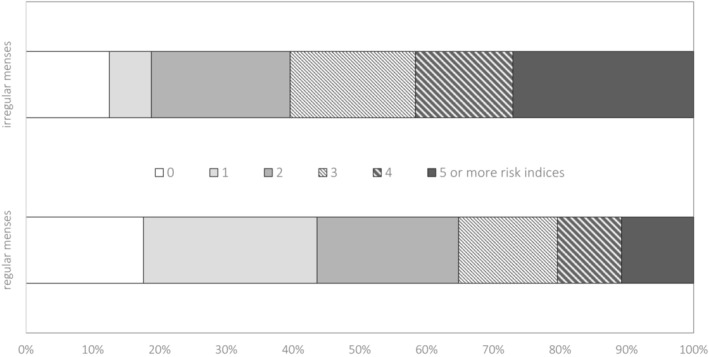
Comparison of women with and without regular menses at age 15 years and count of total number of the 9 measured clinical indicators of risk by ordinal logistic regression: odds ratio=2.82 (95% CI, 1.62–4.91), P<0.001.
Discussion
The present study adds to research conducted at the interface of reproductive and cardiometabolic health for women. Variability in menstruation onset, cycles, and duration of phases has only recently been leveraged to explore risk for health outcomes and generate causal hypotheses linking ovarian hormones to health and disease. The data from the present study add to this literature, 13 , 14 , 15 , 16 , 17 and extend the findings by demonstrating prospective associations between an early, easily measured indicator of menstrual cycle irregularity and risk to cardiometabolic health in early adulthood.
Using prospectively collected data from a representative, mostly Black cohort of young women from the city of Pittsburgh, age at menarche had limited impact on later health, but menstrual cycle irregularity in adolescence was associated with multiple objective biomarkers of cardiometabolic health in early adulthood using both continuous measures and clinical indicators of pre‐disease. There were no race differences in reported rates of cycle irregularity, and few race differences in measured biomarkers; levels of triglycerides were lower and waist circumference was greater for Black compared with White women. Given that our sample comprised mostly Black women, and that the incidence of, and morbidity and mortality resulting from cardiometabolic disease are higher for Black American women compared with White women, we view our results as potentially relevant to reducing the racial disparities in women's cardiovascular and cardiometabolic health. Empirically derived early risk indicators that can be used to increase surveillance, engage in behavioral and lifestyle preventive interventions, and, if needed, introduce pharmacologic management may reduce the morbidity and mortality due to poor cardiovascular health that young Black women are currently facing.
A plausible interpretation of the present findings is that menstrual irregularity at age 15 years was symptomatic of a syndrome associated with risk for later cardiac and metabolic disease, such as polycystic ovarian syndrome. 27 , 28 Our measure of cycle irregularity did not specify whether cycles occurred over short or long durations; polycystic ovarian syndrome is characterized by oligomenorrhea, or infrequent periods, nor did we have data from medical records regarding polycystic ovarian syndrome or other medical problems, but this is a goal for future work. Distribution of adipose tissue may play a role in both menstrual cycle irregularity and cardiometabolic health. In some studies, higher body mass index is associated with earlier age at menarche, 29 , 30 but no studies have shown associations between body mass index and menstrual cycle irregularity in women living in the Unites States, to the best of our knowledge. Moreover, we note that the reliability of body mass index as a measure of adiposity and as a marker of risk for adiposity‐related health problems for Black populations is questionable. 31 , 32 , 33 Thus, future research on the interface between reproductive and cardiometabolic health, including adiposity, should include other anthropometric (eg, skinfold thickness) or scanning (eg, dual x‐ray absorptiometry) measures.
The observed association between menstrual cycle irregularity and cardiometabolic health may fall within a longer causal chain linking risk factors to broad dimensions of health. Results from studies that include measures of exposure to structural, systemic, and interpersonal racism and health indices, for example, have shown impacts on multiple systems including vascular, metabolic, immune, endocrine, and hormonal systems. 34 Stress and behavioral health such as nutrition, sleep, and physical activity are also associated with disruptions in menstruation. 35 The likelihood that menstrual cycle dysregulation is impacted by racism‐related stressors does not negate its usefulness as an early indicator of risk for Black women. Most Black women do not develop cardiometabolic diseases, but when they do the morbidity and mortality is high. Reliable, early indicators of risk will be useful in preventing disease.
