Abstract
Background
There is limited evidence on the potential negative metabolic health impacts of prolonged and uninterrupted sedentary bouts in structurally disadvantaged youth. This study investigated associations between sedentary bout variables and metabolic health markers in the Hispanic Community Health Study/SOL Youth (Study of Latino Youth).
Methods and Results
SOL Youth was a population‐based cohort of 1466 youth (age range, 8–16 years; 48.5% female); 957 youth were included in the analytic sample based on complete data. Accelerometers measured moderate‐to‐vigorous physical activity (MVPA), total sedentary time, and sedentary bout patterns (daily time spent in sedentary bouts ≥30 minutes, median sedentary bout duration, and number of daily breaks from sedentary time). Clinical measures included body mass index, waist circumference, fasting glucose, glycated hemoglobin, fasting insulin, and the homeostasis model assessment of insulin resistance. After adjusting for sociodemographics, total sedentary time, and MVPA, longer median bout durations and fewer sedentary breaks were associated with a greater body mass index percentile (bbouts=0.09 and bbreaks=−0.18), waist circumference (bbouts=0.12 and bbreaks=−0.20), and fasting insulin (bbouts=0.09 and bbreaks=−0.21). Fewer breaks were also associated with a greater homeostasis model assessment of insulin resistance (b=−0.21). More time in bouts lasting ≥30 minutes was associated with a greater fasting glucose (b=0.18) and glycated hemoglobin (b=0.19).
Conclusions
Greater accumulation of sedentary time in prolonged and uninterrupted bouts had adverse associations with adiposity and glycemic control over and above total sedentary time and MVPA. Findings suggest interventions in Hispanic/Latino youth targeting both ends of the activity spectrum (more MVPA and less prolonged/uninterrupted sedentary patterns) may provide greater health benefits than those targeting only MVPA.
Keywords: children, glucose, insulin, obesity, physical activity
Subject Categories: Lifestyle
Nonstandard Abbreviations and Acronyms
- HCHS/SOL
Hispanic Community Health Study/Study of Latinos
- HOMA‐IR
Homeostatic Model Assessment for Insulin Resistance
- MVPA
moderate‐to‐vigorous physical activity
- SOL Youth
Study of Latino Youth
Clinical Perspective.
What Is New?
Long periods of uninterrupted sedentary time had detrimental associations with adiposity and glycemic control in Hispanic/Latino youth.
These associations were observed after accounting for total sedentary time and moderate‐to‐vigorous physical activity.
What Are the Clinical Implications?
Breaking up long periods of sedentary time may be an important intervention target in addition to increasing moderate to vigorous physical activity in Hispanic/Latino youth.
Strategies for breaking up prolonged periods of sedentary time should include an increase in breaks from sitting and a reduction in the amount of time spent sitting for >30 minutes.
Evidence suggests high amounts of sedentary time are detrimentally associated with metabolic health in youth. 1 However, while these associations have been consistently observed for reported sedentary and screen time, many studies investigating device (eg, accelerometer) measured sedentary time have failed to find such associations. 1 , 2 , 3 , 4 , 5 This inconsistency in evidence between reported and device measures has impeded the development of clear pediatric health guidelines around sedentary time. In adults, the link between device measured sedentary time and metabolic health is better established, including when accounting for differences in moderate‐to‐vigorous physical activity (MVPA). 6 , 7 , 8 Research has also shown activity patterns in childhood can carry into adulthood, 9 , 10 , 11 suggesting lifelong metabolic health risks may be increased.
The lack of consistent associations between device measured sedentary time and metabolic health in youth may be in part attributable to the sedentary time variables that have been investigated. The accumulation of sedentary time in prolonged/uninterrupted (eg, periods of sedentary time lasting >20 or 30 minutes) as opposed to brief and interrupted bouts, often referred to as “sedentary bout patterns,” has emerged as an important risk factor for poor metabolic health in adults. 12 , 13 Some studies have even shown effect modification whereby deleterious associations between the total volume of sedentary time and metabolic health are stronger among those whose sedentary time is accumulated in more prolonged/uninterrupted bouts. 14 , 15 Laboratory studies comparing prolonged sedentary bouts and shortened sedentary bouts with the same volume of total sedentary time have found that shortened bouts are beneficial for maintenance of metabolic health. 16 , 17 , 18 These effects have been attributed to brief muscle contractions associated with breaking up continuous sedentary bouts putatively improving blood flow and improving glucose homeostasis.
Despite this evidence in adults, relatively few studies in youth have investigated sedentary bout patterns versus total sedentary time. 3 , 19 While existing pattern‐focused studies in youth have failed to show consistent associations with health markers, many have operationalized sedentary bout patterns with limited variables, often investigating only breaks in sedentary time. Variables that quantify the duration and frequency of sedentary bouts (eg, time spent in long bouts) warrant more investigation to advance understanding on the clinical relevance of sedentary bout patterns. 20 Many studies of sedentary bout patterns in youth have also failed to account for total sedentary time or MVPA. Accounting for these factors is important for disentangling the potential role of sedentary bout patterns from that of total sedentary time and MVPA in relation to metabolic health.
In addition to these gaps in the literature, most studies have focused on adiposity measures, and there is a need for more studies investigating glycemic markers, which are critical for understanding diabetes risk. Furthermore, no studies have included large population‐based samples of Hispanic/Latino youth. Sedentary health research is critical among Hispanic/Latino individuals, as they encompass the largest racial and ethnic group in the United States after non‐Hispanic White 21 and experience high rates of metabolic disease such as obesity and diabetes because of structural health barriers. 22 , 23 , 24 , 25 Hispanic/Latino youth have been shown to engage in high amounts of prolonged and uninterrupted sedentary time. 26 Device measured total sedentary time has been investigated in the Hispanic Community Health Study/SOL Youth (Study of Latinos Youth) cohort and was not associated with body mass index (BMI), waist circumference, or glycemic markers after adjusting for differences in MVPA. 27 Given prior evidence on the link between prolonged/uninterrupted bout patterns and metabolic health in adults, an important next step is to investigate these associations in SOL Youth.
The purpose of the present study was to investigate associations of sedentary bout pattern variables with metabolic health markers in SOL Youth. It was hypothesized that more prolonged and uninterrupted sedentary bout patterns would be deleteriously associated with adiposity and glycemic control beyond measures of total sedentary time and MVPA, which would improve understanding of the importance of limiting time in prolonged/uninterrupted sedentary bouts relative to limiting total sedentary time to support metabolic health among youth.
Methods
Study Population and Sampling Design
The HCHS/SOL (Hispanic Community Health Study/Study of Latinos) enrolled a population‐based cohort of Hispanic/Latino adults from 2008 to 2011 (N=16 415, ages 18–74 years) in Chicago, IL; Miami, FL; Bronx, NY; and San Diego, CA. Each site used a 2‐stage area probability sample of households within census block groups across 4 strata based on Hispanic/Latino concentration and socioeconomic status. 28 , 29 Sampling weights were calculated to account for disproportionate sampling of population subgroups. The current analyses used data from SOL Youth, an ancillary study that enrolled 1466 youth (8 to 16 years old, 2012–2014) whose parents/caregivers were participants in HCHS/SOL. 30 , 31 The study was conducted with approval from the institutional review boards at all participating institutions. Written informed consent and assent were obtained from parents/caregivers and their children, respectively. The data that support the findings of this study are available from the HCHS/SOL Publications Committee and corresponding author upon reasonable request.
