Abstract
Background
Despite its high prevalence and clinical significance, clinical measurement of lipoprotein(a) is rare but has not been systematically quantified. We assessed the prevalence of lipoprotein(a) testing overall, in those with various cardiovascular disease (CVD) conditions and in those undergoing cardiac testing across 6 academic medical centers associated with the University of California, in total and by year from 2012 to 2021.
Methods and Results
In this observational study, data from the University of California Health Data Warehouse on the number of individuals with unique lipoprotein(a) testing, unique CVD diagnoses (using International Classification of Diseases, Tenth Revision [ICD‐10], codes), and other unique cardiac testing were collected. The proportion of total individuals, the proportion of individuals with a given CVD diagnosis, and the proportion of individuals with a given cardiac test and lipoprotein(a) testing any time during the study period were calculated. From 2012 to 2021, there were 5 553 654 unique adults evaluated in the University of California health system, of whom 18 972 (0.3%) had lipoprotein(a) testing. In general, those with lipoprotein(a) testing were more likely to be older, men, and White race, with a greater burden of CVD. Lipoprotein(a) testing was performed in 6469 individuals with ischemic heart disease (2.9%), 836 with aortic stenosis (3.1%), 4623 with family history of CVD (3.3%), 1202 with stroke (1.7%), and 612 with coronary artery calcification (6.1%). For most conditions, the prevalence of testing in the same year as the diagnosis of CVD was relatively stable, with a small upward trend over time. Lipoprotein(a) testing was performed in 10 753 individuals (1.8%) who had lipid panels, with higher rates with more specialized testing, including coronary computed tomography angiography (6.8%) and apolipoprotein B (63.0%).
Conclusions
Lipoprotein(a) testing persists at low rates, even among those with diagnosed CVD, and remained relatively stable over the study period.
Keywords: lipoprotein(a), prevention, risk factors, testing
Subject Categories: Cardiovascular Disease, Risk Factors, Primary Prevention, Secondary Prevention, Genetics
Nonstandard Abbreviations and Acronyms
- AS
aortic stenosis
- FHx
family history of cardiovascular disease
- IHD
ischemic heart disease
- PCSK9i
proprotein convertase subtilisin/kexin type 9 inhibitor
- PVD
peripheral vascular disease
- UC
University of California
- UCHDW
University of California Health Data Warehouse
Clinical Perspective.
What Is New?
In a large study of 6 academic health systems in California from 2012 to 2021, only 0.3% of adults had lipoprotein(a) testing.
Lipoprotein(a) testing was low even among those with a family (3.3%) or personal history of cardiovascular disease (<4%). Of those with lipid panels, only 1.8% had lipoprotein(a) tested.
What Are the Clinical Implications?
Lipoprotein(a) remains an underrecognized risk factor for cardiovascular disease, and further efforts are needed to raise awareness and increase implementation of lipoprotein(a) testing when appropriate.
Elevated lipoprotein(a) is a well‐established risk factor for multiple cardiovascular diseases (CVDs), especially coronary artery disease and aortic stenosis (AS). 1 Lipoprotein(a) is also associated with ischemic stroke, 2 , 3 peripheral vascular disease (PVD), 4 and heart failure (HF). 5 The accumulation of epidemiologic and genetic studies strongly supports a causal role for lipoprotein(a) in CVD in diverse populations, even with low levels of low‐density lipoprotein cholesterol. 6 There is also growing evidence that lipoprotein(a) lowering translates to clinical benefit. 7 , 8 Lipoprotein(a) levels of >30 and >50 mg/dL are estimated to be present in 35% to 40% and 24% to 29% of the global population, respectively. 9 There is consensus among the major international guidelines that lipoprotein(a) is an independent and genetically determined risk factor that enhances CVD risk, and all guidelines provide at least a relative indication for lipoprotein(a) testing in those with a family history of premature CVD. However, the guidelines differ in their recommendations for which other specific populations should be tested for lipoprotein(a). The European Society of Cardiology and Canadian Cardiovascular Society recommend screening all adults for elevated lipoprotein(a) at least once in their lifetime. 10 , 11 The American Heart Association/American College of Cardiology, National Lipid Association, and American Association of Clinical Endocrinology recommend lipoprotein(a) testing in patients with premature CVD, especially if not explained by other major risk factors. In addition, the National Lipid Association and American Association of Clinical Endocrinology recommend checking lipoprotein(a) in patients at risk for progressive AS. 12 , 13 , 14
Despite the availability of these guidelines, lipoprotein(a) remains an underappreciated CVD risk factor, and rates of testing in the United States are anecdotally low but have not been well quantified. We aimed to evaluate the prevalence of lipoprotein(a) testing in all individuals in the study population, individuals with several CVD diagnoses, and those undergoing other selected cardiac testing. Addressing these questions will help to clarify the current baseline for lipoprotein(a) testing and identify opportunities to improve testing rates when clinically appropriate.
