Abstract
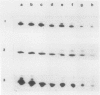
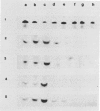
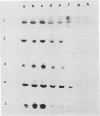
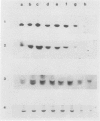
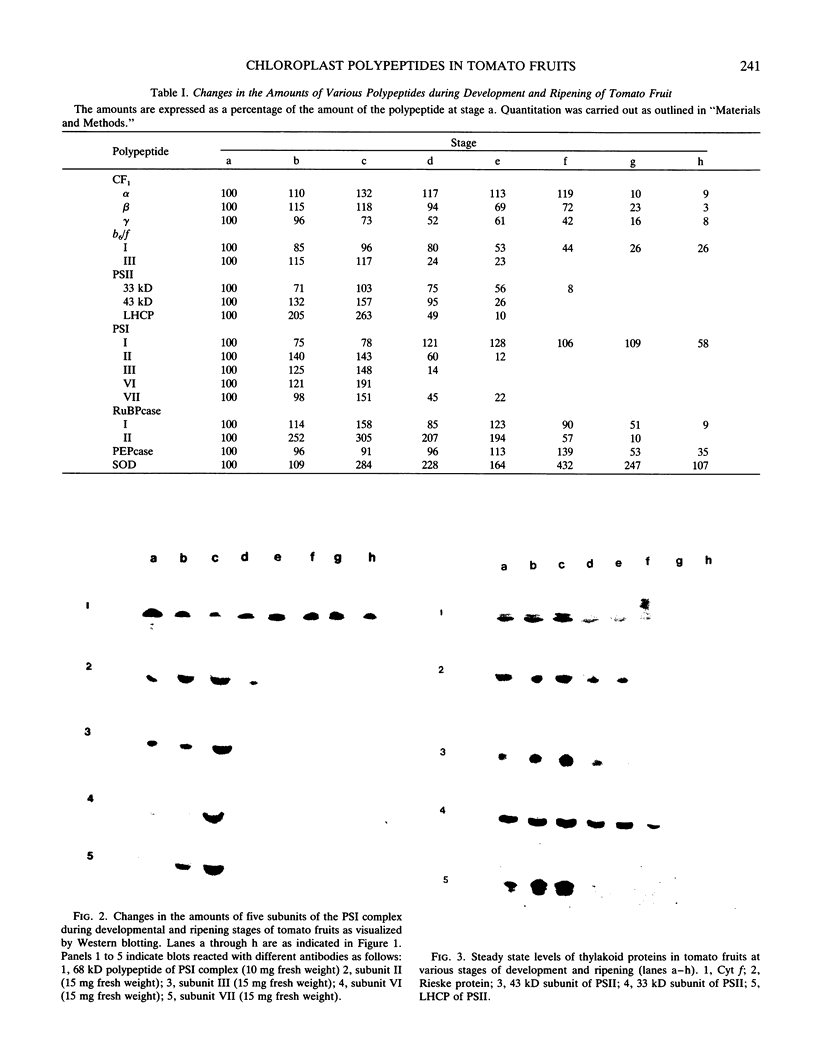
During maturation and ripening of tomato (Lycopersicon esculentum, cv Tamar) fruits, there are differential changes in the steady state levels of chloroplast proteins. Western blot analysis indicated that with the exception of the core polypeptide of photosystem I (PSI) (subunit I) the whole complex disappears during the transition of chloroplast to chromoplast. The amounts of the core polypeptide of photosystem II (PSII) (43 kilodaltons) and the light harvesting chlorophyll protein complex increase during maturation and decrease thereafter. In contrast, the 33 kilodalton subunit of PSII is found at the highest levels from the early recorded stages and decreases gradually until late stages of ripening. The level of cytochrome f decreases slowly during the maturation and ripening process, whereas the Rieske protein of the same complex disappears at a faster rate. There are also differential changes in the subunits of the chloroplast coupling factor·ATPase complex; α and β subunits increase during maturation, whereas the level of the γ subunit is already maximal at the earliest recorded stage of development and depleted thereafter. The two subunits of the ribulose-1,5 bisphosphate carboxylase increase in abundance during chloroplast maturation and gradually disappear after the transition from chloroplast to chromoplast. However, there are substantial differences in the rates of increase and disappearance of the large and small subunits of this enzyme. This imbalance is attributed to different regulation of nuclear and chloroplast gene expression. In addition, the steady state levels of chloroplastic superoxide dismutase and phosphoenolpyruvate carboxylase have been followed. Both enzymes reach their maxima at the final stages of ripening. This increase coincides with the climacteric rise of CO2 release.