Measures
Device Sedentary Time and Bout Patterns
Youth were requested to wear an Actical accelerometer (198–0200‐03; Respironics Co. Inc, Bend, OR) on the hip for 7 days during waking hours. 32 The Choi algorithm 33 was used to remove nonwear time. Valid days were those with ≥8 hours and ≤16 hours of wear time. 34 The upper limit was used because some participants wore the device overnight, and in‐ and out‐of‐bed information was not collected. Those who did not wear the accelerometer for ≥1 weekday and ≥1 weekend day were excluded. Sedentary time was defined using a cut point of <72 counts applied to 60‐second epochs. 32 , 35 , 36 MVPA was defined using a cut point of ≥441 counts applied to 15‐second epochs. 32
Sedentary bouts were defined as periods of sedentary time lasting ≥1 minute, and a break in sedentary time was defined as any time a sedentary minute was followed by a nonsedentary minute (no allowance for interruptions, ie, no tolerance). Sedentary bout pattern variables included time in sedentary bouts lasting ≥30 minutes (min/day), the median duration of all sedentary bouts (minutes), and the number of daily breaks in sedentary time (Table 1). These 3 variables were selected based on a correlational analysis comparing 7 total variables, which also included mean bout duration, usual bout duration, alpha, and the fragmentation index. 37 , 38 , 39 The 3 variables had the lowest intervariable correlations and generally had the lowest correlations with total sedentary time, thus capturing relatively distinct aspects of the sedentary bout pattern. Sedentary time was divided by total wear time to derive the percentage of wear time spent sedentary (hereafter referred to as total sedentary time). Data for each variable were aggregated to the participant level by computing the weighted average daily value, defined as ([mean value across valid weekdays×5]+[mean value across valid weekend days×2])÷7.
Table 1.
Descriptions and Characteristics of Sedentary Bout Pattern Variables Explored for Inclusion in the Present Study, SOL Youth
| Variable name (units) | Description | Range based on the 5th and 95th percentile | Correlation* with other pattern variables | Correlation* with total sedentary time† |
|---|---|---|---|---|
| Included variables | ||||
| Time in sedentary bouts ≥30 min (min/day) | The amount of time (min/day) the youth spent in sedentary bouts lasting ≥30 min without interruption. This variable reflects the right tail of the bout distribution‡, showing time spent in long bouts, and is not necessarily reflective of the person's typical bout duration or frequency of sedentary breaks. | 35.3–327.4 | r=0.63 with median bout duration; r=−0.62 with sedentary breaks | r=0.82 |
| Median sedentary bout duration (min) | A central tendency measure of the bout distribution used to reflect the typical duration (minutes/bout) of the youth's sedentary bouts. The median may be more appropriate than the mean because the bout distribution is right‐skewed‡, and thus the median is less impacted by the duration of long bouts, resulting in a lower correlation with time in bouts ≥30‐min. | 2.0–5.9 | r=0.63 with time in bouts ≥30 min; r=−0.55 with sedentary breaks | r=0.63 |
| Sedentary breaks (breaks/d) | A frequency measure that reflects how often (n/day) the youth ended a sedentary bout, equivalent to the total number of daily sedentary bouts. Because sedentary breaks occur for all bouts across the bout distribution‡ (regardless of the bout duration), this variable is less reflective of the amount of time the youth spent in sedentary bouts that lasted ≥30 min or the youth's typical bout duration because it does not consider when the breaks occurred (ie, the extent to which the breaks interrupted longer versus shorter bouts). | 46.4–95.7 | r=−0.62 with time in bouts ≥30 min; r=−0.55 with median bout duration | r=−0.56 |
| Excluded variables | ||||
| Mean sedentary bout duration (min) | A central tendency measure of the bout distribution used to reflect the typical duration (in minutes) of the youth's sedentary bouts. Because the bout distribution is most commonly right‐skewed‡, the mean may not be the most appropriate measure of central tendency, though it is often included in sedentary pattern research. | 4.5–12.8 | r=0.78 with time in bouts ≥30 min; r=0.92 with median bout duration; r=−0.66 with sedentary breaks | r=0.70 |
| Usual sedentary bout duration 37 (min) | The bout duration (in min) at which 50% of all sedentary time was accumulated, with greater values reflecting a higher tendency toward longer bouts. Usual bout duration is more impacted by time spent in long bouts (ie, the right tail of the bout distribution‡) than measures of central tendency and thus can be highly correlated with the amount of time the youth spent in sedentary bouts that lasted ≥30 min. | 7.7–26.9 | r=0.94 with time in bouts ≥30 min; r=0.71 with median bout duration; r=−0.74 with sedentary breaks | r=0.81 |
| Alpha 38 | Indicates the slope of the youth's bout distribution based on a power law function‡, with lower values reflect more time in prolonged bout lengths. Alpha is unitless and can be difficult to interpret. Alpha is most correlated with the amount of time the youth spent in sedentary bouts that lasted ≥30 min and has a similar correlation with usual bout duration. | 1.6–2.1 | r=−0.75 with time in bouts ≥30 min; r=−0.61 with median bout duration; r=0.63 with sedentary breaks | r=−0.90 |
| Fragmentation index 39 | Also known as break rate, calculated as number of sedentary breaks divided by total hours of sedentary time and thus is interdependent (and often highly correlated) with sedentary breaks and total sedentary time. | 4.9–13.9 | r=−0.86 with time in bouts ≥30 min; r=−0.63 with median bout duration; r=0.71 with sedentary breaks | r=−0.91 |
SOL Youth indicates Study of Latino Youth.
Values reflect Pearson correlation coefficients.
Total sedentary reflected the percent of wear time spent sedentary.
A histogram of an individual's sedentary bout distribution, with bout duration plotted on the x axis and number of bouts (bout density) plotted on the y axis, is most commonly right‐skewed, having the shape of a power law distribution. 38
Metabolic Health Markers
The adiposity measures included body mass index (BMI) percentiles based on age and sex, 40 and waist circumference calculated as the average of 3 measurements conducted by trained staff. Height and weight were measured using a wall‐mounted stadiometer (SECA 222, Germany) and digital scale (Tanita Body Composition Analyzer, TBF 300, Japan). The glucose measures included fasting glucose and glycated hemoglobin (HbA1c), and the insulin measures included fasting insulin and the Homeostatic Model Assessment for Insulin Resistance (HOMA‐IR), 41 which have been described previously. 22 , 27 All blood specimens were collected in the morning under fasting conditions and processed at HCHS/SOL Central Laboratory.
Sociodemographic and Other Individual Characteristics
Sociodemographic characteristics were provided by parents/caregivers and included age, sex, place of birth (born in the 50 US states or DC, y/n), Hispanic/Latino background (non‐Mexican or Mexican heritage), annual household income (≤$20K, >$20–$40K, or >$40K), and parent/caregiver's highest level of education (no high school diploma or General Educational Development, at most high school diploma or General Educational Development, or greater than high school diploma or General Educational Development). Parents/caregivers also responded to the Pubertal Development Scale, 42 which was mapped to the Tanner stages based on both adrenal and gonadal scores. 43
Statistical Analysis
All analyses were performed in R 44 using package survey 45 to account for the complex sampling design including sampling weights, stratification (crossing of high/low Hispanic/Latino concentration versus high/low socioeconomic status), and clustering (US census block groups). Descriptive statistics were calculated to summarize the participant and population characteristics, and Pearson correlation coefficients were used to evaluate associations between each pair of independent (sedentary pattern) variables (ie, with one another) and between each pair of dependent (health marker) variables. The primary analyses involved testing associations of each sedentary pattern variable with each metabolic health marker in separate linear regression models adjusting for covariates. Although the present research focused on sedentary bout patterns rather than total sedentary time, associations between total sedentary time and each metabolic health marker are also presented or referenced.
