Abstract
Background
We aimed to compare statin monotherapy and upfront combination therapy of statin and ezetimibe in patients with acute coronary syndromes (ACSs).
Methods and Results
The study included consecutive patients with ACS included in the PL‐ACS (Polish Registry of Acute Coronary Syndromes), which is a national, multicenter, ongoing, prospective observational registry that is mandatory for patients with ACS hospitalized in Poland. Data were matched using the Mahalanobis distance within propensity score matching calipers. Multivariable stepwise logistic regression analysis, including all variables, was next used in propensity score matching analysis. Finally, 38 023 consecutive patients with ACS who were discharged alive were included in the analysis. After propensity score matching, 2 groups were analyzed: statin monotherapy (atorvastatin or rosuvastatin; n=768) and upfront combination therapy of statin and ezetimibe (n=768 patients). The difference in mortality between groups was significant during the follow‐up and was present at 1 (5.9% versus 3.5%; P=0.041), 2 (7.8% versus 4.3%; P=0.019), and 3 (10.2% versus 5.5%; P=0.024) years of follow‐up in favor of the upfront combination therapy, as well as for the overall period. For the treatment, rosuvastatin significantly improved prognosis compared with atorvastatin (odds ratio [OR], 0.790 [95% CI, 0.732–0.853]). Upfront combination therapy was associated with a significant reduction of all‐cause mortality in comparison with statin monotherapy (OR, 0.526 [95% CI, 0.378–0.733]), with absolute risk reduction of 4.7% after 3 years (number needed to treat=21).
Conclusions
The upfront combination lipid‐lowering therapy is superior to statin monotherapy for all‐cause mortality in patients with ACS. These results suggest that in high‐risk patients, such an approach, rather than a stepwise therapy approach, should be recommended.
Keywords: acute coronary syndrome, combination therapy, lipids, monotherapy, prevention
Subject Categories: Epidemiology, Secondary Prevention, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- HIJ‐PROPER
Heart Institute of Japan Proper Level of Lipid Lowering With Pitavastatin and Ezetimibe in Acute Coronary Syndrome
- IMPROVE‐IT
Improved Reduction of Outcomes: Vytorin Efficacy International Trial
- KOS‐Zawał
Managed Care After Acute Myocardial Infarction in Poland
- LLT
lipid‐lowering therapy
- PL‐ACS
Polish Registry of Acute Coronary Syndromes
- PSM
propensity score matching
- RACING
Randomized Comparison of Efficacy and Safety of Lipid‐Lowering With Statin Monotherapy Versus Statin/Ezetimibe Combination for High‐Risk Cardiovascular Diseases
Clinical Perspective.
What Is New?
We aimed to assess which strategy, monotherapy versus upfront combination lipid‐lowering therapy of statin and ezetimibe, is superior in the context of the reduction of all‐cause mortality in patients with acute coronary syndrome.
It is the first such analysis that presents results based on real‐world data.
We confirmed that already after 1.5 months, the difference in the reduction of all‐cause mortality was significant, with absolute risk reduction of 4.7% after 3 years (number needed to treat=21).
What Are the Clinical Implications?
Because the strategy of lipid‐lowering therapy recommended in the European guidelines has not been sufficient to increase the percentage of patients achieving the low‐density lipoprotein cholesterol goal (only one‐third in Europe), in 2021, the International Lipid Expert Panel recommended introducing the upfront combination therapy in a selected group of patients at very high and extremely high cardiovascular risk.
However, the approach of upfront combination therapy has had limited data to support it.
In this analysis, we proved that upfront combination lipid‐lowering therapy is superior to statin monotherapy for all‐cause mortality, which questions the recommended stepwise approach.
Atherosclerotic cardiovascular disease is still the most important cause of cardiovascular death. 1 Among the principles of pharmacotherapy of acute coronary syndromes (ACSs), there is a need for intensive lipid‐lowering therapy (LLT). Preventing dyslipidemia, as a modifiable risk factor, remains crucial to improve morbidity and mortality. 2 , 3 The therapeutic goal is, however, difficult to achieve, with only less than one‐third of patients being on the goal (irrespective of the risk) and only 18% of very high‐risk individuals being on the goal of <55 mg/dL (1.4 mmol/L). 4 The main rules of LLT are “the earlier, the better” and “the lower, the better for longer.” 5 , 6 Therefore, in many cases, combination therapy remains the only way to be effective. 7 , 8 The 2019 European Society of Cardiology/European Atherosclerosis Society guidelines categorize patients after ACS as very high‐risk patients, defining the goals for low‐density lipoprotein cholesterol (LDL‐C) reduction to be <55 mg/dL (1.4 mmol/L). 7 The procedure to achieve the target according to European Society of Cardiology guidelines is to start with monotherapy, and when it is not sufficient to add other LLT (after 4–6 weeks). 7 In this case, the recommended strategy is the combination with ezetimibe (class IIa), and if still not at target, the addition of a PCSK9 (proprotein convertase subtilisin/kexin 9) inhibitor is recommended (class 1).
However, because of the fact that this strategy is not sufficient enough to increase the percentage of patients being on LDL‐C goal, and in consequence to effectively reduce the risk of cardiovascular events and mortality (even 20% in the first year after ACS), since 2021, most experts suggest immediate/upfront combination therapy of statin and ezetimibe, which also allows, in case of lack of LDL‐C target achievement, practitioners to introduce PCSK9 inhibitors only after 4 to 6 weeks. 8 In April 2021, the experts within the International Lipid Expert Panel 8 for the first time recommended that, because of large difficulties in reaching treatment goals, 9 , 10 , 11 there was a large need to extend the definition of the extremely high‐risk patients and to introduce the upfront combination therapy in a selected group of patients at very high cardiovascular risk. 8 Therefore, with the aim to reduce the delay in therapeutic target achievement, the International Lipid Expert Panel experts recommend initiation of the upfront combination therapy in 4 patient groups: those with very high LDL‐C baseline levels unable to achieve goals with statin monotherapy, patients with ACS and concomitant familial hypercholesterolemia, those at extremely high risk, and those already treated optimally before hospitalization. 8 This approach was next approved by other societies 12 and expert groups, which even suggested considering upfront combination therapy in all patients at very high cardiovascular disease risk. 13
Therefore, the aim of our analysis was to assess which strategy, monotherapy or upfront combination LLT, is superior in the context of the reduction of all‐cause mortality in patients with ACS based on the data from the Polish Registry of Acute Coronary Syndromes (PL‐ACS).