Full text
PDF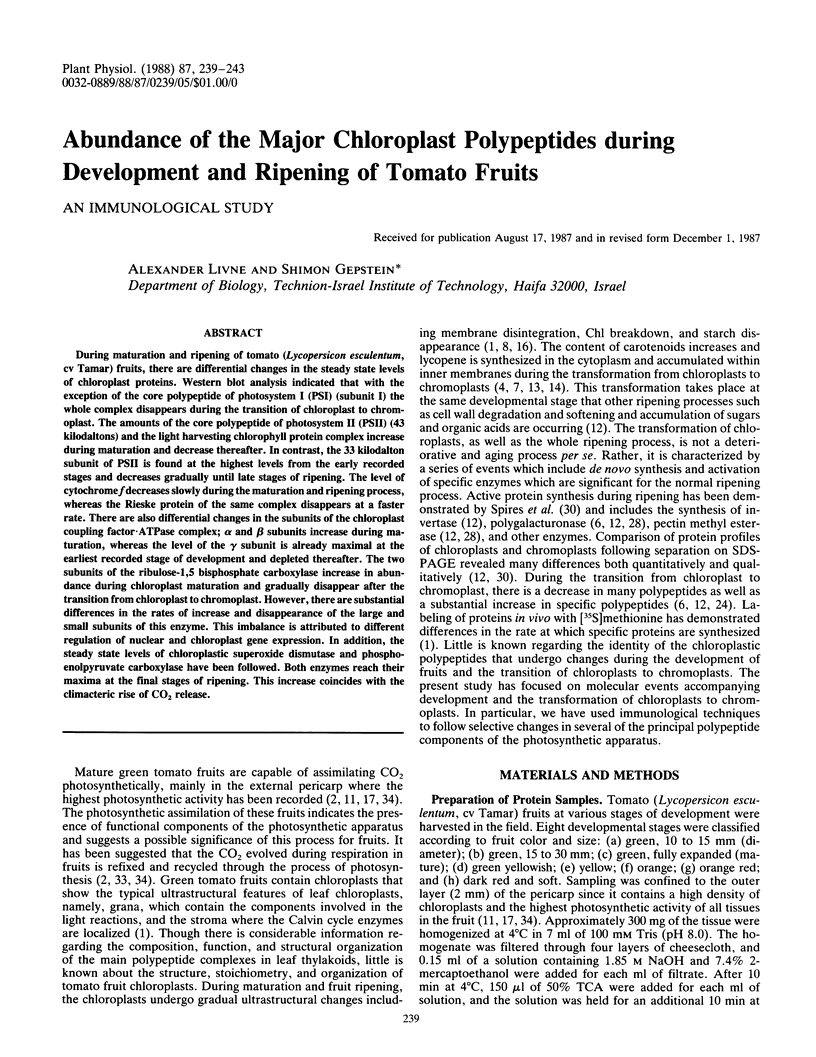



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-David H., Nelson N., Gepstein S. Differential Changes in the Amount of Protein Complexes in the Chloroplast Membrane during Senescence of Oat and Bean Leaves. Plant Physiol. 1983 Oct;73(2):507–510. doi: 10.1104/pp.73.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostal H. C., Leopold A. C. Gibberellin delays ripening of tomatoes. Science. 1967 Dec 22;158(3808):1579–1580. doi: 10.1126/science.158.3808.1579. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farineau J. Light versus Dark Carbon Metabolism in Cherry Tomato Fruits: II. Relationship Between Malate Metabolism and Photosynthetic Activity. Plant Physiol. 1977 Dec;60(6):877–880. doi: 10.1104/pp.60.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E., Hauska G. Identification of the polypeptides in the cytochrome b6/f complex from spinach chloroplasts with redox-center-carrying subunits. J Bioenerg Biomembr. 1982 Dec;14(5-6):405–424. doi: 10.1007/BF00743067. [DOI] [PubMed] [Google Scholar]
- Laval-Martin D. Light versus Dark Carbon Metabolism in Cherry Tomato Fruits: I. Occurrence of Photosynthesis. Study of the Intermediates. Plant Physiol. 1977 Dec;60(6):872–876. doi: 10.1104/pp.60.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liveanu V., Yocum C. F., Nelson N. Polypeptides of the oxygen-evolving photosystem II complex. Immunological detection and biogenesis. J Biol Chem. 1986 Apr 25;261(12):5296–5300. [PubMed] [Google Scholar]
- Rott R., Nelson N. Purification and immunological properties of proton-ATPase complexes from yeast and rat liver mitochondria. J Biol Chem. 1981 Sep 10;256(17):9224–9228. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu J. C., Yelenosky G., Bausher M. G. Photosynthetic Activity in the Flower Buds of ;Valencia' Orange (Citrus sinensis [L.] Osbeck). Plant Physiol. 1985 Jun;78(2):420–423. doi: 10.1104/pp.78.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel E., Gepstein S. Immunological evidence for the presence of ribulose bisphosphate carboxylase in guard cell chloroplasts. Plant Physiol. 1985 Jul;78(3):586–590. doi: 10.1104/pp.78.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]