Methods
Data Collection
We used data from the University of California Health Data Warehouse (UCHDW) for this observational study. The UCHDW contains deidentified data on patients from 6 academic medical centers within the University of California (UC), dating back to 2012. We collected the number of unique adults evaluated in the UC health system from 2012 to 2021 (both by year and over the whole study period). We collected the number of adults (aged ≥18 years) with relevant CVD diagnoses (including AS, carotid stenosis, coronary artery calcification, family history of CVD [FHx], HF, ischemic heart disease [IHD], PVD, and stroke), as well as diabetes and diagnoses that may affect lipoprotein(a) levels (chronic kidney disease/end‐stage renal disease, hypothyroidism, menopause, and statin use) based on International Classification of Diseases, Tenth Revision (ICD‐10), codes (Table S1). We also collected the number of individuals with relevant cardiac testing (including apolipoprotein B‐100, coronary computed tomography angiography, hs‐CRP [high‐sensitivity C‐reactive protein], lipid panels, and lipoprofile nuclear magnetic resonance) based on Current Procedural Terminology codes. We collected the number of individuals with lipoprotein(a) testing from 2012 to 2021, demographics and the number with the above diagnoses and tests among those with lipoprotein(a) testing, and those without lipoprotein(a) testing. Data on the types of assays used for lipoprotein(a) measurement were not available. Finally, we collected the number of participants on lipid‐lowering therapy at baseline and at 1 year in those with lipoprotein(a) testing, in those without lipoprotein(a) testing, and in those with lipid panel testing. Baseline was defined as time of first lipoprotein(a) test, time of first visit, or time of first lipid panel, respectively. The data that support the findings of this study are available from the corresponding author upon reasonable request. The study protocol was approved by the UC San Diego Human Research Protections Program. Informed consent was not required because of the retrospective nature of this study.
Statistical Analysis
We compared characteristics of individuals with lipoprotein(a) testing over the study period with those without lipoprotein(a) testing using χ2 testing for categorical variables and t‐testing or Mann‐Whitney U tests. We then calculated the overall rate of testing among all unique individuals seen in the University of California health system over the study period and the rate of testing by year. For the analysis of the whole study period, each individual was only counted once from 2012 to 2021. For the analysis of testing by year, each individual was only counted once per year. We tabulated the number of unique individuals with each diagnosis, biomarker, or imaging test by year, then tabulated the number of individuals with at least 1 lipoprotein(a) measurement any time during the study period. The number of individuals who had a lipoprotein(a) measurement in the same year as their CVD diagnosis, biomarker, or imaging test was also tabulated. The proportions with lipoprotein(a) testing by year overall, by year of incident CVD diagnosis, and by year of cardiac testing were compared using χ2 tests. For each diagnosis or test, the first event from 2012 to 2021 was counted; thus, each individual was counted only once for each analysis. For analyses by year, data are presented from 2013 to 2021 as only partial data were available for 2012. The prevalence of lipid‐lowering therapy at baseline and at 1 year was compared using the McNemar test.
Analyses were conducted using R version 4.1 (R Core Team 2021). A 2‐sided P<0.05 was considered statistically significant. One author (H.S.B.) had full access to all the data in the study and takes responsibility for their integrity and the data analysis.
Results
In the study period, the UCHDW contained data from 5 553 654 unique adult individuals (each individual counted once from 2012 to 2021), of whom 590 026 (10.6%) had lipid profile testing, 141 128 (2.5%) had a diagnosis of FHx, 222 472 (4.0%) had a diagnosis of IHD, 57 699 (1.0%) had a diagnosis of PVD, 27 216 (0.5%) had a diagnosis of AS, and 68 914 (1.2%) had a diagnosis of stroke from 2012 to 2021. Of all unique patients seen in the UC health system during the study period, 18 972 (0.3%) had a lipoprotein(a) test any time from 2012 to 2021, and an additional 9599 repeated tests were performed. The rate of lipoprotein(a) testing per year increased over time, with 514 tests (0.05%) in 2012 to 4144 tests (0.19%; P<0.001) in 2021. For this analysis, individuals were counted at most once per year (Figure 1).