Methods
Ethical review and approval were waived for this study because of retrospective analysis of the prospective PL‐ACS.
Data Source
The PL‐ACS is a national, multicenter, ongoing, prospective observational registry that includes data on patients hospitalized with ACS in Poland. It is a joint project of the Silesian Center of Heart Diseases in Zabrze and the Polish Ministry of Health, in cooperation with the National Health Fund. 14 The registry was founded in October 2003, and in May 2004, it was harmonized with the European Cardiology Audit and Registration Data Standards. 14 Participation in the registry is mandatory for every hospital treating patients with ACS in Poland. Full details on the rationale and methods of the PL‐ACS have been previously described. 14 Before enrollment, informed consent was signed by all participants. Institutional review board approval was obtained for all participants. The authors declare that all supporting data are available within the article. The primary end point was all‐cause mortality, assessed at 1, 2, and 3 years of observation. No other cardiovascular end points (eg, cardiovascular mortality and nonfatal myocardial infarction and stroke) are available in the registry. The median follow‐up was 3 years.
Population
The study included consecutive patients with ACS included in PL‐ACS, who were discharged alive. At the time of this analysis, the registry included 810 511 patients with ACS (Figure 1). Because information on LDL‐C levels has been collected only since 2016, and it is not required to enter these data for patients based on the registry design, data on LDL‐C concentrations and outcomes were available for 38 023 patients. Data on ezetimibe have been available in the registry since 2017 (Figure 1).
Figure 1. Study flowchart.
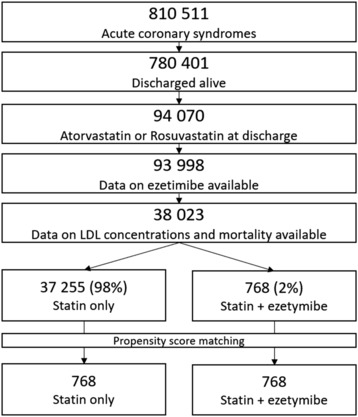
LDL indicates low‐density lipoprotein.
Data on comorbidities, history of coronary interventions, and concomitant diseases were collected. Patients with heart failure were assessed using New York Heart Association and Killip‐Kimball classifications. Data on medications, including a history of statin and ezetimibe use, were also collected. In all patients' physical examinations, laboratory testing (including low‐density lipoprotein concentrations) and echocardiography were performed. Neither historical laboratory testing nor dosage of statins (and other medications) was collected as it was not a part of the protocol.
Statistical Analysis
Continuous variables were presented as means and SDs. Categorical variables were shown as percentages. Groups were compared using the parametric Student t‐test for continuous variables and the χ2 test for categorical data.
The study patients were matched to achieve similar: age, sex, left ventricular ejection fraction, heart rate, systolic blood pressure, LDL‐C levels, ACS diagnosis (ST‐segment elevation myocardial infarction versus non–ST‐segment elevation ACS), history of hypertension, diabetes, myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, peripheral arterial disease, cardiac arrest, current smoking, atrial fibrillation, New York Heart Association 3 or 4 class (versus 1 or 2 class), Killip 3 or 4 class (versus 1 or 2 class), inotrope treatment in hospital, angiotensin‐converting enzyme inhibitors/angiotensin receptor blocker/angiotensin receptor–neprilysin inhibitor, β‐blocker treatment at discharge, rosuvastatin (versus atorvastatin), left main disease, multivessel coronary disease, and percutaneous coronary intervention in the acute phase of ACS.
Data were matched using the Mahalanobis distance within propensity score matching (PSM) calipers. The caliper radius was set to 0.2*sigma. 15 , 16 Finally, the propensity score–matched groups included 768 individuals each. Kaplan‐Meier curves after PSM were drawn to show the cumulative survival in 3‐year observation. Survival between the groups before PSM was compared using the log‐rank test; and after PSM, it was compared using the Wilcoxon test for nonpaired samples. The standardized mean differences for groups after PSM were calculated. The matched cohorts were considered well balanced for a given variable if the absolute standardized mean difference between the cohorts was ≤0.1. Multivariable analysis, including all variables used in PSM analysis, was performed with forward stepwise logistic regression P<0.05 for inclusion and P>0.05 for exclusion in the whole study group. For the categorical variables, the null values were imputed by a random new category. For continuous variables (missing rates were <0.5%), median imputation was performed. Statistical significance was defined as 2‐sided P<0.05. All statistical analyses were performed using TIBCO Statistica software (TIBCO Statistica, v. 13.3.0; TIBCO Software Inc, Palo Alto, CA).
Results
Among 810 511 patients included in the ACS registry because of ACS, 780 401 (96.3%) were discharged from the hospital. From those patients, we had data for 93 023 (11.92%) subjects who were discharged on atorvastatin or rosuvastatin with the potential addition of ezetimibe prescribed. Because of procedural circumstances (see Methods), data on LDL‐C concentration and outcome were present in 38 023 patients (Figure 1). Thus, the study finally included 38 023 consecutive patients with ACS who were discharged alive, for whom data on low‐density lipoprotein concentrations and use of statin and ezetimibe (n=768 [2%]) were available (Figure 1). After, the propensity score–matching monotherapy with statins (atorvastatin or rosuvastatin) and combination therapy with ezetimibe was used in 768 patients each (Figure 1).
