Abstract
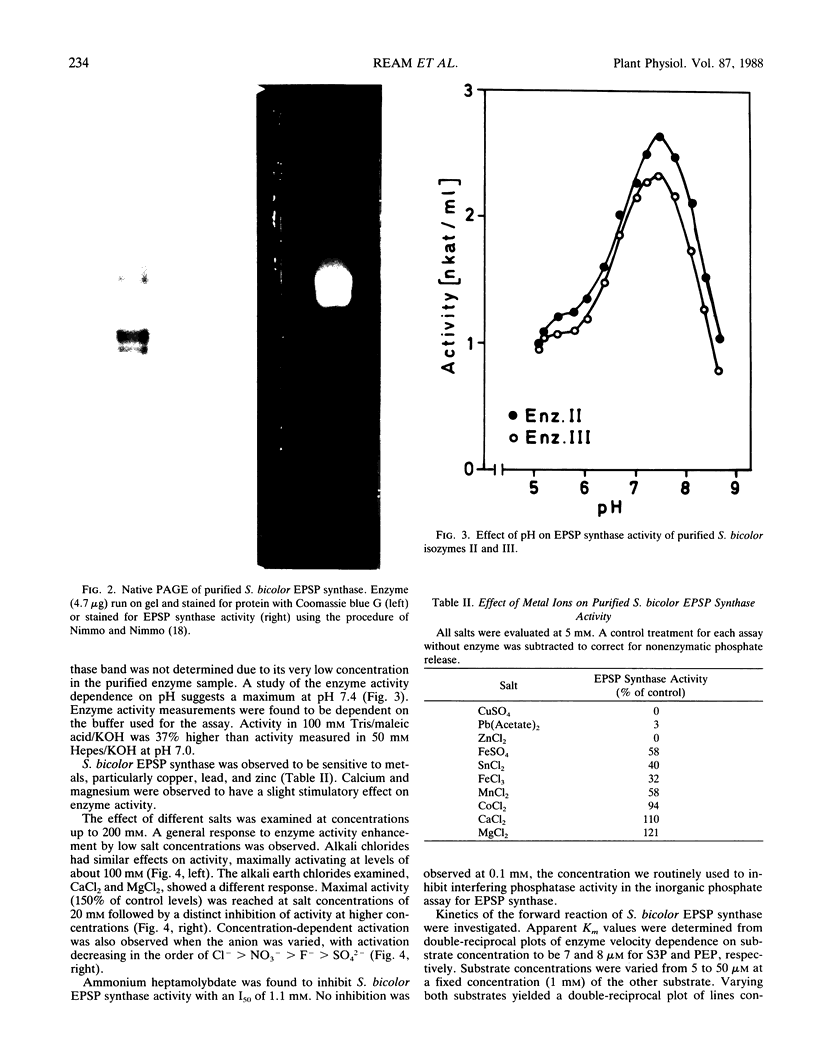
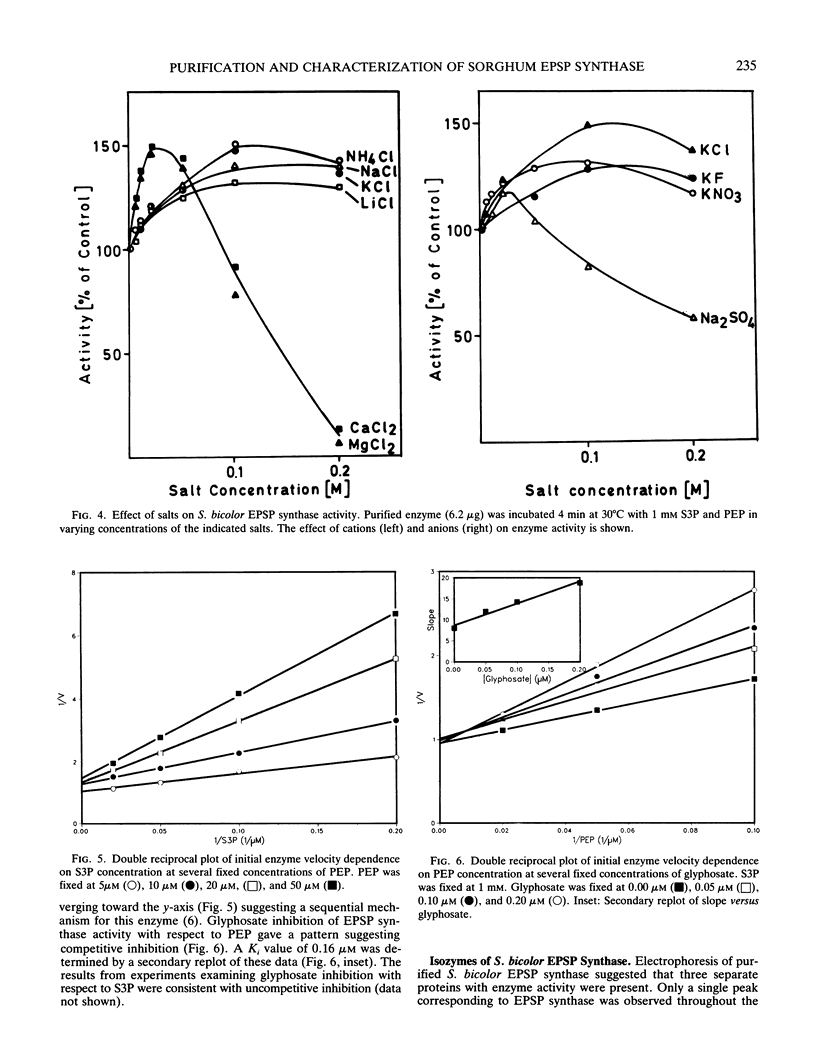
5-Enolpyruvylshikimate-3-phosphate (EPSP) synthase (3-phospho-shikimate 1-carboxyvinyltransferase; EC 2.5.1.19) was purified 1300-fold from etiolated shoots of Sorghum bicolor (L.) Moench. Native polyacrylamide gel electrophoresis revealed three barely separated protein bands staining positive for EPSP synthase activity. The native molecular weight was determined to be 51,000. Enzyme activity was found to be sensitive to metal ions and salts. Apparent Km values of 7 and 8 micromolar were determined for the substrates shikimate-3-phosphate and phosphoenolpyruvate (PEP), respectively. The herbicide glyphosate was found to inhibit the enzyme competitively with respect to PEP (Ki = 0.16 micromolar). Characterization studies support the conclusion of a high degree of similarity between EPSP synthase from S. bicolor, a monocot, and the enzyme from dicots. A similarity to bacterial EPSP synthase is also discussed. Three EPSP synthase isozymes (I, II, III) were elucidated in crude homogenates of S. bicolor shoots by high performance liquid chromatography. The major isozymes, II and III, were separated and partially characterized. No significant differences in pH activity profiles and glyphosate sensitivity were found. This report of isozymes of EPSP synthase from S. bicolor is consistent with other reports for shikimate pathway enzymes, including EPSP synthase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boocock M. R., Coggins J. R. Kinetics of 5-enolpyruvylshikimate-3-phosphate synthase inhibition by glyphosate. FEBS Lett. 1983 Apr 5;154(1):127–133. doi: 10.1016/0014-5793(83)80888-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Della-Cioppa G., Bauer S. C., Klein B. K., Shah D. M., Fraley R. T., Kishore G. M. Translocation of the precursor of 5-enolpyruvylshikimate-3-phosphate synthase into chloroplasts of higher plants in vitro. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6873–6877. doi: 10.1073/pnas.83.18.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb L. D. Conservation and duplication of isozymes in plants. Science. 1982 Apr 23;216(4544):373–380. doi: 10.1126/science.216.4544.373. [DOI] [PubMed] [Google Scholar]