Figure 1. Unique Lp(a) testing by year.
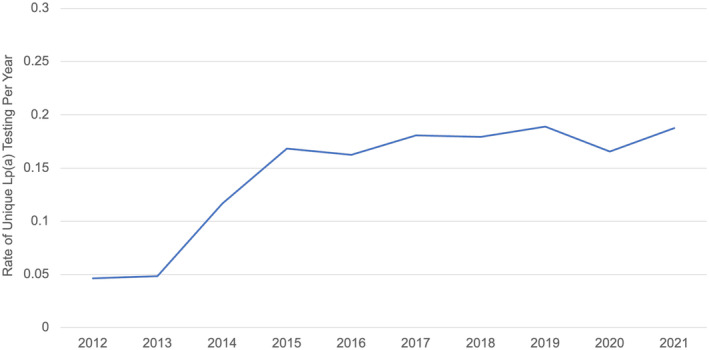
The rates of unique individuals with Lp(a) testing per year from 2012 to 2021 are shown. Unique individuals were tabulated per year; thus, each individual was counted at most once per year. Lp(a) indicates lipoprotein(a).
Characteristics of patients with and without lipoprotein(a) testing are shown in the Table. In general, those with lipoprotein(a) testing had a higher burden of cardiovascular risk factors, including older age, being men, and higher prevalence of CVD conditions and other cardiovascular testing. Those with lipoprotein(a) testing were more likely to be White race and less likely to be from other racial and ethnic groups, including Asian, Black, or Hispanic/Latino. In addition, those with lipoprotein(a) testing were significantly more likely to be on statin therapy.
Table 1.
Characteristics of Patients With and Without Lipoprotein(a) Testing
| Characteristic | Lipoprotein(a) testing (n=18 972) | No lipoprotein(a) testing (n=5 534 682) | Difference in means/proportions (95% CI) | P value |
|---|---|---|---|---|
| Age, y | 63 (16) | 51 (19) | 12.0 (11.7 to 12.3) | <0.001 |
| Female sex | 8399 (44.3) | 3 047 233 (55.1) | −10.8 (−11.5 to −10.1) | <0.001 |
| Race | <0.001 | |||
| American Indian or Alaska Native | 59 (0.3) | 21 608 (0.4) | −0.1 (−0.2 to 0.003) | |
| Asian | 1624 (8.6) | 494 342 (8.9) | −0.3 (−0.8 to 0.03) | |
| Black | 616 (3.2) | 264 433 (4.8) | −1.6 (−1.8 to −1.3) | |
| Native Hawaiian or other Pacific Islander | 87 (0.5) | 28 544 (0.5) | 0 (−0.2 to 0.0) | |
| White | 12 500 (65.9) | 2 620 250 (47.3) | 18.6 (17.9 to 19.2) | |
| Other or unknown | 4086 (21.5) | 2 105 505 (38.0) | −16.5 (−17.1 to −15.9) | |
| Ethnicity | <0.001 | |||
| Hispanic or Latino | 1691 (8.9) | 832 238 (15.0) | −6.1 (−6.5 to −5.7) | |
| Diagnoses | ||||
| Aortic stenosis | 611 (3.2) | 5083 (0.1) | 3.1 (2.9 to 3.4) | <0.001 |
| Coronary artery calcification | 361 (1.9) | 689 (0.01) | 1.9 (1.7 to 2.1) | <0.001 |
| Chronic kidney disease | 1547 (8.2) | 40 117 (0.7) | 7.5 (7.0 to 7.8) | <0.001 |
| End‐stage renal disease | 399 (2.1) | 11 852 (0.2) | 1.9 (1.7 to 2.1) | <0.001 |
| Carotid stenosis | 739 (3.9) | 6688 (0.1) | 3.8 (3.5 to 4.1) | <0.001 |
| Diabetes | 2590 (13.7) | 133 262 (2.4) | 11.3 (10.8 to 11.7) | <0.001 |
| Family history of CVD | 3428 (18.1) | 1873 (0.03) | 18.1 (17.5 to 18.6) | <0.001 |
| Heart failure | 1465 (7.7) | 29 013 (0.5) | 7.2 (6.8 to 7.6) | <0.001 |
| Hypothyroidism | 2246 (11.8) | 59 902 (1.1) | 10.7 (10.3 to 11.2) | <0.001 |
| Ischemic heart disease | 5059 (26.7) | 56 971 (1.0) | 25.7 (25.0 to 26.3) | <0.001 |
| Menopause | 1162 (6.1) | 14 271 (0.3) | 5.8 (5.5 to 6.2) | <0.001 |
| Peripheral vascular disease | 677 (3.6) | 10 227 (0.2) | 3.4 (3.1 to 3.7) | <0.001 |