Patients' Main Characteristics
Patient baseline characteristics, comorbidities, New York Heart Association classification, Killip‐Kimball classification, clinical data, and data on pharmacotherapy are presented in Table 1. The group with statin monotherapy was more numerous with 37 255 patients (98%); the patients were older (66.8 [11.1] versus 63.5 [10.7] years; P<0.001) but had fewer comorbidities. The prevalence of hypertension, dyslipidemia, and history of coronary syndromes, including ACS, treated or not with percutaneous coronary intervention, coronary artery bypass grafting, history of heart failure, and history of peripheral arterial disease was significantly higher in patients treated with statin versus those on the combination therapy. There were no differences between groups for the presence of diabetes, stroke history, or chronic obstructive pulmonary disease. More patients with statin monotherapy were in higher New York Heart Association class; there were no differences in the number of current smokers between the groups. Patients on statin monotherapy had slightly lower ejection fraction (48.7% [10.1%] versus 49.5% [9.7%]; P=0.02), but higher baseline heart rate and systolic blood pressure (77.8 [18.0] versus 75.6 [17.2] beats per minute [P<0.001] and 140.4 (25.2) versus 138.0 (24.9) mm Hg [P=0.009], respectively). The baseline low‐density lipoprotein was lower in the group where the patients were treated with statins only (113 [52.2] versus 131.8 [71.7] mg/dL; P<0.001) (Table 1).
Table 1.
Main Characteristics of Patients Treated With Statin Monotherapy and With Combination Therapy Before and After PSM
| Before PSM | After PSM | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Statin monotherapy | Statin+ezetimibe | P value | Statin monotherapy | Statin+ezetimibe | P value | SMD |
| No. | 37 255 | 768 | … | 768 | 768 | … | … |
| Age, y | 66.8 (11.1) | 63.5 (10.7) | <0.001 | 64.0 (11.2) | 63.5 (10.7) | 0.33 | 0.01 |
| Men, n (%) | 24 834 (66.4) | 512 (66.7) | 0.99 | 509 (66.3) | 512 (66.7) | 0.93 | −0.01 |
| STEMI, n (%) | 12 263 (32.9) | 192 (25.0) | <0.001 | 188 (24.5) | 192 (25.0) | 0.81 | 0.01 |
| Hypertension, n (%) | 26 595 (71.4) | 580 (75.5) | 0.01 | 594 (77.3) | 580 (75.5) | 0.40 | −0.04 |
| Dyslipidemia, n (%) | 17 423 (46.8) | 511 (66.5) | <0.001 | 526 (68.5) | 511 (66.5) | 0.41 | −0.04 |
| Diabetes, n (%) | 10 545 (28.3) | 228 (29.7) | 0.40 | 217 (28.3) | 228 (29.7) | 0.54 | 0.03 |
| History of CAD, n (%) | 11 607 (31.2) | 407 (53.0) | <0.001 | 410 (53.4) | 407 (53.0) | 0.88 | −0.06 |
| History of MI, n (%) | 7978 (21.4) | 314 (40.9) | <0.001 | 329 (39.0) | 314 (40.9) | 0.44 | −0.04 |
| History of PCI, n (%) | 7722 (20.7) | 312 (40.6) | <0.001 | 327 (42.6) | 312 (40.6) | 0.44 | −0.04 |
| History of CABG, n (%) | 1812 (4.9) | 78 (10.2) | <0.001 | 84 (10.9) | 78 (10.2) | 0.62 | −0.03 |
| History of HF, n (%) | 2964 (8.0) | 76 (9.9) | 0.05 | 88 (11.5) | 76 (9.9) | 0.32 | −0.05 |
| History of AF, n (%) | 4152 (11.1) | 59 (7.7) | 0.003 | 56 (7.3) | 59 (7.7) | 0.77 | 0.01 |
| History of CKD, n (%) | 2350 (6.3) | 53 (6.9) | 0.14 | 60 (7.8) | 53 (6.9) | 0.49 | −0.03 |
| History of PAD, n (%) | 1942 (5.2) | 62 (8.1) | <0.001 | 56 (7.3) | 62 (8.1) | 0.57 | 0.03 |
| History of stroke, n (%) | 1768 (4.8) | 41 (5.3) | 0.45 | 36 (4.7) | 41 (5.3) | 0.64 | 0.03 |
| History of COPD, n (%) | 1584 (4.3) | 27 (3.5) | 0.32 | 19 (2.5) | 27 (3.5) | 0.23 | 0.06 |
| NYHA class III‐IV, n (%) | 1425 (3.8) | 13 (1.7) | 0.003 | 11 (1.4) | 13 (1.7) | 0.64 | 0.02 |
| Killip class III‐IV, n (%) | 1068 (2.9) | 16 (2.1) | 0.45 | 11 (1.4) | 16 (2.1) | 0.33 | 0.05 |
| ACEI/ARB/ARNI, n (%) | 31 526 (84.6) | 690 (89.8) | <0.001 | 689 (89.7) | 690 (89.8) | 0.93 | 0.01 |
| β‐Blocker, n (%) | 32 759 (88.0) | 687 (89.5) | 0.21 | 702 (91.4) | 687 (89.5) | 0.19 | −0.07 |
| Rosuvastatin, n (%) | 12 068 (32.4) | 557 (72.5) | <0.001 | 559 (72.8) | 557 (72.5) | 0.91 | −0.01 |
| Inotropes, n (%) | 983 (2.6) | 16 (2.1) | 0.34 | 18 (2.3) | 16 (2.1) | 0.73 | −0.02 |
| Cardiac arrest, n (%) | 714 (1.9) | 14 (1.8) | 0.85 | 17 (2.2) | 14 (1.8) | 0.59 | −0.03 |
| Current smokers, n (%) | 10 437 (28.0) | 197 (25.7) | 0.15 | 176 (22.9) | 197 (25.7) | 0.21 | 0.01 |
| EF, % | 48.7 (10.1) | 49.5 (9.7) | 0.02 | 49.6 (10.2) | 49.5 (9.7) | 0.88 | −0.01 |
| Heart rate, bpm | 77.8 (18.0)] | 75.6 (17.2) | <0.001 | 75.3 (15.9) | 75.6 (17.2) | 0.75 | 0.02 |
| Systolic BP, mm Hg | 140.4 (25.2) | 138.0 (24.9) | 0.009 | 138.6 (24.3) | 138.0 (24.9) | 0.61 | −0.03 |
| LDL, mg/dL | 113.0 (52.2) | 131.8 (71.7) | <0.001 | 126.1 (94.6) | 131.8 (71.7) | 0.19 | 0.07 |
| MVD, n (%) | 15 699 (42.1) | 382 (49.7) | <0.001 | 383 (49.9) | 382 (49.7) | 0.96 | −0.01 |
| LM disease, n (%) | 2371 (6.4) | 68 (8.9) | 0.01 | 71 (9.2) | 68 (8.9) | 0.79 | −0.01 |
| PCI, n (%) | 29 288 (78.7) | 651 (84.8) | <0.001 | 641 (83.5) | 651 (84.8) | 0.49 | 0.04 |