The analyses for the association between each sedentary pattern variable and each metabolic health marker involved 5 models. Model 1 adjusted for age, sex, place of birth, Hispanic/Latino background, household income, parent/caregiver education, Tanner stage, site/city, min/day of accelerometer wear time, number of wear days, and proportion of wear days that were weekdays. Model 2 also adjusted for total sedentary time (percent of wear time spent sedentary), and Model 3 also adjusted for MVPA (min/day). Models 4 and 5 included the same covariates as Model 3 but tested effect modification using multiplicative interactions (sedentary pattern variable x total sedentary time in Model 4 and sedentary pattern variable x MVPA in Model 5). The purpose of these interaction tests was to explore whether sedentary pattern – health associations differed across low and high levels of total sedentary time or MVPA, as has been observed in some prior research. 46 Two types of regression coefficients were calculated for each model. The first, b1, was based on dependent and independent variables that were standardized as z‐scores. Benchmarks for interpreting the magnitude of these coefficients were small (b1=0.10), small‐to‐moderate (b1=0.20), and moderate (b1=0.30). 47 The second, b2, reflected the difference in the dependent (health marker) variable, using its raw/original unit, for every 1 SD difference in the independent (sedentary pattern) variable (ie, using a z‐score). Primary emphasis was placed on interpretation of the Model 3 (rather than Model 1 or Model 2) associations that were over and above total sedentary time and MVPA. Significance was interpreted as P<0.05 or the weighted 95% CI not spanning 0, except that a more conservative value of P<0.01 was used to interpret the regression coefficients for the interaction terms to minimize Type I error because of the number of tests.
Of the 1466 SOL Youth participants, 222 were excluded because they did not have any valid days of accelerometer wear time, and 287 were excluded because they did not have ≥1 valid weekday and ≥1 valid weekend day, for a final analytic sample of 957. To account for missing data because of nonadherence to the accelerometer protocol, inverse probability weights were calculated based on the sociodemographic and individual characteristics mentioned in the measures section. 48 , 49 The final weight was a product of the inverse probability weight and sampling weight. Imputation was used to account for missing data on covariates. Household income, parent/caregiver education, and place of birth were missing for 4.6% of all participants and imputed based on other variables when possible or using the sample mode. Missing Tanner stage values were computed using the gonadal score only (n=10) or the adrenal score only (n=163), or imputed using a regression model comprising age, sex, weight percentile, and BMI percentile (n=105). The blood measures (dependent variables) were missing for 48 to 55 individuals in the analytic sample, and values were left as missing. As a sensitivity analysis, we repeated Models 1 to 3 detailed above using an informed missingness approach. This involved imputing the sample mean when accelerometer, adiposity, glucose, or insulin values were missing. These models used the full sample of 1466 participants with the original sampling weights and additionally adjusted for whether the participant had missing values for any of the included variables (yes/no).
Results
The participant and population characteristics are presented in Table 2. Results not shown were as follows: Associations between insulin and adiposity measures were stronger (r=0.43–0.59) than associations between the glucose and adiposity measures (r=0.10–0.11) and associations between the glucose and insulin measures (r=0.14–0.36). When adjusted for MVPA and the Model 1 covariates, total sedentary time did not have a statistically significant or meaningful association with BMI percentile (b1=−0.08; 95% CI, −0.21 to 0.05) or waist circumference (b1=−0.03; 95% CI, −0.15 to 0.08), or with any of the other metabolic health markers as reported previously. 27
Table 2.
Cohort and Population Characteristics, SOL Youth (n=957)
| n | Unweighted mean or % | Unweighted SD | Weighted mean or % | Weighted SE | |
|---|---|---|---|---|---|
| Field center (site) | |||||
| Bronx | 248 | 25.9% | … | 33.5% | 2.6% |
| Chicago | 279 | 29.2% | … | 17.3% | 1.8% |
| Miami | 180 | 18.8% | … | 14.2% | 1.7% |
| San Diego | 250 | 26.1% | … | 35.0% | 3.2% |
| Sociodemographic | |||||
| Age, y | 957 | 11.8 | 2.5 | 12.08 | 0.13 |
| Tanner stage | 957 | 3.6 | 1.3 | 3.67 | 0.06 |
| Sex | |||||
| Female | 498 | 52.0% | … | 48.5% | 2.5% |
| Male | 459 | 48.0% | … | 51.5% | 2.5% |
| Place of birth | |||||
| Not US‐born | 228 | 23.8% | … | 21.0% | 2.0% |
| US‐born | 729 | 76.2% | … | 79.0% | 2.0% |
| Hispanic background | |||||
| Non‐Mexican | 479 | 50.1% | … | 48.8% | 3.0% |
| Mexican | 478 | 49.9% | … | 51.2% | 3.0% |
| Parent income | |||||
| ≤$20k | 508 | 53.1% | … | 52.4% | 3.0% |
| >$20k–$40k | 306 | 32.0% | … | 32.2% | 2.9% |
| >$40k | 143 | 14.9% | … | 15.4% | 2.0% |
| Parent education | |||||
| <High school | 373 | 39.0% | … | 38.2% | 2.9% |
| High school or equivalent | 261 | 27.3% | … | 30.2% | 3.0% |
| >High school | 323 | 33.8% | … | 31.6% | 2.5% |
| Accelerometer covariates | |||||
| No. of wear days | 957 | 5.3 | 1.8 | 5.2 | 0.1 |
| Proportion of weekdays | 957 | 0.65 | 0.13 | 0.66 | 0.01 |
| Wear time, min/d | 957 | 760.9 | 85.8 | 763.4 | 3.9 |
| MVPA, min/d | 957 | 42.1 | 27.8 | 42.6 | 1.3 |
| Sedentary, min/d | 957 | 507.8 | 95.8 | 514.7 | 4.2 |
| Total sedentary time (% of wear time) | 957 | 66.7% | 10.1% | 67.4% | 0.4% |
| Sedentary bout pattern variables | |||||
| Time in bouts ≥30 min, min/d | 957 | 156.3 | 91.3 | 163.5 | 4.2 |
| Median bout duration, min/bout | 957 | 3.5 | 1.7 | 3.5 | 0.1 |
| Sedentary breaks, n/day | 957 | 71.4 | 15.0 | 70.6 | 0.7 |
| Metabolic health markers | |||||
| BMI percentile, % | 957 | 72.6 | 28.1 | 72.5 | 1.5 |
| Waist circumference, cm | 957 | 77.0 | 15.0 | 76.8 | 0.7 |
| Glucose, mg/dL | 907 | 91.8 | 7.0 | 91.5 | 0.4 |
| HbA1c, % | 909 | 5.2 | 0.3 | 5.2 | 0.02 |
| Insulin, pmol/L | 902 | 90.4 | 63.3 | 87.0 | 2.8 |
| HOMA‐IR, mass units | 902 | 3.5 | 2.6 | 3.3 | 0.1 |
BMI indicates body mass index; HOMA‐IR, homeostatic model assessment for insulin resistance; MVPA, moderate‐to‐vigorous physical activity; and SOL, Study of Latino Youth.
Adiposity Measures
Longer median bout durations and fewer sedentary breaks had a statistically significant association with a greater BMI percentile and waist circumference when adjusting for total sedentary time and MVPA (ie, in Model 3; Table 3). Model 3 effect sizes (ie, regression coefficients) were small and small‐to‐moderate (|b1|=0.09 to 0.20) and were larger for sedentary breaks than for median bout duration. Every 1 minute in median bout duration was associated with a higher BMI by 1.5 percentile and larger waist circumference by 1.1 cm (Figures 1A and 1C). Every 10 sedentary breaks were associated with a lower BMI by 3.4 percentile and smaller waist circumference by 2.0 cm (Figures 1B and 1D). A low number of breaks (1 SD below the mean, ≈56.4 breaks/day) corresponded with a BMI at the 76.6 percentile and waist circumference of 79.3 cm, while a high number of breaks (1 SD above the mean, ≈86.3 breaks/day) corresponded with a BMI at the 66.6 percentile and waist circumference of 73.4 cm.
Table 3.
