Abstract
Background
Studies demonstrated sex differences in outcomes following acute myocardial infarction, with women more likely to develop heart failure (HF). Sacubitril/valsartan has been shown to reduce cardiovascular death and HF hospitalizations in patients with HF with reduced ejection fraction.
Methods and Results
A total of 5661 patients (1363 women [24%]) with acute myocardial infarction complicated by reduced left ventricular ejection fraction (≤40%), pulmonary congestion, or both and ≥1 of 8 risk‐augmenting factors were randomized to receive sacubitril/valsartan or ramipril. The primary outcome was cardiovascular death or incident HF. Baseline characteristics, clinical outcomes, and safety events were compared according to sex, a prespecified subgroup. Female participants were older and had more comorbidities. After multivariable adjustment, women and men were at similar risks for cardiovascular death or all‐cause death. Women were more likely to have first HF hospitalization (hazard ratio [HR], 1.34 [95% CI, 1.05–1.70]; P=0.02) and total HF hospitalizations (HR, 1.39 [95% CI, 1.05–1.84]; P=0.02). Sex did not significantly modify the treatment effect of sacubitril/valsartan compared with ramipril on the primary outcome (P for interaction=0.11).
Conclusions
In contemporary patients who presented with reduced left ventricular ejection fraction, pulmonary congestion, or both, following acute myocardial infarction, women had a higher incidence of HF during follow‐up. Sex did not modify the treatment effect of sacubitril/valsartan relative to ramipril.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02924727.
Keywords: heart failure, myocardial infarction, sacubitril/valsartan, sex differences
Subject Categories: Acute Coronary Syndromes, Coronary Artery Disease, Heart Failure
Nonstandard Abbreviations and Acronyms
- CEC
clinical events committee
- PARADISE‐MI
Prospective ARNI (Angiotensin Receptor–Neprilysin Inhibitor) Versus ACE (Angiotensin‐Converting Enzyme) Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After Myocardial Infarction
- VALIANT
Valsartan in Acute Myocardial Infarction
Clinical Perspective.
What Is New?
In a contemporary clinical trial cohort of patients with high‐risk myocardial infarction, women were older with more comorbidities. Women and men had similar rates of all‐cause mortality and cardiovascular death.
Women were more likely to have heart failure hospitalization after high‐risk myocardial infarction, over a median follow‐up duration of 22 months.
Sacubitril/valsartan was well tolerated in both men and women.
What Are the Clinical Implications?
Clinicians should pay close attention to the increased risk of heart failure hospitalization after high‐risk myocardial infarction to mitigate adverse clinical outcomes.
Historically, women were less likely to receive guideline‐directed medical therapy after myocardial infarction. Sacubitril/valsartan was safe and well tolerated in both sexes and should not be withheld in women when clinically indicated.
Heart disease is a leading cause of mortality in women. 1 Once thought to be primarily a problem in men, ischemic heart disease is also known to afflict women with similar incidence, albeit at an older age. 2 Until recently, at a population level, women had higher mortality from cardiovascular disease than men, with ischemic heart disease as a major contributor. 1
Prior studies have shown sex differences in outcomes following myocardial infarction (MI), although the results have been inconsistent. Some studies showed higher mortality in women after MI, whereas others found such differences only existed in certain types of MI or were attenuated after adjusting for differences in patient characteristics. 3 , 4 , 5 In the VALIANT (Valsartan in Acute Myocardial Infarction) trial, women experienced a higher risk of heart failure (HF) following MI; mortality and risk of recurrent MI or stroke did not differ significantly between men and women after adjusting for baseline differences. 6 However, since the VALIANT trial, there have been significant changes in the management of MI with or without HF. With the adoption of timely primary percutaneous coronary intervention (PCI), increased use of medical therapies, such as statins, β‐blockers, angiotensin‐converting enzyme inhibitors (ACEis), and angiotensin receptor blockers, mortality and other cardiovascular outcomes following MI have improved. 7
The angiotensin receptor–neprilysin inhibitor (ARNI) sacubitril/valsartan reduced cardiovascular death and HF hospitalization compared with an ACEi in patients with HF with reduced ejection fraction. 8 In patients with HF with preserved ejection fraction, sacubitril/valsartan reduced HF hospitalizations compared with an angiotensin receptor blocker, valsartan, in patients with an ejection fraction below normal. 9 This benefit was particularly pronounced in women versus men. 10 PARADISE‐MI (Prospective ARNI Versus ACE Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After Myocardial Infarction) provides a contemporary population to evaluate potential sex differences in patient characteristics and outcomes following MI, and it determines whether women derive a greater benefit from an ARNI following MI.