| CABG direct, n (%) | 1413 (3.8) | 15 (2.0) | 0.01 | 18 (2.3) | 15 (2.0) | 0.60 | −0.03 |
| Postdischarge all‐cause mortality, n (%) | |||||||
| 1‐y Follow‐up | 2511 (6.7) | 27 (3.5) | <0.001 | 45 (5.9) | 27 (3.5) | 0.041 | … |
| 2‐y Follow‐up | 3650 (9.8) | 33 (4.3) | <0.001 | 60 (7.8) | 33 (4.3) | 0.019 | … |
| 3‐y Follow‐up | 4506 (12.1) | 42 (5.5) | <0.001 | 78 (10.2) | 42 (5.5) | 0.024 | … |
| Overall follow‐up | 4871 (13.1) | 42 (5.5) | <0.001 | 83 (10.8) | 42 (5.5) | 0.022 | … |
Data are given as mean (SD) unless otherwise indicated. ACEI indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; BP, blood pressure; bpm, beats per minute; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; HF, heart failure; LDL, low‐density lipoprotein; LM, left main; MI, myocardial infarction; MVD, multivessel coronary artery disease; NYHA, New York Heart Association; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; PSM, propensity score matching; SMD, standardized mean difference; and STEMI, ST‐segment–elevation MI.
Monotherapy Versus Upfront Combination Therapy and Mortality
The group of patients on the combination therapy (statin plus ezetimibe) was shown to have significantly lower postdischarge all‐cause mortality (before matching) than patients treated with a statin alone (5.5% versus 12.1%; P<0.001), with absolute risk reduction (ARR) of 6.6% after 3 years of follow‐up (Table 1).
The multivariable analysis performed before PSM (Table 2) showed that age was positively and significantly associated with the risk of mortality (odds ratio [OR], 1.065 [95% CI, 1.061–1.069]); male sex also significantly increased the risk. The risk of mortality for a patient with concomitant chronic kidney disease was >2‐fold greater (OR, 2.100 [95% CI, 1.894–2.330]). Other diseases that increased the risk of mortality were diabetes, peripheral arterial disease, heart failure, atrial fibrillation, chronic obstructive pulmonary disease, and previous myocardial infarction or stroke (Table 2). Low ejection fraction, the use of angiotensin‐converting enzyme inhibitors, angiotensin receptor blocker, or angiotensin receptor–neprilysin inhibitor, and systolic blood pressure were inversely associated with the mortality risk. Another factor that significantly contributed to increased all‐cause mortality was smoking (OR, 1.196 [95% CI, 1.093–1.308]). For the LLT, rosuvastatin significantly improved prognosis compared with atorvastatin (OR, 0.790 [95% CI, 0.732–0.853]), and the combination of LLT with statin and ezetimibe reduced mortality in comparison to monotherapy with a statin (OR, 0.526 [95% CI, 0.378–0.733]).
Table 2.
Multivariable Analysis to Identify the Variables Affecting All‐Cause Mortality in the 3‐Year Follow‐Up
| Variable | OR | 95% CI | P value | |
|---|---|---|---|---|
| Age, per 1‐y increase | 1.065 | 1.061 | 1.069 | <0.0001 |
| EF, per 1% increase | 0.967 | 0.963 | 0.970 | <0.0001 |
| History of CKD | 2.100 | 1.894 | 2.330 | <0.0001 |
| Heart rate | 1.009 | 1.007 | 1.011 | <0.0001 |
| History of PAD | 1.591 | 1.410 | 1.794 | <0.0001 |
| History of diabetes | 1.435 | 1.336 | 1.541 | <0.0001 |
| ACEI/ARB/ARNI | 0.687 | 0.630 | 0.749 | <0.0001 |
| History of stroke | 1.677 | 1.482 | 1.898 | <0.0001 |
| PCI in MI | 0.715 | 0.661 | 0.774 | <0.0001 |
| NYHA class 3–4 (vs 1–2) | 1.574 | 1.377 | 1.799 | <0.0001 |
| History of MI | 1.285 | 1.190 | 1.389 | <0.0001 |
| Killip class 3–4 (vs 1–2) | 1.621 | 1.391 | 1.888 | <0.0001 |
| Rosuvastatin (vs atorvastatin) | 0.790 | 0.732 | 0.853 | <0.0001 |
| History of COPD | 1.464 | 1.278 | 1.677 | <0.0001 |
| History of AF | 1.231 | 1.123 | 1.349 | <0.0001 |
| Systolic BP, per 1–mm Hg increase | 0.997 | 0.996 | 0.998 | <0.0001 |
| Current smoking | 1.196 | 1.093 | 1.308 | <0.0001 |
| Multivessel coronary disease | 1.149 | 1.072 | 1.231 | <0.0001 |
| Statin+ezetimibe (vs statin only) | 0.526 | 0.378 | 0.733 | <0.0001 |
| History of HF | 1.208 | 1.089 | 1.339 | <0.0001 |
| Male sex | 1.131 | 1.051 | 1.218 | 0.001 |
| Inotropes | 1.305 | 1.092 | 1.558 | 0.003 |
Variables: β‐blocker at admission, ST‐segment–elevation myocardial infarction vs non–ST‐segment–elevation acute coronary syndrome, history of hypertension, dyslipidemia, cardiac arrest, history of coronary artery disease, history of PCI, history of coronary artery bypass grafting, left main disease, and low‐density lipoprotein concentrations were included in the model but eventually were dropped out in the stepwise analysis. ACEI indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; BP, blood pressure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; HF, heart failure; MI, myocardial infarction; NYHA, New York Heart Association; OR, odds ratio; PAD, peripheral arterial disease; and PCI, percutaneous coronary intervention.