- Helmerhorst E., Stokes G. B. Microcentrifuge desalting: a rapid, quantitative method for desalting small amounts of protein. Anal Biochem. 1980 May 1;104(1):130–135. doi: 10.1016/0003-2697(80)90287-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewendon A., Coggins J. R. Purification of 5-enolpyruvylshikimate 3-phosphate synthase from Escherichia coli. Biochem J. 1983 Jul 1;213(1):187–191. doi: 10.1042/bj2130187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo H. G., Nimmo G. A. A general method for the localization of enzymes that produce phosphate, pyrophosphate, or CO2 after polyacrylamide gel electrophoresis. Anal Biochem. 1982 Mar 15;121(1):17–22. doi: 10.1016/0003-2697(82)90551-6. [DOI] [PubMed] [Google Scholar]
- Padgette S. R., Huynh Q. K., Aykent S., Sammons R. D., Sikorski J. A., Kishore G. M. Identification of the reactive cysteines of Escherichia coli 5-enolpyruvylshikimate-3-phosphate synthase and their nonessentiality for enzymatic catalysis. J Biol Chem. 1988 Feb 5;263(4):1798–1802. [PubMed] [Google Scholar]
- Rubin J. L., Gaines C. G., Jensen R. A. Glyphosate Inhibition of 5-Enolpyruvylshikimate 3-Phosphate Synthase from Suspension-Cultured Cells of Nicotiana silvestris. Plant Physiol. 1984 Jul;75(3):839–845. doi: 10.1104/pp.75.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. G. Acid phosphatase from tobacco leaves. Arch Biochem Biophys. 1966 Oct;117(1):1–9. doi: 10.1016/0003-9861(66)90118-4. [DOI] [PubMed] [Google Scholar]
- Steinrücken H. C., Amrhein N. 5-Enolpyruvylshikimate-3-phosphate synthase of Klebsiella pneumoniae 2. Inhibition by glyphosate [N-(phosphonomethyl)glycine]. Eur J Biochem. 1984 Sep 3;143(2):351–357. doi: 10.1111/j.1432-1033.1984.tb08379.x. [DOI] [PubMed] [Google Scholar]
- Steinrücken H. C., Amrhein N. The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1207–1212. doi: 10.1016/0006-291x(80)90547-1. [DOI] [PubMed] [Google Scholar]
- Steinrücken H. C., Schulz A., Amrhein N., Porter C. A., Fraley R. T. Overproduction of 5-enolpyruvylshikimate-3-phosphate synthase in a glyphosate-tolerant Petunia hybrida cell line. Arch Biochem Biophys. 1986 Jan;244(1):169–178. doi: 10.1016/0003-9861(86)90106-2. [DOI] [PubMed] [Google Scholar]