| Statin use | 7614 (40.1) | 421 626 (7.6) | 32.5 (31.8 to 33.2) | <0.001 |
| Stroke | 1031 (5.4) | 23 510 (0.4) | 5.0 (4.7 to 5.3) | <0.001 |
| Testing | ||||
| apoB | 4457 (23.5) | 174 (0.003) | 23.5 (22.9 to 24.1) | <0.001 |
| CCTA | 897 (4.7) | 2350 (0.04) | 4.7 (4.4 to 5.0) | <0.001 |
| hs‐CRP | 8588 (45.3) | 8483 (0.2) | 45.1 (44.4 to 45.8) | <0.001 |
| Lipid panel | 9821 (51.8) | 60 782 (1.1) | 50.7 (50.0 to 51.4) | <0.001 |
| Lipoprofile NMR | 1558 (8.2) | 1873 (0.03) | 8.2 (7.8 to 8.6) | <0.001 |
| LDL‐C, mg/dL | 95 (61–138) | 102 (79–127) | … | <0.001 |
The table shows characteristics of those with lipoprotein(a) testing compared with the those without lipoprotein(a) testing any time from 2012 to 2021. For those with lipoprotein(a) testing, characteristics are at the time of lipoprotein(a) testing. For those without lipoprotein(a) testing, characteristics are at the time of first visit in the database. Values are presented as number (percentage), mean (SD), or median (quartile 1–quartile 3). apoB indicates apolipoprotein B‐100; CCTA, coronary computed tomography angiography; CVD, cardiovascular disease; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; and NMR, nuclear magnetic resonance.
Rates of unique lipoprotein(a) testing between 2012 and 2021 in adults with incident CVD diagnosis are shown in Table S2, with <4% in each CVD condition evaluated, with the exception of coronary artery calcification (6.1% of individuals). Notably, the rates of lipoprotein(a) testing in individuals with FHx, IHD, PVD, AS, and stroke were 3.3%, 2.9%, 2.0%, 3.1%, and 1.8%, respectively. The rate of testing for carotid stenosis was 3.2% and the rate of testing for HF was 1.9%. The prevalence of lipoprotein(a) measurements differed among the CVD conditions evaluated (P<0.001). Lipoprotein(a) testing rates remained relatively stable over the study period for all diagnoses evaluated, with an upward trend peaking in 2017 to 2018 that was statistically significant (P<0.001) for AS, carotid stenosis, FHx, HF, and IHD (Figure 2A). Similar results were seen for lipoprotein(a) testing in the same year as an incident diagnosis of FHx (1.6%), IHD (1.2%), PVD (0.5%), AS (1.2%), carotid stenosis (1.1%), HF (0.8%), and stroke (0.8%) throughout the entire study period (Figure 2B). Testing rates appeared to increase from 2014 to 2017 for all conditions, and then declined after 2017 with an increase again in 2021 (Figure 2). Similar low testing rates were seen in individuals with diagnoses associated with increased CVD risk, such as diabetes and advanced renal disease, as well as those with other diagnoses (ie, end‐stage renal disease, hypothyroidism, and menopause) and statin use, which may influence an individual's baseline genetically determined lipoprotein(a) level (Table S3).
Figure 2. Lp(a) testing in patients with CVD diagnosis.