After applying PSM, the difference in mortality between groups remained significant at 1 (5.9% versus 3.5%; P=0.041; ARR, 2.4%), 2 (7.8% versus 4.3%; P=0.019; ARR, 3.5%) and 3 (10.2% versus 5.5%; P=0.024; ARR, 4.7%; number needed to treat=21) years, as well as for the overall period (10.8% versus 5.5%; P=0.022; ARR, 5.3%; number needed to treat=19) (Table 1).
Kaplan‐Meier event rates for the primary end point (all‐cause mortality) assessed at 1 year were 3.5% in the combination therapy group and 5.9% in the monotherapy group (Figure 2). For the 2‐year observation, it was 4.3% and 7.8%, respectively, whereas for the 3‐year observation, the difference was growing and constituted as 5.5% versus 10.2%. The benefit appeared to emerge at ≥52 days (Figure 2).
Figure 2. Kaplan‐Meier curves with 52‐day and 1‐, 2‐, and 3‐year postdischarge all‐cause mortality.

Discussion
The combination of LLT with statin and ezetimibe administered in patients after ACS significantly reduced mortality during the 3‐year follow‐up compared with monotherapy with atorvastatin or rosuvastatin, with the clinical benefit observed for every 21st patient. More important, the significant effect of the upfront combination therapy appeared early, at 52 days of treatment. In those patients treated with statins, monotherapy rosuvastatin proved to be superior to atorvastatin for the reduction of the primary end point.
One of the first randomized controlled trials, which assessed combination LLT was the IMPROVE‐IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). 17 The study included 18 144 patients randomized to combination therapy (ezetimibe and simvastatin; 9067 patients) and simvastatin monotherapy (9077 patients). The primary end point, defined as cardiovascular death, ACS, coronary revascularization in <30 days, or stroke, was less frequent in the combination therapy group compared with monotherapy group (32.7% versus 34.7%; hazard ratio [HR], 0.936 [95% CI, 0.89–0.99]; P=0.016). Contrary to our results, there was no difference between the groups for all‐cause mortality (15.4% versus 15.3%; HR, 0.99 [95% CI, 0.91–1.07]; P=0.11). The authors also demonstrated that the beneficial effect of combined therapy was irrespective of baseline values of LDL‐C. 17 However, the observed benefit was much higher in those at higher cardiovascular disease risk, for example, in those with diabetes (ARR, 5.5%; HR, 0.86 [95% CI, 0.78–0.94]; P=0.023), or in those with a stroke before randomization (ARR, 8.6% for stroke of any cause [number needed to treat=12; HR, 0.60 {95% CI, 0.38–0.95}; P=0.030]; and ARR, 7.6% for ischemic stroke [number needed to treat=13; HR, 0.52 {95% CI, 0.31–0.86}; P=0.011]). 17 , 18
Another study, the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial, assessed the thickness of intima‐media of the carotid arteries in patients with familial hypercholesterolemia on monotherapy with simvastatin and on combination therapy with simvastatin with ezetimibe. 19 They showed no significant difference between the groups for the difference from baseline in the intima‐media thickness (P=0.29). All‐cause mortality between groups (as well as observed major adverse cardiac events) was also similar (however, with only a few observed events: 1 patient in the monotherapy group versus 2 patients in the combination therapy group). 19 The study was also limited by the relatively small number of included patients (n=720) and a short period of observation (24 months). 19 A similarly low number of major adverse cardiac events was observed in study by Hibi et al, which compared monotherapy versus combination LLT (no deaths versus 3 all‐cause deaths; P=0.50). 20 Patients were randomized to pitavastatin with ezetimibe (n=50) or pitavastatin alone (n=53). After a 10‐month follow‐up, the combination LLT was more effective in LDL‐C reduction than monotherapy with pitavastatin (−45.7% versus −28.8%; P<0.0001); however, there was no difference in the primary end point, defined as percentage change in atheroma coronary plaque volume between groups (combination therapy versus monotherapy statin group, −5.1% versus −6.2%; P=0.66). 20 A combination of pitavastatin with ezetimibe was also assessed in the HIJ‐PROPER (Heart Institute of Japan Proper Level of Lipid Lowering With Pitavastatin and Ezetimibe in Acute Coronary Syndrome) trial. 21 The trial demonstrated that aggressive coronary revascularization along with LDL‐C decrease to a target value <70 mg/dL (1.8 mmol/L) with the combination therapy was not superior to pitavastatin monotherapy for the reduction of cardiovascular events. On the other hand, combination therapy appeared to be significantly more effective in cardiovascular event reduction in patients with higher cholesterol absorption, represented by elevated pretreatment sitosterol. 21
A study by Liu et al showed a similar number of major adverse cardiac events between the combination therapy group (atorvastatin, 10 mg, with ezetimibe, 10 mg; n=114) and the double‐dose atorvastatin group (20 mg; n=116) (23.2% versus 19.8%, respectively; P=0.55). 22 The primary end point was defined as cardiac death, ACS, or unplanned revascularization, and the follow‐up lasted 1 year. The risk of major adverse cardiac events was similar between groups (HR, 1.12 [95% CI, 0.51–2.55]; P=0.74). 22 A combination of atorvastatin with ezetimibe versus atorvastatin with placebo was also assessed in the OCTIVUS trial in a group of 230 octogenarian patients with ACS. 23 The primary end point, the change in necrotic core, did not differ significantly between groups. However, the secondary end points, total and percentage atheroma volumes, were reduced only with the combination treatment. 23 Another study, which assessed atherosclerotic plaque volume by comparing patients on the combined therapy versus statin monotherapy, showed no difference in LDL‐C reduction at 2‐year observation, with significantly lower levels of LDL‐C after 1 year on the combined therapy. 24 There was also no change in atherosclerotic plaque volume after 2 years of follow‐up.