Methods
Data Sharing
The sponsor of PARADISE‐MI is committed to sharing access to patient‐level data and supporting clinical documents from eligible studies with qualified external researchers. These requests are reviewed and approved by an independent review panel based on scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The trial data availability is according to the criteria and process described. 11
Study Design and Patient Characteristics
The design and results of PARADISE‐MI have been previously published. 7 , 12 Briefly, PARADISE‐MI was a multicenter, double‐blind, active‐comparator, randomized trial evaluating the efficacy and safety of sacubitril/valsartan compared with ramipril. A total of 5661 patients (1363 women [24.0%]) were randomized in a 1:1 manner to receive sacubitril/valsartan or ramipril, with a median follow‐up of 22 months. The key eligibility criteria included adults without a history of HF, who had a spontaneous MI within 0.5 to 7 days before randomization associated with a reduced left ventricular ejection fraction (LVEF; ≤40%), pulmonary congestion, or both, and with at least 1 of 8 risk‐augmenting factors (age ≥70 years, diabetes, previous MI, estimated glomerular filtration rate [eGFR] <60 mL/min per 1.73 m2, atrial fibrillation, LVEF <30% associated with index MI, Killip class III or IV, or ST‐segment–elevation MI without reperfusion within 24 hours after presentation). 12 Key exclusion criteria included clinical instability within 24 hours before randomization, an eGFR <30 mL/min per 1.73 m2, serum potassium level >5.2 mmol/L, and inability to take an ACEi or an angiotensin receptor blocker. Medications were titrated to a target dose of ramipril, 5 mg twice daily, and sacubitril/valsartan, 97/103 mg twice daily, over 3 to 4 weeks. 7 The PARADISE‐MI Women subtrial is a prespecified subgroup analysis of PARADISE‐MI evaluating potential sex differences in clinical characteristics and outcomes following acute MI. The trial protocol for PARADISE‐MI was approved by the institutional review board at each trial center, and trial participants gave informed consent.
End Points and Follow‐Up
The primary composite outcome in PARADISE‐MI was cardiovascular death or incident HF, defined as hospitalization for HF or outpatient symptomatic HF requiring intravenous or sustained oral diuretic therapy. Secondary outcomes included the composite of cardiovascular death or HF hospitalization; the composite of HF hospitalization or outpatient HF; the composite of cardiovascular death, nonfatal MI, or nonfatal stroke; cardiovascular death; and all‐cause death. Clinical outcomes were reported by the primary site investigator and were adjudicated (using clearly defined end points) by an independent clinical events committee (CEC) blinded to treatment allocation. 7 For the primary composite end point, cardiovascular death or incident HF, both CEC‐adjudicated and investigator‐reported events were analyzed. The coronary composite end point of death from coronary heart disease, nonfatal MI, hospitalization for angina, or coronary revascularization was included as an exploratory outcome.
For the total (first and recurrent) event analysis, CEC‐adjudicated end points included cardiovascular death and total cardiovascular events (HF hospitalization, MI, or stroke), cardiovascular death and total HF events (HF hospitalization or outpatient HF), total HF hospitalizations, total outpatient HF events, and total HF events. Investigator‐reported cardiovascular death and total HF events, total HF hospitalizations, total outpatient HF events, and total HF events were also examined.
For safety end points, rates of adverse events, serious adverse events, and drug discontinuation attributable to adverse events were compared between men and women. Key adverse events of interest include hypotension, cough, angioedema, hyperkalemia, hepatotoxicity, hypersensitivity, renal impairment, cognitive impairment, risk of malignancy, and statin drug‐drug interaction. Laboratory abnormalities of elevated serum creatinine, serum potassium, aspartate aminotransferase, and alanine aminotransferase above predefined thresholds were also compared between men and women.