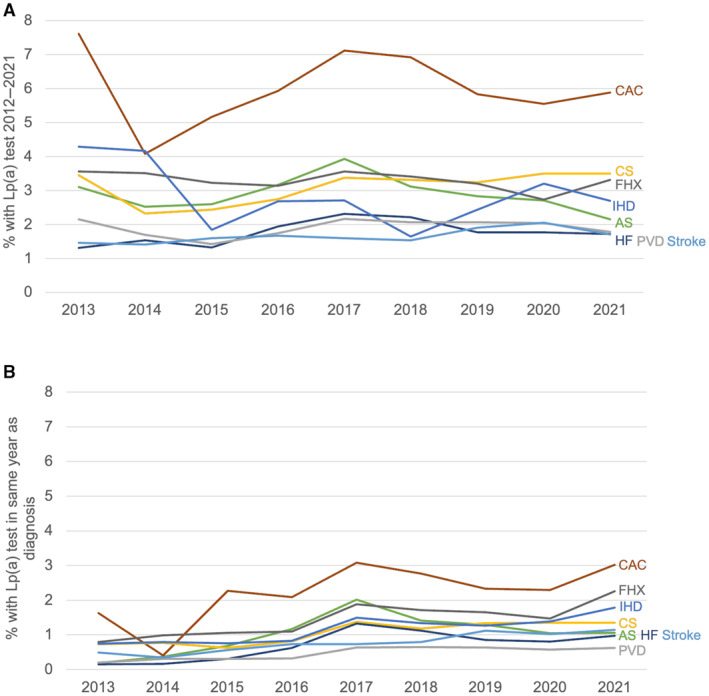
A, The rate of unique Lp(a) testing any time during the study period (2012–2021) by year of incident CVD diagnosis for multiple CVD conditions (P<0.05 for AS, CS, FHx, HF, and IHD). B, The rate of unique Lp(a) testing in the same year as incident CVD diagnosis for multiple CVD conditions (P<0.05 for all except CAC). AS indicates aortic stenosis; CAC, coronary artery calcification; CS, carotid stenosis; CVD, cardiovascular disease; FHx, family history of CVD; HF, heart failure; IHD, ischemic heart disease; Lp(a), lipoprotein(a); and PVD, peripheral vascular disease.
Testing rates were higher among individuals who also had additional biomarker or imaging testing for CVD risk stratification and varied by test (P<0.001). The proportions of adults who had lipid panel, apolipoprotein B‐100, lipoprofile nuclear magnetic resonance, hs‐CRP, and coronary computed tomography angiography testing who were also tested for lipoprotein(a) were 1.8%, 63.0%, 15.8%, 10.4%, and 6.8%, respectively (Table S2). Rates of incident lipoprotein(a) testing among individuals undergoing apolipoprotein B‐100 testing increased over time (P<0.001), peaking in 2015 and then declining and plateauing. Rates of testing among those undergoing lipoprofile nuclear magnetic resonance also increased over time (P<0.001), peaking in 2017, then declining. Among those undergoing coronary computed tomography angiography, rates of lipoprotein(a) testing peaked in 2017, then declined and plateaued (P<0.001). Testing among those undergoing lipid panel and hs‐CRP testing remained relatively stable over time, with slight upward and downward trends (all P<0.001; Figure 3).
Figure 3. Lp(a) testing in patients with other cardiovascular testing.
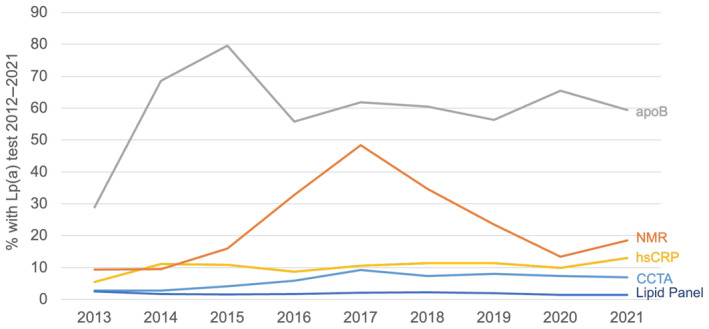
The figure demonstrates the rate of unique Lp(a) testing any time during the study period (2012–2021) by year of incident related cardiac testing (P<0.001 for all biomarker testing). apoB indicates apolipoprotein B‐100; CCTA, coronary computed tomography angiography; hs‐CRP, high‐sensitivity C‐reactive protein; Lp(a), lipoprotein(a); and NMR, nuclear magnetic resonance.