We acknowledge that direction of effect cannot be established within our study design. We do not have measures of cardiometabolic health, such as glucose levels and blood pressure, in adolescence. Objective measures of cardiometabolic health assessed as early as age 11 years have been associated with later metabolic syndrome in early adulthood; 36 thus, it is possible that alterations or disruptions in cardiometabolic systems preceded the disruptions in menstrual cycles that we observed at age 15 years. Testing the relative utility of menstrual cycle irregularities in the context of other health indices such as blood pressure, blood sugars, as well as behavioral and social determinants of health throughout adolescence is needed to fully articulate the developmental unfolding of potentially interrelated risk factors.
We note several limitations to the present study. First, we focused on a single time point for the assessment of cardiometabolic biomarkers due to the availability of data. Although the blood draws were obtained after an overnight fast, the participants' sleep, exercise, and diet in the past 24 hours could have impacted blood levels of cardiometabolic biomarkers, and repeated assessments over several months may have yielded stronger measures of cardiometabolic health. The fact that differences in resting blood pressure, measured in a controlled laboratory environment, also were observed between women with and without cycle regularity in adolescence provides some greater confidence that significant differences in blood biomarkers were meaningful. Second, we used a single question about length of cycle to define menstrual irregularity and did not validate the girls' self‐reports. Third, given the small representation of White women and an even smaller number of Latina women, our results cannot be generalized to groups other than Black American women.
Despite these limitations, the observed association between a single assessment of cycle irregularity in adolescence and multiple measures of cardiometabolic health in early adulthood in a sample comprising mostly Black American women is compelling. Assessing menstrual cycle irregularity is an easily scalable tool that can be deployed in multiple health care settings to initiate further and repeated screening, and with further research may help reveal causal pathways to racial disparities in Black women's cardiometabolic health.
Sources of Funding
National Institutes of Health grants R01 MH56630, HL157787, and R01 HL137246 supported the work presented in this article.
Disclosures
None.
Acknowledgments
The authors thank the caregivers and girls of the Pittsburgh Girls Study and the young women who participated in the PGS‐HEArT Substudy.
This manuscript was sent to Tiffany M. Powell‐Wiley, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 7.
References
- 1. Siokatas G, Papatheodorou I, Daiou A, Lazou A, Hatzistergos KE, Kararigas G. Sex‐related effects on cardiac development and disease. J Cardiovasc Develop Disease. 2022;9:90. doi: 10.3390/jcdd9030090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cho L, Davis M, Elgendy I, Epps K, Lindley KJ, Mehta PK, Michos ED, Minissian M, Pepine C, Viola Vaccarino V, et al. Summary of updated recommendations for primary prevention of cardiovascular disease in women: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75:2602–2618. doi: 10.1016/j.jacc.2020.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elder P, Sharma G, Gulati M, Michos ED. Identification of female‐specific risk enhancers throughout the lifespan of women to improve cardiovascular disease prevention. Am J Prevent Cardiol. 2020;2:100028. doi: 10.1016/j.ajpc.2020.100028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr, Willis M, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–e423. doi: 10.1161/CIR.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 5. Cardel MI, Guo Y, Sims M, Dulin A, Miller D, Chi X, Pavela G, DeBoer MD, Gurka MJ. Objective and subjective socioeconomic status associated with metabolic syndrome severity among African American adults in Jackson Heart Study. Psychoneuroendocrinology. 2020;117:104686. doi: 10.1016/j.psyneuen.2020.104686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep. 2019;68:1–77. [PubMed] [Google Scholar]