Statistical Analysis
Demographics, baseline characteristics, and baseline medications were compared between men and women using the χ2 test for comparison of categorical variables and t‐test for comparison of normally distributed continuous variables. Efficacy analyses were performed according to the intention‐to‐treat principle. The association between sex and the primary composite outcome, its components, and the secondary outcomes was evaluated using Cox proportional hazards regression models in a time‐to‐first event analysis. To assess whether sex modified the treatment effect of sacubitril/valsartan relative to ramipril, a sex‐by‐treatment interaction term was included in models adjusted for PCI use at baseline and geographic region, and it was stratified by type of MI. Total (first and recurrent) events were analyzed using a negative binomial model with a Weibull baseline intensity function. Models were adjusted for age, self‐reported race, geographic region, number of risk‐augmenting factors, baseline LVEF, intravenous treatment for pulmonary congestion, history of prior MI, hypertension, diabetes, current tobacco use, eGFR, type of index MI, ST‐segment–elevation MI (STEMI) without reperfusion within 24 hours of presentation, PCI at baseline, Killip class, and treatment assignment. Proportional hazards assumptions were assessed via Schoenfeld residuals.
Adverse events were compared between men and women using the χ2 test for comparison of categorical variables. Statistical significance was assessed using a 2‐sided α level of 0.05 without adjustment for multiplicity. All statistical analyses were conducted using Stata software, version 16 (StataCorp, College Station, TX).
Results
Patient Characteristics
There were significant differences in baseline characteristics between men (n=4298) and women (n=1363) in PARADISE‐MI. Women were older, had higher LVEF, were more likely to present with pulmonary congestion, and had >1 risk‐augmenting factor (Table 1).
Table 1.
Baseline Characteristics in Women Versus Men
| Characteristic | Men (n=4298) | Women (n=1363) | P value |
|---|---|---|---|
| Age, y | 62.5±11.4 | 67.8±10.9 | <0.001 |
| Race, n (%) | 0.01 | ||
| Asian | 755 (17.6) | 198 (14.5) | |
| Black | 51 (1.2) | 24 (1.8) | |
| White | 3200 (74.5) | 1063 (78.0) | |
| Other* | 292 (6.8%) | 78 (5.7%) | |
| Region, n (%) | <0.001 | ||
| Asia/Pacific and others | 880 (20.5) | 222 (16.3) | |
| Central Europe | 1104 (25.7) | 395 (29.0) | |
| Latin America | 501 (11.7) | 178 (13.1) | |
| North America | 383 (8.9) | 145 (10.6) | |
| Western Europe | 1430 (33.3) | 423 (31.0) | |
| Heart rate, bpm | 75.3±11.7 | 76.8±11.9 | <0.001 |
| Pulse pressure, mm Hg | 46.5±11.5 | 49.3±12.3 | <0.001 |
| Systolic blood pressure, mm Hg | 120.5±13.2 | 122.0±13.5 | <0.001 |
| Diastolic blood pressure, mm Hg | 74.1±9.7 | 72.7±9.8 | <0.001 |
| Body mass index, kg/m2 | 28.0±4.7 | 28.4±5.8 | 0.01 |
| Left ventricular ejection fraction, % | 36.1±9.2 | 38.0±9.9 | <0.001 |
| Pulmonary congestion, n (%) | 2258 (52.5) | 798 (58.5) | <0.001 |
| >1 Risk‐augmenting factor, n (%) | 2130 (49.6) | 824 (60.5) | <0.001 |
| Medical history, n (%) | |||
| Prior MI | 764 (17.8) | 156 (11.4) | <0.001 |
| Prior CABG or PCI | 780 (18.1) | 154 (11.3) | <0.001 |
| Prior stroke | 180 (4.2) | 83 (6.1) | 0.01 |
| Hypertension | 2667 (62.1) | 1009 (74.0) | <0.001 |
| Diabetes | 1776 (41.3) | 625 (45.9) | 0.003 |
| Current smoking | 985 (22.9) | 211 (15.5) | <0.001 |
| Atrial fibrillation/flutter | 607 (14.1) | 177 (13.0) | 0.41 |
| Estimated GFR, mL/min per 1.73 m2 | 73.2±21.8 | 67.6±23.7 | <0.001 |
| Qualifying MI: STEMI | 3328 (77.4) | 963 (70.7) | <0.001 |