Meta‐analysis of 12 studies of patients with atherosclerotic cardiovascular disease by Shaya et al summarized that combination therapy with statin and ezetimibe provided an additional LDL‐C reduction versus monotherapy with a statin (mean difference, − 21.86 mg/dL; P<0.0001) after 6 months of treatment. 25 The same beneficial effect was observed in patients with recent ACS, with mean treatment difference of −19.19 mg/dL (95% CI, −25.22 to −13.16 mg/dL; P<0.0001). 25 In the meta‐analysis by Zhan et al, including 23 499 patients (26 randomized controlled trials), the authors showed a 6% (0.94 [95% CI, 0.90–0.98]) significant reduction of major adverse events with the combination therapy compared with statin monotherapy. 26 They also showed that adding ezetimibe to statins reduced the risk of nonfatal myocardial infarction (risk ratio [RR], 0.88 [95% CI, 0.81–0.95]) and nonfatal stroke (RR, 0.83 [95% CI, 0.71–0.97]). Analysis of all‐cause mortality (8 studies, with 21 222 patients) as an end point showed no difference between groups (RR, 0.98 [95% CI, 0.91–1.05]). 26
The recently published RACING (Randomized Comparison of Efficacy and Safety of Lipid‐Lowering With Statin Monotherapy Versus Statin/Ezetimibe Combination for High‐Risk Cardiovascular Diseases) trial, a randomized, noninferiority, open‐label trial, including patients with atherosclerotic cardiovascular disease, compared 1894 patients on combined LLT (ezetimibe with moderate‐intensity statin) with 1886 patients on statin monotherapy (high intensity). 27 The composite primary end point, defined as cardiovascular death or major events and nonfatal stroke, occurred with similar frequency in both groups (9.1% versus 9.9%, respectively; HR, 0.92 [95% CI, 0.75–1.13]; P=0.43). However, lower levels of LDL‐C (<70 mg/dL [1.8 mmol/L]) were obtained more often in the combination group than in monotherapy group (for 1‐, 2‐, and 3‐year observations: in 73%, 75%, and 72% of patients versus 55%, 60%, and 58% of patients, respectively; P<0.0001). Another observed benefit of the upfront combination therapy was a lower frequency of discontinuation or dose reduction in those treated with the combination therapy than in the monotherapy group (88 patients [4.8%] versus 150 patients [8.2%]; P<0.0001). 27 The results of the RACING trial support the main principle of LLT: the lower, the better for longer and, above all, the earlier on LDL‐C goal, the better, showing noninferiority of combination therapy to monotherapy (with insignificant ARR, 0.8%), as well as better efficacy and adherence. 27 Our data show additionally that upfront combination LLT is superior to statin monotherapy for all‐cause mortality. This undermines the stepwise approach presented in the recent European Society of Cardiology 2021 Prevention guidelines, 28 suggesting that high‐risk patients should be treated with the upfront combination strategy. Such an approach was also suggested in previous experts' articles, 29 , 30 , 31 including the position article of the International Collaborative Group of the National College of French Cardiologists, which recommends the upfront combination LLT in post‐ACS patients who are at the highest cardiovascular risk. 29
Our study has important strengths, which include the following: (1) The population in our study was a real‐world cohort treated because of ACS in multiple centers in Poland with a relatively high number of included patients, as well as a relatively long‐time follow‐up. (2) To the best of our knowledge, it is the first such analysis that presented results based on real‐world data. Therefore, the observation from our retrospective analysis remains highly valuable. Despite this, further prospective studies would be beneficial to confirm the positive role of immediate combined therapy with a maximally tolerated dose of statin with ezetimibe initiated in the acute phase of myocardial infarction.
This analysis has some obvious limitations. One of them is the lack of data on the dosage of statins in both groups, and thus we could not fully assess the effectiveness of the treatment used. However, all centers that took part in the registry were adjusting pharmacotherapy to individual patients, and the treatment was conducted strictly in accordance with the current European guidelines. In addition, the available data from the PolASPIRE study suggest that Polish patients with ACS are mostly prescribed a high‐dose statin (67.9%), with the mean dose of atorvastatin 46.2±27.2 mg/d, and rosuvastatin 19.5±11.1 mg/d 32 ; and in the recent KOS‐Lipid study, based on the data from the KOS‐Zawal (Managed Care After Acute Myocardial Infarction) study in Poland, the authors showed that 83.4% of patients are on the high‐intensity statin therapy at hospital discharge. 33 The registry does not also include information on diet, lifestyle, and other factors that can confound the findings; similarly, data on LDL‐C concentration in the follow‐up are not available. There is also no information on patients' compliance, possible therapy switching in the follow‐up, and drug‐related adverse effects gathered in PL‐ACS. Another limitation is an attempt to post hoc adjustment used in propensity score matching, which may miss the balance between all the factors that may play a role in the circumstances of the registry and clinical management of patients. We were also not able to evaluate the referral bias, mainly because ezetimibe, which has been available in Poland since 2006, was severely underused (usually in ≈1% of patients). 34 Finally, we cannot address the effects of possibly clinically significant factors (eg, some concomitant diseases and changes of the parameters during the follow‐up), which were not considered but may affect the analysis outcomes.
In conclusion, the main principles of LLT are that the lower, the better for longer, and above all, the earlier on the LDL‐C target, the better. Therefore, the short‐term benefits of immediate initiation of upfront combination therapy as demonstrated by lower LDL‐C levels are obvious. In our multicenter analysis, based on the real‐world data, we proved, for the first time, that upfront combination LLT is superior to statin monotherapy for all‐cause mortality. It undermines the stepwise approach, confirming the benefit of using the upfront combination strategy in patients with very high cardiovascular disease risk.
Sources of Funding
The Polish Registry of Acute Coronary Syndromes (PL‐ACS) was funded by the Ministry of Health. This analysis was written independently; no company or institution supported it financially. No professional writer was involved in the preparation of this article.