Among those with lipoprotein(a) testing, the rate of statin use at baseline was 40.1% (n=7614), which decreased to 39.5% (n=7493; P<0.001) at 1 year. The rate of ezetimibe use, however, increased from 5.6% (n=1062) to 6.7% (n=1267; P<0.001), and the rate of PCSK9i (proprotein convertase subtilisin/kexin type 9 inhibitors) also increased from 1.7% (n=329) to 2.2% (n=409; P<0.001). Among those without lipoprotein(a) testing, the rates of use of all 3 therapies declined at 1 year (7.6% [n=421 626] versus 5.9% [n=325 245] [P<0.001] for statin use; 0.5% [n=27 075] versus 0.3% [n=17 501] [P<0.001] for ezetimibe; and 0.04% [n=2476] versus 0.03% [n=1691] [P<0.001] for PCSK9i). For those with lipid panel testing, the rates of use of statin therapy (29.9% [n=176 379] versus 25.8% [n=152 311]; P<001) and ezetimibe (1.6% [n=9475] versus 1.6% [n=9263]; P<0.001) also declined, whereas the rate of PCSK9i use increased from 0.2% (n=1475) to 0.3% (n=1680; P<0.001).
Discussion
In this large study of >5.5 million adult patients seen across 6 academic health systems in California, lipoprotein(a) testing rates were low, with an overall prevalence of 0.3% and prevalence of <4% among patients with CVD (Figure 4). Rates of testing were low even among those with a family history of CVD, despite this being a population for whom there is guideline consensus for checking lipoprotein(a). Lipoprotein(a) testing rates were higher among patients who also had more specialized tests, suggesting that lipoprotein(a) measurements are used more in specialized versus general clinical practice. There were only modest and nonlinear increases in lipoprotein(a) testing over the study period. There were also notable disparities in lipoprotein(a) testing, which was performed less often in younger individuals, women, and racial and ethnic minorities.
Figure 4. Summary of key results.
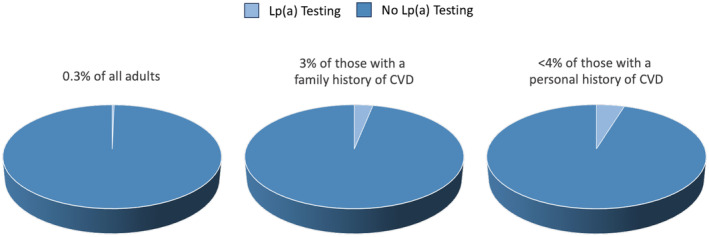
Percentage of all adults, those with a family history of CVD, and those with a personal history of CVD who have had Lp(a) testing. CVD indicates cardiovascular disease; and Lp(a), lipoprotein(a).
This study enumerates how underrecognized lipoprotein(a) is and how underdiagnosed elevated lipoprotein(a) is despite its high prevalence, with >20% of the general population corresponding to an estimated 64 million individuals in the United States and 1.4 billion individuals worldwide. Several barriers to more widespread lipoprotein(a) testing may exist and provide targets for improvement, including the following: (1) lack of awareness of lipoprotein(a) as a CVD risk factor, (2) lack of awareness of guideline indications for lipoprotein(a) testing, (3) lack of awareness of how to assess and manage risk in patients with elevated lipoprotein(a), (4) lack of implementation programs for lipoprotein(a) testing, (5) concerns about insurance coverage, and (6) uncertainty related to the variations in clinically available assays that reported lipoprotein(a) levels. Improved dissemination of the guidelines is needed, although there is also a need for more consistency across the major societal guidelines to provide clinicians with more certainty on when to use lipoprotein(a). Many high‐quality review articles discussing the mechanistic, epidemiologic, and human population genetic studies linking lipoprotein(a) to CVD, including the recent American Heart Association and European atherosclerosis society scientific statements on lipoprotein(a), exist and can be used for education. 6 , 15 These statements also review evidence‐based clinical approaches on how to test for lipoprotein(a), incorporate lipoprotein(a) into atherosclerotic CVD risk assessment and manage risk associated with elevated lipoprotein(a), and they describe implications for cascade screening. In addition to education, implementation of practice changes would be important to increase lipoprotein(a) testing rates. This was exemplified in a prior study by our group, observing that the rate of lipoprotein(a) testing among individuals with AS at a single academic center increased from 8.5% to a peak of 24.2% between 2010 and 2020 as a result of educational efforts and use of order sets in the electronic medical record, demonstrating that targeted efforts can meaningfully impact rates of testing. 16 Last, although targeted lipoprotein(a)‐lowering therapies are not yet clinically available, broader recognition of elevated lipoprotein(a) would facilitate enrollment of adequate numbers of diverse participants in existing or planned clinical trials evaluating such therapies.