- 7. Okunrintemi V, Tibuakuu M, Virani SS, Sperling LS, Volgman AS, Gulati M, Cho L, Leucker TM, Blumenthal RS, Michos ED. Sex differences in the age of diagnosis for cardiovascular disease and its risk factors among US adults: trends from 2008 to 2017, the Medical Expenditure Panel Survey. J Am Heart Assoc. 2020;9:e018764. doi: 10.1161/JAHA.120.018764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang X, Zhu J, Kim JH, Sumerlin TS, Feng Q, Yu J. Metabolic health and adiposity transitions and risks of type 2 diabetes and cardiovascular diseases: a systematic review and meta‐analysis. Diabetol Metab Syndr. 2023;15:60. doi: 10.1186/s13098-023-01025-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calcaterra V, Cena H, Magenes VC, Vincenti A, Comola G, Beretta A, Di Napoli I, Zuccotti G. Sugar‐sweetened beverages and metabolic risk in children and adolescents with obesity: a narrative review. Nutrients. 2023;15:702. doi: 10.3390/nu15030702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wickrama KA, Lee TK, O'Neal CW. Stressful life experiences in adolescence and cardiometabolic risk factors in young adulthood. J Adolesc Health. 2015;56:456–463. doi: 10.1016/j.jadohealth.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 11. Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–2178. doi: 10.1161/CIRCULATIONAHA.117.029652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doom JR, Mason SM, Suglia SF, Clark CJ. Pathways between childhood/adolescent adversity, adolescent socioeconomic status, and long‐term cardiovascular disease risk in young adulthood. Soc Sci Med. 2017;188:166–175. doi: 10.1016/j.socscimed.2017.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goossens GH, Jocken JWE, Blaak EE. Sexual dimorphism in cardiometabolic health: the role of adipose tissue, muscle, and liver. Nat Rev Endocrinol. 2021;17:47–66. doi: 10.1038/s41574-020-00431-8 [DOI] [PubMed] [Google Scholar]
- 14. Adam TC, Drummen M, Macdonald I, Jalo E, Siig‐Vestentoft P, Martinez JA, Handjiev‐Darlenska T, Brand‐Miller J, Poppitt S, Stratton G, et al. Association of psychobehavioral variables with HOMA‐IR and BMI differs for men and women with prediabetes in the PREVIEW lifestyle intervention. Diabetes Care. 2021;44:1491–1498. doi: 10.2337/dc21-0059 [DOI] [PubMed] [Google Scholar]
- 15. Cordola Hsu AR, Ames SL, Xie B, Peterson DV, Garcia L, Going SB, Wong ND, Anton‐Culver H. Sociodemographic and metabolic risk characteristics associated with metabolic weight categories in the Women's Health Initiative. Cardiovasc Endocrinol Metab. 2020;9:42–48. doi: 10.1097/XCE.0000000000000194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roach RE, Helmerhorst FM, Lijfering WM, Stijnen T, Algra A, Dekkers OM. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015;2015:CD011054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang ET, Cirillo PM, Vittinghoff E, Bibbins‐Domingo K, Cohn BA, Cedars MI. Menstrual irregularity and cardiovascular mortality. J Clin Endocrinol Metab. 2011;9:e114–e118. doi: 10.1210/jc.2010-1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ogunmoroti O, Osibogun O, Kolade OB, Ying W, Sharma G, Vaidya D, Michos ED. Multiparity is associated with poorer cardiovascular health among women from the Multi‐Ethnic Study of Atherosclerosis. Am J Obstet Gynecol. 2019;221:631.e1–631.e16. doi: 10.1016/j.ajog.2019.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kiconco S, Teede HJ, Earnest A, Loxton D, Joham AE. Menstrual cycle regularity as a predictor for heart disease and diabetes: findings from a large population‐based longitudinal cohort study. Clin Endocrinol. 2022;96:605–616. doi: 10.1111/cen.14640 [DOI] [PubMed] [Google Scholar]