Associations of Sedentary Bout Patterns With Metabolic Health Markers in Hispanic/Latino youth, SOL Youth (n=902–957)
| Sedentary bout pattern variable (independent variable) | ||||||
|---|---|---|---|---|---|---|
| Time in bouts ≥30 min, z‐score | Median bout duration, z‐score | Sedentary breaks, z‐score | ||||
| Metabolic health marker (dependent variable) and model | b1 (95% CI) | b2 (95% CI) | b1 (95% CI) | b2 (95% CI) | b1 (95% CI) | b2 (95% CI) |
| BMI percentile (n=957) | ||||||
| Model 1 | 0.03 (−0.06 to 0.12) | 0.80 (−1.82 to 3.41) | 0.07 (0.02 to 0.12)* | 2.01 (0.57 to 3.45)* | −0.04 (−0.15 to 0.06) | −1.26 (−4.27 to 1.76) |
| Model 2 | −0.03 (−0.17 to 0.11) | −0.83 (−4.83 to 3.17) | 0.07 (0.02 to 0.12)* | 1.86 (0.46 to 3.26)* | −0.02 (−0.14 to 0.11) | −0.49 (−4.06 to 3.09) |
| Model 3 | 0.04 (−0.11 to 0.19) | 1.25 (−2.96 to 5.45) | 0.09 (0.03 to 0.15)* | 2.56 (0.89 to 4.23)* | −0.18 (−0.34 to −0.02)* | −5.03 (−9.45 to −0.61)* |
| Waist circumference, cm (n=957) | ||||||
| Model 1 | 0.04 (−0.05 to 0.14) | 0.68 (−0.71 to 2.06) | 0.12 (0.06 to 0.18)* | 1.76 (0.83 to 2.68)* | −0.08 (−0.18 to 0.02) | −1.18 (−2.67 to 0.31) |
| Model 2 | −0.08 (−0.22 to 0.05) | −1.23 (−3.27 to 0.81) | 0.10 (0.04 to 0.15)* | 1.46 (0.63 to 2.29)* | −0.02 (−0.13 to 0.09) | −0.32 (−2.01 to 1.37) |
| Model 3 | −0.01 (−0.14 to 0.13) | −0.09 (−2.14 to 1.96) | 0.12 (0.06 to 0.19)* | 1.87 (0.91 to 2.82)* | −0.20 (−0.33 to −0.06)* | −2.95 (−4.94 to −0.97)* |
| Glucose, mg/dL (n=907) | ||||||
| Model 1 | 0.07 (−0.04 to 0.17) | 0.46 (−0.27 to 1.19) | −0.03 (−0.08 to 0.02) | −0.18 (−0.54 to 0.17) | −0.02 (−0.14 to 0.10) | −0.15 (−0.97 to 0.67) |
| Model 2 | 0.14 (−0.01 to 0.29) | 0.97 (−0.04 to 1.99) | −0.04 (−0.10 to 0.02) | −0.27 (−0.68 to 0.14) | −0.03 (−0.15 to 0.10) | −0.18 (−1.07 to 0.71) |
| Model 3 | 0.18 (0.02 to 0.35)* | 1.28 (0.11 to 2.46)* | −0.03 (−0.09 to 0.03) | −0.21 (−0.65 to 0.24) | −0.10 (−0.28 to 0.07) | −0.72 (−1.92 to 0.48) |
| HbA1c, % (n=909) | ||||||
| Model 1 | 0.04 (−0.08 to 0.15) | 0.01 (−0.02 to 0.04) | −0.03 (−0.11 to 0.06) | −0.01 (−0.03 to 0.02) | −0.01 (−0.15 to 0.13) | 0.00 (−0.04 to 0.04) |
| Model 2 | 0.15 (−0.04 to 0.33) | 0.04 (−0.01 to 0.10) | −0.01 (−0.11 to 0.08) | 0.00 (−0.03 to 0.02) | −0.04 (−0.22 to 0.13) | −0.01 (−0.07 to 0.04) |
| Model 3 | 0.19 (0.00 to 0.37)* | 0.06 (0.00 to 0.11)* | −0.01 (−0.09 to 0.08) | 0.00 (−0.03 to 0.02) | −0.12 (−0.32 to 0.08) | −0.03 (−0.09 to 0.03) |
| Insulin, pmol/L (n=902) | ||||||
| Model 1 | 0.12 (0.02 to 0.23)* | 7.77 (1.22 to 14.33)* | 0.13 (0.02 to 0.23)* | 7.99 (1.58 to 14.41)* | −0.13 (−0.25 to −0.01)* | −8.45 (−16.11 to −0.80)* |
| Model 2 | −0.02 (−0.16 to 0.12) | −1.30 (−10.23 to 7.62) | 0.07 (0.00 to 0.14) | 4.48 (−0.22 to 9.17) | −0.04 (−0.18 to 0.09) | −2.79 (−11.15 to 5.57) |
| Model 3 | 0.05 (−0.10 to 0.19) | 3.10 (−6.13 to 12.34) | 0.09 (0.00 to 0.19)* | 5.92 (0.00 to 11.84)* | −0.21 (−0.37 to −0.04)* | −13.07 (−23.68 to −2.46)* |
| HOMA‐IR, mass units (n=902) | ||||||
| Model 1 | 0.12 (0.02 to 0.22)* | 0.32 (0.06 to 0.58)* | 0.12 (0.02 to 0.22)* | 0.30 (0.04 to 0.56)* | −0.13 (−0.25 to −0.01)* | −0.33 (−0.64 to −0.03)* |
| Model 2 | 0.00 (−0.14 to 0.13) | −0.01 (−0.36 to 0.34) | 0.06 (−0.01 to 0.14) | 0.16 (−0.03 to 0.36) | −0.05 (−0.18 to 0.08) | −0.12 (−0.46 to 0.22) |
| Model 3 | 0.07 (−0.08 to 0.21) | 0.17 (−0.20 to 0.54) | 0.09 (−0.01 to 0.18) | 0.22 (−0.02 to 0.46) | −0.21 (−0.37 to −0.04)* | −0.54 (−0.97 to −0.11)* |
b1–regression coefficient with both the independent and dependent variables standardized as z scores; b2–regression coefficient with only the independent variable standardized as a z score; BMI indicates body mass index; HbA1c, glycated hemoglobin; HOMA‐IR‐ homeostatic model assessment for insulin resistance; and SOL Youth, Study of Latino Youth. Model 1: Adjusted for age, sex, place of birth, Hispanic background, household income, parent/caregiver education, Tanner stage, site, min/day of accelerometer wear time, number of wear days, and proportion of wear days that were weekdays. Model 2: Adjusted for the same covariates as Model 1, plus total sedentary time. Model 3: Adjusted for the same covariates as Model 2, plus moderate‐to‐vigorous physical activity.
Significant with P<0.05 or weighted 95% CI that does not span 0.
Figure 1. Partial residual plots showing associations between sedentary pattern variables (x‐axis) and metabolic health markers (y‐axis) from SOL Youth (n=902–957).
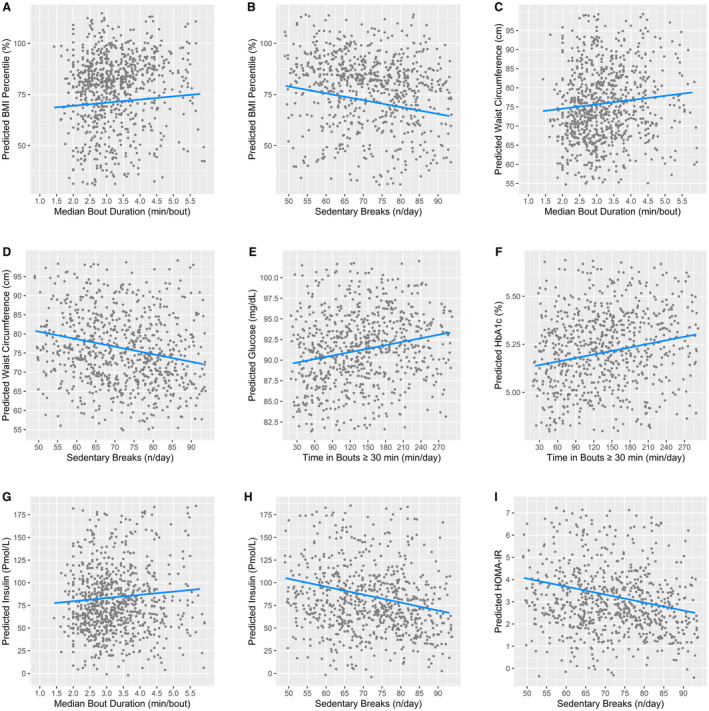
Only significant associations from Table 3 were plotted (P<0.05 or weighted 95% CI that does not span 0). All y‐axes and x‐axes were scaled to range 1.5 SD below the mean to 1.5 SD above the mean for the corresponding variable. The predicted values for each dependent variable were estimated from Model 3 with all variables in their original unit (not z‐scores). Covariates were age, sex, place of birth, Hispanic/Latino background, site, Tanner stage, household income, parent/caregiver education, accelerometer wear time, number of valid accelerometer wear days, proportion of valid days from week days, total sedentary time, and moderate‐to‐vigorous physical activity. Plots were created using the R package ‘visreg’ version 2.7.0 and show differences in the dependent variable across values of the independent variable when holding all other variables constant at either the mean value for continuous variables and binary factors, or the most representative group (ie, the group whose mean was closest to the pooled sample mean) for multi‐categorical variables, using the ‘cond’ argument. The most representative sites were Chicago (panels A, B, E), Bronx (panels C, D, F), and San Diego (panels G through I). The most representative parent education level was high school or equivalent for all but panels ( E) and ( F) (greater than high school). BMI indicates body mass index; HbA1c, glycated hemoglobin; HOMA‐IR, homeostatic model assessment for insulin resistance; min, minutes; and SOL Youth, Study of Latino Youth.