Disclosures
Dr Niedziela discloses the following relationships: speaker's bureau: Novartis, Swixx BioPharma, Berlin Chemie, and Boehringer Ingelheim. Dr Jankowski discloses the following relationships: speaker's bureau: Novartis, Sanofi, Servier, and Zentiva; consultancy to Novartis and Sanofi; and grants from Novartis, Sanofi, Servier, and Zentiva. Dr Dudek has received honoraria and grant/research support from: Amgen, AstraZeneca, Cardinal Health, Gedeon Richter, and Sanofi. Dr Banach discloses the following relationships: speaker's bureau: Amgen, Daichii Sankyo, KRKA, Polpharma, Novartis, Sanofi‐Aventis, Teva, and Zentiva; consultancy to Adamed, Amgen, Daichii Sankyo, Esperion, NewAmsterdam, Novartis, and Sanofi‐Aventis; and grants from Amgen, Daichii Sankyo, Mylan/Viatris, Novartis, Sanofi, and Valeant. The remaining authors have no disclosures to report.
Preprint posted on SSRN, February 21, 2023. doi: https://doi.org/10.2139/ssrn.4357916.
This article was sent to Amgad Mentias, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 9.
See Editorial by YaQoub et al.
REFERENCES
- 1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, et al. Low‐density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561. doi: 10.1016/S0140-6736(16)31357-5 [DOI] [PubMed] [Google Scholar]
- 4. Ray KK, Molemans B, Schoonen WM, Giovas P, Bray S, Kiru G, Murphy J, Banach M, De Servi S, Gaita D, et al. EU‐wide cross‐sectional observational study of lipid‐modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol. 2021;28:1279–1289. doi: 10.1093/eurjpc/zwaa047 [DOI] [PubMed] [Google Scholar]
- 5. Penson PE, Pirro M, Banach M. LDL‐C: lower is better for longer–even at low risk. BMC Med. 2020;18:320. doi: 10.1186/s12916-020-01792-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cybulska B, Kłosiewicz‐Latoszek L, Penson PE, Nabavi SM, Lavie CJ, Banach M. How much should LDL cholesterol be lowered in secondary prevention? Clinical efficacy and safety in the era of PCSK9 inhibitors. Prog Cardiovasc Dis. 2021;67:65–74. doi: 10.1016/j.pcad.2020.12.008 [DOI] [PubMed] [Google Scholar]
- 7. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 8. Banach M, Penson PE, Vrablik M, Bunc M, Dyrbus K, Fedacko J, Gaita D, Gierlotka M, Jarai Z, Magda SL, et al. Optimal use of lipid‐lowering therapy after acute coronary syndromes: a position paper endorsed by the International Lipid Expert Panel (ILEP). Pharmacol Res. 2021;166:105499. doi: 10.1016/j.phrs.2021.105499 [DOI] [PubMed] [Google Scholar]
- 9. Banach M, Penson PE. Lipid‐lowering therapies: better together. Atherosclerosis. 2021;320:P86–P88. doi: 10.1016/j.atherosclerosis.2021.01.009 [DOI] [PubMed] [Google Scholar]
- 10. Banach M, Penson PE. Statins and LDL‐C in secondary prevention–so much progress, so far to go. JAMA Netw Open. 2020;3:e2025675. doi: 10.1001/jamanetworkopen.2020.25675 [DOI] [PubMed] [Google Scholar]
- 11. Vrablik M, Seifert B, Parkhomenko A, Banach M, Jóźwiak JJ, Kiss RG, Gaita D, Rašlová K, Zachlederova M, Bray S, et al. Lipid‐lowering therapy use in primary and secondary care in Central and Eastern Europe: DA VINCI observational study. Atherosclerosis. 2021;334:66–75. doi: 10.1016/j.atherosclerosis.2021.08.035 [DOI] [PubMed] [Google Scholar]
- 12. Banach M, Burchardt P, Chlebus K, Dobrowolski P, Dudek D, Dyrbus K, Gąsior M, Jankowski P, Jóźwiak J, Kłosiewicz‐Latoszek L, et al. PoLA/CFPiP/PCS/PSLD/PSD/PSH guidelines on diagnosis and therapy of lipid disorders in Poland 2021. Arch Med Sci. 2021;17:1447–1547. doi: 10.5114/aoms/141941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ray KK, Reeskamp LF, Laufs U, Banach M, Mach F, Tokgözoğlu LS, Connolly DL, Gerrits AJ, Stroes ESG, Masana L, et al. Combination lipid‐lowering therapy as first‐line strategy in very high‐risk patients. Eur Heart J. 2022;43:830–833. doi: 10.1093/eurheartj/ehab718 [DOI] [PubMed] [Google Scholar]
- 14. Poloński L, Gasior M, Gierlotka M, Kalarus Z, Cieśliński A, Dubiel JS, Gil RJ, Ruzyłło W, Trusz‐Gluza M, Zembala M, et al. Polish Pegistry of Acute Coronary Syndromes (PL‐ACS). Characteristics, treatments and outcomes of patients with acute coronary syndromes in Poland. Kardiol Pol. 2007;65:861–864. [PubMed] [Google Scholar]
- 15. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. doi: 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baltar VT, Sousa CA, Westphal MF. Mahalanobis' distance and propensity score to construct a controlled matched group in a Brazilian study of health promotion and social determinants. Rev Bras Epidemiol. 2014;17(3):668–679. doi: 10.1590/1809-4503201400030008 [DOI] [PubMed] [Google Scholar]
- 17. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 18. Bohula EA, Wiviott SD, Giugliano RP, Blazing MA, Park JG, Murphy SA, White JA, Mach F, Van de Werf F, Dalby AJ, et al. Prevention of stroke with the addition of ezetimibe to statin therapy in patients with acute coronary syndrome in IMPROVE‐IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation. 2017;136:2440–2450. doi: 10.1161/CIRCULATIONAHA.117.029095 [DOI] [PubMed] [Google Scholar]
- 19. Kastelein JJP, Akdim F, Stroes ESG, Zwinderman AH, Bots ML, Stalenhoef AF, Visseren FL, Sijbrands EJ, Trip MD, Stein EA, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–1443. doi: 10.1056/NEJMoa0800742 [DOI] [PubMed] [Google Scholar]