- 20. Lee JJ, Cook‐Wiens G, Johnson BD, Braunstein GD, Berga SL, Stanczyk FZ, Pepine CJ, Bairey Merz CN, Shufelt CL. Age at menarche and risk of cardiovascular disease outcomes: findings from the National Heart Lung and Blood Institute‐sponsored Women's Ischemia Syndrome Evaluation. J Am Heart Assoc. 2019;8:e012406. doi: 10.1161/JAHA.119.012406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rostami Dovom M, Ramezani Tehrani F, Djalalinia S, Cheraghi L, Behboudi Gandavani S, Azizi F. Menstrual cycle irregularity and metabolic disorders: a population‐based prospective study. PLoS One. 2016;11:e0168402. doi: 10.1371/journal.pone.0168402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glueck CJ, Woo JG, Khoury PR, Morrison JA, Daniels SR, Wang P. Adolescent oligomenorrhea (age 14‐19) tracks into the third decade of life (age 20‐28) and predicts increased cardiovascular risk factors and metabolic syndrome. Metabolism. 2015;64:539–553. doi: 10.1016/j.metabol.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 23. Ford ND, Robbins CL, Hayes DK, Ko JY, Loustalot F. Prevalence, treatment, and control of hypertension among US women of reproductive age by race/Hispanic origin. Am J Hypertens. 2022;35:723–730. doi: 10.1093/ajh/hpac053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 25. Wenger NK, Lloyd‐Jones DM, Elkind MSV, Fonarow GC, Warner JJ, Alger HM, Cheng S, Kinzy C, Hall JL, Roger VL, et al. Call to action for cardiovascular disease in women: epidemiology, awareness, access, and delivery of equitable health care: a presidential advisory from the American Heart Association. Circulation. 2022;145:e1059–e1071. doi: 10.1161/CIR.0000000000001071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keenan K, Hipwell A, Chung T, Stepp S, Stouthamer‐Loeber M, Loeber R, McTigue K. The Pittsburgh Girls Study: overview and initial findings. J Clin Child Adolesc Psychol. 2010;39:506–521. doi: 10.1080/15374416.2010.486320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends Cardiovasc Med. 2020;30:399–404. doi: 10.1016/j.tcm.2019.08.010 [DOI] [PubMed] [Google Scholar]
- 28. Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132:321–336. doi: 10.1097/AOG.0000000000002698 [DOI] [PubMed] [Google Scholar]
- 29. Reagan PB, Salsberry PJ, Fang MZ, Gardner WP, Pajer K. African‐American/white differences in the age of menarche: accounting for the difference. Soc Sci Med. 2012;75:1263–1270. doi: 10.1016/j.socscimed.2012.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schuh SM, Kadie J, Rosen MP, Sternfeld B, Reijo Pera RA, Cedars MI. Links between age at menarche, antral follicle count, and body mass index in African American and European American women. Fertil Steril. 2019;111:122–131. doi: 10.1016/j.fertnstert.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 31. Banack HR, Bea JW, Chen Z, Blew RM, Nicholas S, Stefanick M, Wild RA, Manson JE, Odegaard AO. Longitudinal patterns of abdominal visceral and subcutaneous adipose tissue, total body composition, and anthropometric measures in postmenopausal women: results from the Women's Health Initiative. Int J Obes. 2023;47:288–296. doi: 10.1038/s41366-023-01266-9 [DOI] [PubMed] [Google Scholar]
- 32. Weaver RG, Beets MW, Brazendale K, Hunt E. Disparities by household income and race/ethnicity: the utility of BMI for surveilling excess adiposity in children. Ethn Health. 2021;26:1180–1195. doi: 10.1080/13557858.2019.1591349 [DOI] [PubMed] [Google Scholar]
- 33. Williams CY, Wylie A, Ghobrial V, Coe CL, Short SJ. Racial differences in the associations between adiposity, placental growth hormone and inflammatory cytokines in pregnant women. Front Endocrinol (Lausanne). 2023;14:1100724. doi: 10.3389/fendo.2023.1100724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller HN, LaFave S, Marineau L, Stephens J, Thorpe RJ Jr. The impact of discrimination on allostatic load in adults: an integrative review of literature. J Psychosom Res. 2021;146:110434. doi: 10.1016/j.jpsychores.2021.110434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ryterska K, Kordek A, Załęska P. Has menstruation disappeared? Functional hypothalamic amenorrhea‐what is this story about? Nutrients. 2021;13:2827. doi: 10.3390/nu13082827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ojanen X, Cheng R, Törmäkangas T, Rappaport N, Wilmanski T, Wu N, Fung E, Nedelec R, Sebert S, Vlachopoulos D, et al. Towards early risk biomarkers: serum metabolic signature in childhood predicts cardio‐metabolic risk in adulthood. EBioMedicine. 2021;72:103611. doi: 10.1016/j.ebiom.2021.103611 [DOI] [PMC free article] [PubMed] [Google Scholar]