Our study is the largest description of the prevalence of lipoprotein(a) measurements in a US population. Prior studies have also demonstrated low rates of testing for lipoprotein(a). In a study of all adults enrolled in the largest health maintenance organization in Israel, only 0.1% of individuals had lipoprotein(a) testing from 2015 to 2021. 17 Another study of >48 000 patients with a history of atherosclerotic CVD evaluated at multiple international sites for enrollment in a clinical trial of a targeted lipoprotein(a)‐lowering therapy observed that 13.9% of such patients previously had lipoprotein(a) measurements. 18 However, the higher prevalence of lipoprotein(a) testing in this study may not reflect general practice patterns in the United States as potential geographic differences were not described, and there may have been selection bias as the sites were selected for a lipoprotein(a) clinical trial. In addition, we observed only small increases in lipoprotein(a) testing from 2012 to 2021. The decline in lipoprotein(a) testing following 2019 was likely attributable to the COVID‐19 pandemic affecting elective health care and testing, a phenomenon that has been observed previously. 16 , 19 Therefore, additional time points will be required to fully understand whether there will be continued uptake of lipoprotein(a) testing, particularly after the publication of major societal guidelines from 2018 to 2021.
Our study has important limitations. The data extracted from the UCHDW rely on ICD‐10 and Current Procedural Terminology codes, which may be subject to misclassification. Given the consistency of lipoprotein(a) testing results, lipoprotein(a) testing may only need to be done once in an individual's lifetime in the absence of therapy affecting levels. Participants in this study may have had testing before the first data collection in 2012, which was not captured as part of this study, leading to subsequent testing not being performed after a CVD diagnosis. If an individual had multiple diagnoses, particularly within the same year, we are not able to determine which diagnosis led to testing. In addition, testing outside of the UC system, such as with outside providers or at independent laboratories, would not be captured in this database. Also, a significant proportion of individuals who had lipoprotein(a) testing did not have lipid panel testing in the UCHDW; we suspect that this is attributable to lipid panel testing being performed by physicians outside of the UC system or at independent commercial laboratories, with patients being referred to hospitals in the UC system for more specialized assessment and care. This is also evidenced by higher rates of lipoprotein(a) testing among those undergoing more specialized cardiac testing, such as apolipoprotein B‐100, nuclear magnetic resonance, and coronary computed tomography angiography. This may affect the generalizability of these results as they reflect the experience of specialized academic centers. Finally, data on the specific assays used for lipoprotein(a) measurements throughout the study period were not available; however, this is unlikely to affect the primary results on the prevalence of testing.
Conclusions
In summary, lipoprotein(a) testing rates are low overall and even in individuals diagnosed with a family or personal history of CVD and those undergoing relevant cardiac testing, and rates have increased minimally over time. Further education is needed, particularly with regard to guideline recommendations for lipoprotein(a) testing and clinical management of patients with elevated lipoprotein(a).
Sources of Funding
Dr Bhatia was partially supported by National Institutes of Health (NIH) grants 1KL2TR001444 and 5T32HL079891 (as part of the University of California San Diego Integrated Cardiovascular Epidemiology Fellowship). Dr Yeang has received research support from NIH grant 1K08HL150271‐01 and Kaneka Corporation. This work was also partially supported by NIH grant UL1TR001442 of Clinical and Translation Science Award funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosures
Dr Bhatia received consulting fees from Kaneka Corporation. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
This article was sent to Samuel S. Gidding, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.031255
For Sources of Funding and Disclosures, see page 8.