Glucose Measures
Time in bouts lasting ≥30 minutes was the only sedentary pattern variable that had a statistically significant association (b1=0.18–0.19) with fasting glucose and HbA1c in Model 3. Every 60 min/day in bouts lasting ≥30 minutes was associated with a higher glucose by 0.84 mg/dL and higher HbA1c by 0.04% (Figures 1E and 1F). A high amount of time spent in bouts ≥30 minutes (≈248 min/day) corresponded with a fasting glucose of 92.8 mg/dL and HbA1c of 5.3%, whereas a low amount of time spent in bouts ≥30 minutes (≈65 min/day) corresponded with a fasting glucose of 90.2 mg/dL and HbA1c of 5.2%.
Insulin Measures
In Model 3, median bout duration had a significant small association with fasting insulin (b1=0.09), and sedentary breaks had a statistically significant small‐to‐moderate association with insulin and HOMA‐IR (both b1=−0.21). Every 1 minute in median bout duration was associated with a higher fasting insulin by 3.5 pmol/L (Figure 1G). High and low median bout durations (≈5.1 minute and ≈1.8 minutes, respectively) corresponded with a fasting insulin of 91.0 and 78.6 pmol/L, respectively. Every 10 sedentary breaks were associated with a lower fasting insulin by 8.7 pmol/L and lower HOMA‐IR by 0.36 (Figures 1H and 1I). A low number of breaks (≈56.4 breaks/day) corresponded with a fasting insulin of 98.3 pmol/L and HOMA‐IR of 3.8, and a high number of breaks (≈86.3 breaks/day) corresponded with a fasting insulin of 72.4 pmol/L and HOMA‐IR of 2.7.
Interactions
None of the 18 sedentary pattern variable × total sedentary time interactions tested in Model 4 were significant, and none of the 18 sedentary pattern variable × MVPA interactions tested in Model 5 were significant using P<0.01 (Table 4).
Table 4.
Interactions of Sedentary Bout Patterns With Total Sedentary Time and With MVPA in Relation to Metabolic Health Markers in Hispanic/Latino Youth, SOL Youth (n=902–957).
| Sedentary bout pattern variable (independent variable) | ||||||
|---|---|---|---|---|---|---|
| Time in bouts ≥30 mins, z‐score | Median bout duration, z‐score | Sedentary breaks, z‐score | ||||
| Metabolic health marker (dependent variable) and model | b1 (95% CI) | b2 (95% CI) | b1 (95% CI) | b2 (95% CI) | b1 (95% CI) | b2 (95% CI) |
| BMI percentile (n=957) | ||||||
| Model 4: sedentary pattern×sedentary time interaction | 0.02 (−0.07, 0.12) | 0.69 (−1.97, 3.35) | −0.07 (−0.17, 0.02) | −2.11 (−4.86, 0.65) | 0.06 (−0.01, 0.13) | 1.64 (−0.36, 3.64) |
| Model 5: sedentary pattern×MVPA interaction | −0.01 (−0.10, 0.07) | −0.38 (−2.84, 2.08) | 0.10 (−0.03, 0.22) | 2.69 (−0.92, 6.29) | −0.05 (−0.14, 0.04) | −1.35 (−3.82, 1.13) |
| Waist circumference, cm (n=957) | ||||||
| Model 4: sedentary pattern×sedentary time interaction | 0.08 (−0.02, 0.17) | 1.17 (−0.25, 2.60) | −0.05 (−0.16, 0.06) | −0.74 (−2.39, 0.91) | 0.05 (−0.02, 0.12) | 0.77 (−0.25, 1.79) |
| Model 5: sedentary pattern×MVPA interaction | −0.09 (−0.18, 0.00) | −1.34 (−2.65, −0.04) | 0.01 (−0.13, 0.15) | 0.09 (−2.01, 2.19) | −0.02 (−0.09, 0.06) | −0.25 (−1.42, 0.91) |
| Glucose, mg/dL (n=907) | ||||||
| Model 4: sedentary pattern×sedentary time interaction | −0.01 (−0.13, 0.11) | −0.06 (−0.87, 0.76) | 0.07 (−0.02, 0.17) | 0.52 (−0.16, 1.19) | 0.02 (−0.08, 0.12) | 0.15 (−0.52, 0.82) |
| Model 5: sedentary pattern×MVPA interaction | 0.04 (−0.06, 0.15) | 0.29 (−0.43, 1.01) | −0.05 (−0.20, 0.10) | −0.35 (−1.42, 0.72) | −0.04 (−0.13, 0.05) | −0.31 (−0.93, 0.32) |
| HbA1c, % (n=909) | ||||||
| Model 4: sedentary pattern×sedentary time interaction | −0.07 (−0.15, 0.01) | −0.02 (−0.04, 0.00) | 0.08 (−0.02, 0.18) | 0.02 (−0.01, 0.05) | 0.04 (−0.03, 0.11) | 0.01 (−0.01, 0.03) |
| Model 5: sedentary pattern×MVPA interaction | −0.04 (−0.14, 0.06) | −0.01 (−0.04, 0.02) | −0.20 (−0.36, −0.03) | −0.06 (−0.11, −0.01) | 0.01 (−0.08, 0.09) | 0.00 (−0.02, 0.03) |
| Insulin, pmol/L (n=902) | ||||||
| Model 4: sedentary pattern×sedentary time interaction | 0.04 (−0.05, 0.13) | 2.47 (−3.12, 8.06) | −0.06 (−0.17, 0.06) | −3.58 (−10.70, 3.53) | 0.06 (−0.01, 0.13) | 3.73 (−0.63, 8.09) |
| Model 5: sedentary pattern×MVPA interaction | −0.07 (−0.16, 0.01) | −4.55 (−9.94, 0.83) | 0.00 (−0.14, 0.14) | −0.05 (−8.88, 8.78) | 0.01 (−0.07, 0.08) | 0.46 (−4.36, 5.28) |
| HOMA−IR, mass units (n=902) | ||||||
| Model 4: sedentary pattern×sedentary time interaction | 0.04 (−0.05, 0.13) | 0.11 (−0.12, 0.34) | −0.04 (−0.15, 0.06) | −0.12 (−0.40, 0.17) | 0.05 (−0.01, 0.12) | 0.14 (−0.04, 0.32) |
| Model 5: sedentary pattern×MVPA interaction | −0.07 (−0.15, 0.02) | −0.17 (−0.39, 0.05) | 0.00 (−0.14, 0.13) | 0.00 (−0.35, 0.34) | 0.00 (−0.07, 0.08) | 0.01 (−0.18, 0.20) |
b1–regression coefficient with both the independent and dependent variables standardized as z scores; b2–regression coefficient with only the independent variable standardized as a z score; BMI indicates body mass index; CI, confidence interval; HbA1c, glycated hemoglobin; HOMA‐IR, homeostatic model assessment for insulin resistance; and MVPA, moderate‐to‐vigorous physical activity. Models were adjusted for age, sex, place of birth, Hispanic background, household income, parent/caregiver education, Tanner stage, site, min/day of accelerometer wear time, number of wear days, proportion of wear days that were weekdays, total sedentary time, and MVPA. Model 4 interaction: The regression coefficient reflects the multiplicative interaction of the sedentary bout pattern variable x total sedentary time. Model 5 interaction: The regression coefficient reflects the multiplicative interaction of the sedentary bout pattern variable x MVPA.