- 20. Hibi K, Sonoda S, Kawasaki M, Otsuji Y, Murohara T, Ishii H, Sato K, Koshida R, Ozaki Y, Sata M, et al. Effects of ezetimibe‐statin combination therapy on coronary atherosclerosis in acute coronary syndromes. Circ J. 2018;82:757–766. doi: 10.1253/circj.CJ-17-0598 [DOI] [PubMed] [Google Scholar]
- 21. Hagiwara N, Kawada‐Watanabe E, Koyanagi R, Arashi H, Yamaguchi J, Nakao K, Tobaru T, Tanaka H, Oka T, Endoh Y, et al. Low‐density lipoprotein cholesterol targeting with pitavastatin+ezetimibe for patients with acute coronary syndrome and dyslipidaemia: the HIJ‐PROPER study, a prospective, open‐label, randomized trial. Eur Heart J. 2017;38:2264–2276. doi: 10.1093/eurheartj/ehx162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Z, Hao H, Yin C, Chu Y, Li J, Xu D. Therapeutic effects of atorvastatin and ezetimibe compared with double‐dose atorvastatin in very elderly patients with acute coronary syndrome. Oncotarget. 2017;8:41582–41589. doi: 10.18632/oncotarget.15078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hougaard M, Hansen HS, Thayssen P, Antonsen L, Junker A, Veien K, Jensen LO. Influence of ezetimibe in addition to high‐dose atorvastatin therapy on plaque composition in patients with ST‐segment elevation myocardial infarction assessed by serial: intravascular ultrasound with iMap: the OCTIVUS trial. Cardiovasc Revasc Med. 2017;18:110–117. doi: 10.1016/j.carrev.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 24. West AM, Anderson JD, Meyer CH, Epstein FH, Wang H, Hagspiel KD, Berr SS, Harthun NL, DiMaria JM, Hunter JR, et al. The effect of ezetimibe on peripheral arterial atherosclerosis depends upon statin use at baseline. Atherosclerosis. 2011;218:156–162. doi: 10.1016/j.atherosclerosis.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shaya FT, Sing K, Milam R, Husain F, Del Aguila MA, Patel MY. Lipid‐lowering efficacy of ezetimibe in patients with atherosclerotic cardiovascular disease: a systematic review and meta‐analyses. Am J Cardiovasc Drugs. 2020;20:239–248. doi: 10.1007/s40256-019-00379-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhan S, Tang M, Liu F, Xia P, Shu M, Wu X. Ezetimibe for the prevention of cardiovascular disease and all‐cause mortality events. Cochrane Database Syst Rev. 2018;11:CD012502. doi: 10.1002/14651858.CD012502.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oikawa S, Yamashita S, Nakaya N, Sasaki J, Kono S; effect of Fenofibrate and Ezetimibe Combination Treatment on Lipid (EFECTL) Study Investigators . Efficacy and safety of longterm coadministration of fenofibrate and ezetimibe in patients with combined hyperlipidemia: results of the EFECTL study. J Atheroscler Thromb. 2017;24:77–94. doi: 10.5551/jat.35626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim BK, Hong SJ, Lee YJ, Hong SJ, Yun KH, Hong BK, Heo JH, Rha SW, Cho YH, Lee SJ, et al. Long‐term efficacy and safety of moderate‐intensity statin with ezetimibe combination therapy versus high‐intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open‐label, non‐inferiority trial. Lancet. 2022;400:380–390. doi: 10.1016/S0140-6736(22)00916-3 [DOI] [PubMed] [Google Scholar]
- 29. Sabouret P, Lemesle G, Bellemain‐Appaix A, Aubry P, Bocchino PP, Rafflenbeul E, Belle L, Nolan J, Bernardi M, Biondi‐Zoccai G, et al. Post‐discharge and long‐term follow‐up after an acute coronary syndrome: International Collaborative Group of CNCF position paper. Arch Med Sci. 2022;18:839–854. doi: 10.5114/aoms/150321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Averna M, Banach M, Bruckert E, Drexel H, Farnier M, Gaita D, Magni P, März W, Masana L, Mello E Silva A, et al. Practical guidance for combination lipid‐modifying therapy in high‐ and very‐high‐risk patients: a statement from a European Atherosclerosis Society Task force. Atherosclerosis. 2021;325:99–109. doi: 10.1016/j.atherosclerosis.2021.03.039 [DOI] [PubMed] [Google Scholar]
- 31. Banach M, Reiner Z, Cicero AF, Sabouret P, Viigimaa M, Sahebkar A, Postadzhiyan A, Gaita D, Pella D, Penson PE. 2022: the year in cardiovascular disease–the year of upfront lipid lowering combination therapy. Arch Med Sci. 2022;18:1429–1434. doi: 10.5114/aoms/156147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jankowski P, Kozieł P, Setny M, Paniczko M, Haberka M, Banach M, Bacquer D, Backer G, Kotseva K, Wood D, et al. Dyslipidemia management in patients with coronary artery disease. Data from the POLASPIRE survey. J Clin Med. 2021;10:3711. doi: 10.3390/jcm10163711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nowowiejska‐Wiewióra A, Wita K, Mędrala Z, Tomkiewicz‐Pająk L, Bujak K, Mizia‐Stec K, Brzychczy P, Gąsior M, Gąsior Z, Kulbat A, et al. Dyslipidemia treatment and attainment of LDL‐cholesterol treatment goals in patients participating in the Managed Care for Acute Myocardial Infarction Survivors program. Kardiol Pol. 2023;81:359–365. doi: 10.33963/KP.a2023.0045 [DOI] [PubMed] [Google Scholar]
- 34. Dyrbus K, Gasior M, Desperak P, Nowak J, Osadnik T, Banach M. Characteristics of lipid profile and effectiveness of management of dyslipidaemia in patients with acute coronary syndromes—data from the TERCET registry with 19,287 patients. Pharmacol Res. 2019;139:460–466. doi: 10.1016/j.phrs.2018.12.002 [DOI] [PubMed] [Google Scholar]