References
- 1. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. doi: 10.1016/j.jacc.2016.11.042 [DOI] [PubMed] [Google Scholar]
- 2. Langsted A, Nordestgaard BG, Kamstrup PR. Elevated lipoprotein(a) and risk of ischemic stroke. J Am Coll Cardiol. 2019;74:54–66. doi: 10.1016/j.jacc.2019.03.524 [DOI] [PubMed] [Google Scholar]
- 3. Colantonio LD, Bittner V, Safford MM, Marcovina S, Brown TM, Jackson EA, Li M, López JAG, Monda KL, Plante TB, et al. Lipoprotein(a) and the risk for coronary heart disease and ischemic stroke events among black and white adults with cardiovascular disease. J Am Heart Assoc. 2022;11:e025397. doi: 10.1161/jaha.121.025397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Masson W, Lobo M, Barbagelata L, Molinero G, Bluro I, Nogueira JP. Elevated lipoprotein (a) levels and risk of peripheral artery disease outcomes: a systematic review. Vasc Med. 2022;27:385–391. doi: 10.1177/1358863X221091320 [DOI] [PubMed] [Google Scholar]
- 5. Kamstrup PR, Nordestgaard BG. Elevated lipoprotein(a) levels, LPA risk genotypes, and increased risk of heart failure in the general population. JACC Heart Fail. 2016;4:78–87. doi: 10.1016/j.jchf.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 6. Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, Dweck MR, Koschinsky M, Lambert G, Mach F, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43:3925–3946. doi: 10.1093/eurheartj/ehac361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, Fras Z, Goodman SG, Halvorsen S, Hanotin C, et al. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75:133–144. doi: 10.1016/j.jacc.2019.10.057 [DOI] [PubMed] [Google Scholar]
- 8. O'Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni‐Berthold I, Im K, Lira Pineda A, Wasserman SM, Češka R, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139:1483–1492. doi: 10.1161/CIRCULATIONAHA.118.037184 [DOI] [PubMed] [Google Scholar]
- 9. Varvel S, McConnell JP, Tsimikas S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532 359 patients in the United States. Arterioscler Thromb Vasc Biol. 2016;36:2239–2245. doi: 10.1161/atvbaha.116.308011 [DOI] [PubMed] [Google Scholar]
- 10. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 11. Pearson GJ, Thanassoulis G, Anderson TJ, Barry AR, Couture P, Dayan N, Francis GA, Genest J, Grégoire J, Grover SA, et al. 2021 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37:1129–1150. doi: 10.1016/j.cjca.2021.03.016 [DOI] [PubMed] [Google Scholar]
- 12. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:e1182–e1186. doi: 10.1161/CIR.0000000000000698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, Orringer CE. Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13:374–392. doi: 10.1016/j.jacl.2019.04.010 [DOI] [PubMed] [Google Scholar]
- 14. Handelsman Y, Jellinger PS, Guerin CK, Bloomgarden ZT, Brinton EA, Budoff MJ, Davidson MH, Einhorn D, Fazio S, Fonseca VA, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the management of dyslipidemia and prevention of cardiovascular disease algorithm‐2020 executive summary. Endocr Pract. 2020;26:1196–1224. doi: 10.4158/cs-2020-0490 [DOI] [PubMed] [Google Scholar]
- 15. Reyes‐Soffer G, Ginsberg HN, Berglund L, Duell PB, Heffron SP, Kamstrup PR, Lloyd‐Jones DM, Marcovina SM, Yeang C, Koschinsky ML. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42:e48–e60. doi: 10.1161/ATV.0000000000000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhatia HS, Ma GS, Taleb A, Wilkinson M, Kahn AM, Cotter B, Yeang C, DeMaria AN, Patel MP, Mahmud E, et al. Trends in testing and prevalence of elevated Lp(a) among patients with aortic valve stenosis. Atherosclerosis. 2022;349:144–150. doi: 10.1016/j.atherosclerosis.2022.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zafrir B, Aker A, Saliba W. Lipoprotein(a) testing in clinical practice: real‐life data from a large health‐care provider. Eur J Prev Cardiol. 2022;29:e331–e333. doi: 10.1093/eurjpc/zwac124 [DOI] [PubMed] [Google Scholar]
- 18. Nissen SE, Wolski K, Cho L, Nicholls SJ, Kastelein J, Leitersdorf E, Landmesser U, Blaha M, Lincoff AM, Morishita R, et al. Lipoprotein(a) levels in a global population with established atherosclerotic cardiovascular disease. Open Heart. 2022;9:9. doi: 10.1136/openhrt-2022-002060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alexander GC, Tajanlangit M, Heyward J, Mansour O, Qato DM, Stafford RS. Use and content of primary care office‐based vs telemedicine care visits during the COVID‐19 pandemic in the US. JAMA Netw Open. 2020;3:e2021476. doi: 10.1001/jamanetworkopen.2020.21476 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