Sensitivity Analysis
The observed associations were generally consistent when employing an informed missingness modeling approach (Table S1). Of the 9 significant Model 3 associations observed in Table 3, 8 were significant in the sensitivity analysis and had a similar magnitude of association. One significant association became nonsignificant (in bouts lasting ≥30 minutes with HbA1c), and 1 nonsignificant association became significant (median bout duration with HOMA‐IR), though the magnitude of association was similar between the initial model and sensitivity model.
Discussion
In this population‐based cohort of Hispanic/Latino youth aged 8 to 16 years, greater accumulation of sedentary time in prolonged and uninterrupted bout patterns was associated with greater levels of adiposity (higher BMI and waist circumference) and poorer glycemic and insulin values (higher fasting glucose, HbA1c, fasting insulin, and HOMA‐IR). These associations were observed in models adjusting for total sedentary time and MVPA, suggesting that limiting time in prolonged/uninterrupted sedentary bouts may be an important behavioral target in reducing metabolic risks over and above the amount of time spent in MVPA. Considering total sedentary time was unrelated to the metabolic health markers in this cohort after accounting for MVPA, 27 the present findings suggest sedentary bout patterns may play a more important role in youth's metabolic health than total sedentary time, though more evidence is required to establish consensus. Sedentary patterns may also be a more modifiable starting point for some youth who have difficulty with higher intensity physical activity.
Median bout duration and sedentary breaks were consistently associated with the adiposity and insulin markers. This consistency across health markers may be attributable to the interrelationship between higher insulin and greater adiposity, which was shown in this cohort (r=0.43–0.59) and in other studies of youth 50 , 51 and is likely attributable to adipose expansion and elevated insulin promoting one another. 52 The effect sizes for the observed associations were meaningful and comparable with or only slightly smaller than effects of physical activity in a recent meta‐analysis, 53 up to 10 percentile units in BMI, 5.9 cm in waist circumference, 25.9 pmol/L in fasting insulin, and 1.1 units in HOMA‐IR between low (−1 SD) and high (+1 SD) values of the sedentary pattern variable. Though several youth studies have shown associations between sedentary bout patterns and adiposity, many have failed to identify such associations, definitions of sedentary bout patterns have varied widely, and relatively few studies have investigated fasting insulin and HOMA‐IR. 3 , 19 The present findings are more aligned with observational and intervention studies in adults, for which adiposity, insulin markers, and type 2 diabetes have been among the most consistent health factors associated with sedentary patterns, particularly sedentary breaks. 7 , 8 , 54 The differences in findings between the present study and previous youth studies may be partly because of the SOL Youth population having higher rates of sedentary time, obesity, and insulin resistance than other youth populations that have been studied. 22 , 26
Time spent in bouts ≥30 minutes showed fewer associations with health markers compared with median bout duration and sedentary breaks, though more time in bouts ≥30 minutes was associated with higher fasting glucose and HbA1c. The finding that time in bouts ≥30 minutes was associated with different metabolic health markers (glucose and HbA1c) than median bout duration and sedentary breaks (adiposity and insulin) may be attributable to each sedentary pattern variable reflecting a slightly different aspect of the sedentary bout distribution. As shown in Table 1, a high number of breaks and low median bout duration do not necessary reflect a low amount of time spent in prolonged bouts (eg, those lasting ≥30 minutes) but may induce more frequent light activity. Laboratory studies suggest the role of prolonged periods of sedentary time may be somewhat distinct from the role of low energy expenditure in impacting metabolic health, with the former due more to reduced glucose metabolism resulting from reduced contractions in leg and trunk muscles. 16 , 55 This finding warrants more research investigating whether some sedentary bout pattern measures have differential associations with various health markers, using variable selection approaches that aim to capture distinct sedentary pattern variables, such as was done in this study. However, all metabolic health markers investigated play an important role in glucose metabolism and diabetes prevention, so intervention approaches that target favorable changes in multiple aspects of the sedentary bout distribution appear warranted.
None of the interactions tested were significant at P<0.01. Thus, there was no support for effect modification, which has sometimes been shown in previous studies. 46 Adult studies, in particular, have produced evidence suggesting sedentary patterns have the most detrimental associations with health among those with high total sedentary time and low physical activity, 14 , 56 or other interactions.
Strengths, Limitations, and Other Considerations
Study strengths include the investigation of objective measures of metabolic health markers and device‐based measures of multiple sedentary bout pattern variables, with selection based on the lowest intervariable correlations. The study involved a population‐based cohort of Hispanic/Latino youth from 4 US geographic regions, and an ethnic group that experiences health inequities and elevated risk for metabolic diseases (eg, type 2 diabetes) 22 but has generally been excluded from most research. A limitation of basing the sedentary bout pattern variables on accelerometer cut points is that the variables are more likely to reflect bouts of periods of low movement rather than sitting, 57 so limited conclusions can be drawn about sitting patterns. Because BMI percentile and waist circumference are indirect measures of adiposity, more research is needed involving more direct measures (eg, bioelectrical impedance, dual‐energy X‐ray absorptiometry). The cross‐sectional study design prohibited establishment of causality or temporality of associations. While the different pattern variables provide some insight into behavioral targets for interventions, such targets need to be tested in experimental studies, and the variables can be difficult to distill into concise intervention recommendations. Another next step to build on these cross‐sectional findings is to investigate similar research questions in prospective studies of Hispanic/Latino youth, particularly those with the greatest metabolic health risks (eg, those with obesity).
Conclusions
Based on the present findings, interventions that target increases in daily sedentary breaks that are timed appropriately to reduce time spent in longer (eg, 30+ minute) sedentary bouts would be expected to benefit metabolic health in Hispanic/Latino youth. These behavioral targets may be more easily modifiable than higher intensity physical activity, and their benefits may extend beyond the benefits of increasing overall daily MVPA. These findings suggest that more holistic activity‐related interventions targeting both ends of the activity spectrum (more MVPA and less prolonged/uninterrupted sedentary patterns) may provide greater health benefits than those targeting only MVPA.
Sources of Funding
The SOL Youth study was supported by Grant Number R01HL102130 from the National Heart, Lung, and Blood Institute. The children in SOL Youth are drawn from the study of adults: The HCHS/SOL, which was supported by contracts from the National Heart, Lung, and Blood Institute to the University of North Carolina (N01‐HC65233), University of Miami (N01‐HC65234), Albert Einstein College of Medicine (N01‐HC65235), University of Illinois at Chicago (HHSN268201300002I)/Northwestern University (N01‐HC65236), and San Diego State University (N01‐HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the National Heart, Lung, and Blood Institute: National Center on Minority Health and Health Disparities, the National Institute of Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements.
Additional support was provided by the Life Course Methodology Core of the New York Regional Center for Diabetes Translation Research (DK111022–8786). J.A. Carlson, K.M. Perreira, and D. Sotres‐Alvarez received support from the National Heart, Lung, and Blood Institute, R01HL148463, 75N92019D00010, and R01HL102130. C.M. Bejarano received support from the Center for Children's Healthy Lifestyles & Nutrition at Children's Mercy Kansas City and the University of Kansas Medical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or other funders.
Disclosures
None.
Supporting information
Table S1
This manuscript was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028495
For Sources of Funding and Disclosures, see page 12.
REFERENCES
- 1. Tremblay MS, LeBlanc AG, Kho ME, Saunders TJ, Larouche R, Colley RC, Goldfield G, Gorber SC. Systematic review of sedentary behaviour and health indicators in school‐aged children and youth. Int J Behav Nutr Phys Act. 2011;8:98. doi: 10.1186/1479-5868-8-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carson V, Hunter S, Kuzik N, Gray CE, Poitras VJ, Chaput JP, Saunders TJ, Katzmarzyk PT, Okely AD, Connor Gorber S, et al. Systematic review of sedentary behaviour and health indicators in school‐aged children and youth: an update. Appl Physiol Nutr Metab. 2016;41:S240–S265. doi: 10.1139/apnm-2015-0630 [DOI] [PubMed] [Google Scholar]
- 3. Cliff DP, Hesketh KD, Vella SA, Hinkley T, Tsiros MD, Ridgers ND, Carver A, Veitch J, Parrish AM, Hardy LL, et al. Objectively measured sedentary behaviour and health and development in children and adolescents: systematic review and meta‐analysis. Obes Rev. 2016;17:330–344. doi: 10.1111/obr.12371 [DOI] [PubMed] [Google Scholar]
- 4. Biddle SJ, Bengoechea EG, Wiesner G. Sedentary behaviour and adiposity in youth: a systematic review of reviews and analysis of causality. Int J Behav Nutr Phys Act. 2017;14:1–21. doi: 10.1186/s12966-017-0497-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Ekris E, Altenburg TM, Singh AS, Proper KI, Heymans MW, Chinapaw MJ. An evidence‐update on the prospective relationship between childhood sedentary behaviour and biomedical health indicators: a systematic review and meta‐analysis. Obes Rev. 2016;17:833–849. doi: 10.1111/obr.12426 [DOI] [PubMed] [Google Scholar]
- 6. Saunders TJ, McIsaac T, Douillette K, Gaulton N, Hunter S, Rhodes RE, Prince SA, Carson V, Chaput J‐P, Chastin S, et al. Sedentary behaviour and health in adults: an overview of systematic reviews. Appl Physiol Nutr Metab. 2020;45:S197–S217. doi: 10.1139/apnm-2020-0272 [DOI] [PubMed] [Google Scholar]
- 7. Katzmarzyk PT, Powell KE, Jakicic JM, Troiano RP, Piercy K, Tennant B; 2018 Physical Activity Guidelines Advisory Committee . Sedentary behavior and health: update from the 2018 physical activity guidelines advisory committee. Med Sci Sports Exerc. 2019;51:1227–1241. doi: 10.1249/MSS.0000000000001935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dempsey PC, Biddle SJ, Buman MP, Chastin S, Ekelund U, Friedenreich CM, Katzmarzyk PT, Leitzmann MF, Stamatakis E, van der Ploeg HP, et al. New global guidelines on sedentary behaviour and health for adults: broadening the behavioural targets. Int J Behav Nutr Phys Act. 2020;17:1–12. doi: 10.1186/s12966-020-01044-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Ekris E, Wijndaele K, Altenburg TM, Atkin AJ, Twisk J, Andersen LB, Janz KF, Froberg K, Northstone K, Page AS, et al. Tracking of total sedentary time and sedentary patterns in youth: a pooled analysis using the International Children's Accelerometry Database (ICAD). Int J Behav Nutr Phys Act. 2020;17:1–10. doi: 10.1186/s12966-020-00960-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Telama R, Yang X, Viikari J, Valimaki I, Wanne O, Raitakari O. Physical activity from childhood to adulthood: a 21‐year tracking study. Am J Prev Med. 2005;28:267–273. doi: 10.1016/j.amepre.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 11. Telama R. Tracking of physical activity from childhood to adulthood: a review. Obes Facts. 2009;2:187–195. doi: 10.1159/000222244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saunders TJ, Atkinson HF, Burr J, MacEwen B, Skeaff CM, Peddie MC. The acute metabolic and vascular impact of interrupting prolonged sitting: a systematic review and meta‐analysis. Sports Med. 2018;48:2347–2366. doi: 10.1007/s40279-018-0963-8 [DOI] [PubMed] [Google Scholar]
- 13. Owen N, Healy GN, Dempsey PC, Salmon J, Timperio A, Clark BK, Goode AD, Koorts H, Ridgers ND, Hadgraft NT, et al. Sedentary behavior and public health: integrating the evidence and identifying potential solutions. Annu Rev Public Health. 2020;41:265–287. doi: 10.1146/annurev-publhealth-040119-094201 [DOI] [PubMed] [Google Scholar]
- 14. Diaz KM, Goldsmith J, Greenlee H, Strizich G, Qi Q, Mossavar‐Rahmani Y, Vidot DC, Buelna C, Brintz CE, Elfassy T, et al. Prolonged, uninterrupted sedentary behavior and glycemic biomarkers among US Hispanic/Latino adults: the Hispanic community health study/study of Latinos (HCHS/SOL). Circulation. 2017;136:1362–1373. doi: 10.1161/CIRCULATIONAHA.116.026858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keith MD, Virginia JH, Brent H, Natalie C, John E, Monika MS, Steven NB, Steven PH. Patterns of sedentary behavior and mortality in US middle‐aged and older adults. Ann Intern Med. 2017;167:465. doi: 10.7326/M17-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins S, Pomeroy A, Bates LC, Paterson C, Barone Gibbs B, Pontzer H, Stoner L. Sedentary behavior and cardiovascular disease risk: an evolutionary perspective. Front Physiol. 2022;13:962791. doi: 10.3389/fphys.2022.962791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wheeler MJ, Green DJ, Cerin E, Ellis KA, Heinonen I, Lewis J, Naylor LH, Cohen N, Larsen R, Dempsey PC, et al. Combined effects of continuous exercise and intermittent active interruptions to prolonged sitting on postprandial glucose, insulin, and triglycerides in adults with obesity: a randomized crossover trial. Int J Behav Nutr Phys Act. 2020;17:1–11. doi: 10.1186/s12966-020-01057-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Homer AR, Taylor FC, Dempsey PC, Wheeler MJ, Sethi P, Townsend MK, Grace MS, Green DJ, Cohen ND, Larsen RN, et al. Frequency of interruptions to sitting time: benefits for postprandial metabolism in type 2 diabetes. Diabetes Care. 2021;44:1254–1263. doi: 10.2337/dc20-1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verswijveren SJ, Lamb KE, Bell LA, Timperio A, Salmon J, Ridgers ND. Associations between activity patterns and cardio‐metabolic risk factors in children and adolescents: a systematic review. PLoS One. 2018;13:e0201947. doi: 10.1371/journal.pone.0201947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Altenburg TM, Chinapaw MJ. Bouts and breaks in children's sedentary time: currently used operational definitions and recommendations for future research. Prev Med. 2015;77:1–3. doi: 10.1016/j.ypmed.2015.04.019 [DOI] [PubMed] [Google Scholar]
- 21. United States Census Bureau . 2020 Census Statistics Highlight Local Population Changes and Nation's Racial and Ethnic Diversity. 2021.
- 22. Isasi CR, Parrinello CM, Ayala GX, Delamater AM, Perreira KM, Daviglus ML, Elder JP, Marchante AN, Bangdiwala SI, Van Horn L, et al. Sex differences in cardiometabolic risk factors among Hispanic/Latino youth. J Pediatr. 2016;176:121–127. doi: 10.1016/j.jpeds.2016.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention . National diabetes statistics report, 2020. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020:12–15. [Google Scholar]
- 24. Schneiderman N, Llabre M, Cowie CC, Barnhart J, Carnethon M, Gallo LC, Giachello AL, Heiss G, Kaplan RC, LaVange LM, et al. Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic community health study/study of Latinos (HCHS/SOL). Diabetes Care. 2014;37:2233–2239. doi: 10.2337/dc13-2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. U.S. Department of Health & Human Services . NIMHD Minority Health and Health Disparities Research Framework. 2017. https://www.nimhd.nih.gov/about/overview/research‐framework/.
- 26. Bejarano CM, Gallo LC, Castañeda SF, Garcia ML, Sotres‐Alvarez D, Perreira KM, Isasi CR, Daviglus M, Van Horn L, Delamater AM, et al. Patterns of sedentary time in the Hispanic Community Health Study/Study Of Latinos (HCHS/SOL) youth. J Phys Act Health. 2020;18:61–69. doi: 10.1123/jpah.2020-0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strizich G, Kaplan RC, Sotres‐Alvarez D, Diaz KM, Daigre AL, Carnethon MR, Vidot DC, Delamater AM, Perez L, Perreira K, et al. Objectively measured sedentary behavior, physical activity, and cardiometabolic risk in Hispanic youth: Hispanic community health study/study of Latino youth. J Clin Endocrinol Metabol. 2018;103:3289–3298. doi: 10.1210/jc.2018-00356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. LaVange LM, Kalsbeek WD, Sorlie PD, Avilés‐Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan J, et al. Sample design and cohort selection in the Hispanic community health study/study of Latinos. Ann Epidemiol. 2010;20:642–649. doi: 10.1016/j.annepidem.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sorlie PD, Avilés‐Santa LM, Wassertheil‐Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison M, et al. Design and implementation of the Hispanic community health study/study of Latinos. Ann Epidemiol. 2010;20:629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Isasi CR, Carnethon MR, Ayala GX, Arredondo E, Bangdiwala SI, Daviglus ML, Delamater AM, Eckfeldt JH, Perreira K, Himes JH, et al. The Hispanic community children's health study/study of Latino youth (SOL youth): design, objectives, and procedures. Ann Epidemiol. 2014;24:29–35. doi: 10.1016/j.annepidem.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ayala GX, Carnethon M, Arredondo E, Delamater AM, Perreira K, Van Horn L, Himes JH, Eckfeldt JH, Bangdiwala SI, Santisteban DA, et al. Theoretical foundations of the study of Latino (SOL) youth: implications for obesity and cardiometabolic risk. Ann Epidemiol. 2014;24:36–43. doi: 10.1016/j.annepidem.2013.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Romanzini M, Petroski EL, Ohara D, Dourado AC, Reichert FF. Calibration of ActiGraph GT3X, Actical and RT3 accelerometers in adolescents. Eur J Sport Sci. 2014;14:91–99. doi: 10.1080/17461391.2012.732614 [DOI] [PubMed] [Google Scholar]
- 33. Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–364. doi: 10.1249/MSS.0b013e3181ed61a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cain KL, Sallis JF, Conway TL, Van Dyck D, Calhoon L. Using accelerometers in youth physical activity studies: a review of methods. J Phys Act Health. 2013;10:437–450. doi: 10.1123/jpah.10.3.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gallo LC, Roesch SP, McCurley JL, Isasi CR, Sotres‐Alvarez D, Delamater AM, Van Horn L, Arredondo EM, Perreira KM, Buelna C, et al. Youth and caregiver physical activity and sedentary time: HCHS/SOL youth. Am J Health Behav. 2017;41:67–75. doi: 10.5993/AJHB.41.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carson V, Stone M, Faulkner G. Patterns of sedentary behavior and weight status among children. Pediatr Exerc Sci. 2014;26:95–102. doi: 10.1123/pes.2013-0061 [DOI] [PubMed] [Google Scholar]
- 37. Chastin SF, Winkler EA, Eakin EG, Gardiner PA, Dunstan DW, Owen N, Healy GN. Sensitivity to change of objectively‐derived measures of sedentary behavior. Meas Phys Educ Exerc Sci. 2015;19:138–147. doi: 10.1080/1091367X.2015.1050592 [DOI] [Google Scholar]
- 38. Chastin SF, Granat MH. Methods for objective measure, quantification and analysis of sedentary behaviour and inactivity. Gait Posture. 2010;31:82–86. doi: 10.1016/j.gaitpost.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 39. Chastin S, Ferriolli E, Stephens N, C H Fearon K, Greig C. Relationship between sedentary behaviour, physical activity, muscle quality and body composition in healthy older adults. Age Ageing. 2012;41:111–114. doi: 10.1093/ageing/afr075 [DOI] [PubMed] [Google Scholar]
- 40. Kuczmarski RJ. CDC Growth Charts: United States. Centers for Disease Control and Prevention, National Center for Health Statistics; 2000. [Google Scholar]
- 41. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 42. Petersen AC, Crockett L, Richards M, Boxer A. A self‐report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- 43. Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. R Core Team . A language and environment for statistical computing. 2021. http://www.R‐project.org.
- 45. Lumley T. Survey: analysis of complex survey samples. R package version 4.0. 2021.
- 46. Malik JA, Coto J, Pulgaron ER, Daigre A, Sanchez JE, Goldberg RB, Wilson DK, Delamater AM. Sedentary behavior moderates the relationship between physical activity and cardiometabolic risk in young Latino children. Transl Behav Med. 2021;11:1517–1526. doi: 10.1093/tbm/ibab046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 48. Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22:278–295. doi: 10.1177/0962280210395740 [DOI] [PubMed] [Google Scholar]
- 49. Evenson KR, Sotres‐Alvarez D, Deng Y, Marshall SJ, Isasi CR, Esliger DW, Davis S. Accelerometer adherence and performance in a cohort study of US Hispanic adults. Med Sci Sports Exerc. 2015;47:725–734. doi: 10.1249/MSS.0000000000000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lawlor DA, Benfield L, Logue J, Tilling K, Howe LD, Fraser A, Cherry L, Watt P, Ness AR, Smith GD, et al. Association between general and central adiposity in childhood, and change in these, with cardiovascular risk factors in adolescence: prospective cohort study. BMJ (Clinical Research Ed). 2010;341:c6224. doi: 10.1136/bmj.c6224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang R‐C, De Klerk NH, Smith A, Kendall GE, Landau LI, Mori TA, Newnham JP, Stanley FJ, Oddy WH, Hands B, et al. Lifecourse childhood adiposity trajectories associated with adolescent insulin resistance. Diabetes Care. 2011;34:1019–1025. doi: 10.2337/dc10-1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Virtue S, Vidal‐Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome—an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. [DOI] [PubMed] [Google Scholar]
- 53. Hejazi K, Ferrari F. Effects of physical exercise on cardiometabolic biomarkers and inflammatory markers in children: a systematic review and meta‐analysis of randomized controlled trials. Biol Res Nurs. 2022;4:519–529. doi: 10.1177/10998004221099573 [DOI] [PubMed] [Google Scholar]
- 54. Hadgraft NT, Winkler E, Climie RE, Grace MS, Romero L, Owen N, Dunstan D, Healy G, Dempsey PC. Effects of sedentary behaviour interventions on biomarkers of cardiometabolic risk in adults: systematic review with meta‐analyses. Br J Sports Med. 2021;55:144–154. doi: 10.1136/bjsports-2019-101154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hamilton MT, Healy GN, Dunstan DW, Zderic TW, Owen N. Too little exercise and too much sitting: inactivity physiology and the need for new recommendations on sedentary behavior. Curr Cardiovasc Risk Rep. 2008;2:292–298. doi: 10.1007/s12170-008-0054-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ekelund U, Tarp J, Fagerland MW, Johannessen JS, Hansen BH, Jefferis BJ, Whincup PH, Diaz KM, Hooker S, Howard VJ, et al. Joint associations of accelerometer‐measured physical activity and sedentary time with all‐cause mortality: a harmonised meta‐analysis in more than 44 000 middle‐aged and older individuals. Br J Sports Med. 2020;54:1499–1506. doi: 10.1136/bjsports-2020-103270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Carlson JA, Bellettiere J, Kerr J, Salmon J, Timperio A, Verswijveren SJ, Ridgers ND. Day‐level sedentary pattern estimates derived from hip‐worn accelerometer cut‐points in 8–12‐year‐olds: do they reflect postural transitions? J Sports Sci. 2019;37:1899–1909. doi: 10.1080/02640414.2019.1605646 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