| Coronary reperfusion | 3879 (90.3) | 1158 (85.0) | <0.001 |
| STEMI without reperfusion within 24 h | 352 (8.2) | 144 (10.6) | 0.01 |
| Thrombolytic therapy | 207 (4.8) | 46 (3.4) | <0.001 |
| Percutaneous coronary intervention | 3835 (89.2) | 1145 (84.0) | <0.001 |
| Drug‐eluting stent | 3452 (80.3) | 1006 (73.8) | <0.001 |
| Location of MI, n (%) | 0.03 | ||
| Anterior | 2944 (68.5) | 909 (66.7) | |
| Inferior | 810 (18.8) | 243 (17.8) | |
| Other | 544 (12.7) | 211 (15.5) | |
| Killip class ≥II, n (%) | 2383 (57.4) | 818 (61.4) | 0.01 |
| Time to randomization, d | 4.3±1.8 | 4.4±1.7 | 0.03 |
| Dual‐antiplatelet therapy, n (%) | 3984 (92.7) | 1238 (90.8) | 0.03 |
| β‐Blocker, n (%) | 3688 (85.8) | 1139 (83.6) | 0.04 |
| MRA, n (%) | 1780 (41.4) | 558 (40.9) | 0.76 |
| Diuretics, n (%) | 1869 (43.5) | 652 (47.8) | 0.01 |
| Statin, n (%) | 4080 (94.9) | 1290 (94.6) | 0.68 |
| ACE inhibitor/ARB, n (%) | 3360 (78.2) | 1076 (78.9) | 0.55 |
Data are given as mean±SD unless otherwise indicated. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; bpm, beats per minute; CABG, coronary artery bypass grafting; GFR, glomerular filtration rate; MI, myocardial infarction; MRA, mineralocorticoid receptor agonist; PCI, percutaneous coronary intervention; and STEMI, ST‐segment–elevation MI.
Other includes Native American, Pacific Islander, Unknown Race, and Other Race.
Women were less likely to have had a prior stroke, MI, or coronary revascularization but more likely to have hypertension and diabetes (Table 1). Fewer women in PARADISE‐MI were active smokers. Women had a slightly higher systolic blood pressure and lower eGFR than men. Fewer women presented with STEMI, and of those with STEMI, women were less likely to receive reperfusion therapy. Moreover, for those with a STEMI, fewer women than men received reperfusion therapy within 24 hours of presentation (Table 1). Women were more likely to be in Killip class >II (61.4% versus 57.4%; P=0.01) at presentation. At randomization, women and men had similar use of mineralocorticoid receptor antagonists, statins, and ACEis/angiotensin receptor blockers. Women were less likely to be on β‐blockers and dual‐antiplatelet therapy but were more likely to receive diuretic therapy at randomization (Table 1).
Primary Outcomes
The primary outcome (cardiovascular death or incident HF) occurred as 197 CEC‐adjudicated events in women (8.5 per 100 person‐years) and 514 CEC‐adjudicated events in men (6.6 per 100 person‐years), with an unadjusted hazard ratio (HR) of 1.25 (95% CI, 1.06–1.47; P=0.01; Table 2). After adjusting for baseline demographics and risk factors, the association of sex with the primary composite outcome was attenuated (HR, 1.10 [95% CI, 0.92–1.33]; P=0.28). Sex did not significantly modify the treatment effect of sacubitril/valsartan relative to ramipril on the CEC‐adjudicated primary outcome (P for interaction=0.11; Figure 1). Similar results were observed for investigator‐reported outcomes. Women remained at a greater risk of having investigator‐reported primary outcomes (HR, 1.18 [95% CI, 1.01–1.38]; P=0.03) after adjusting for baseline demographics and risk factors (Table 2).
Table 2.
Unadjusted and Adjusted Outcomes in Men and Women
| Men (N=4298) | Women (N=1363) | Unadjusted outcomes | Adjusted outcomes* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | No. of events | Incidence rate (per 100 person‐years) | No. of events | Incidence rate (per 100 person‐years) | HR (95% CI) (reference=men) | P value | HR (95% CI) (reference=men) | P value | P for interaction value† |
| Clinical events committee–adjudicated outcomes | |||||||||
| Primary composite outcome | 514 | 6.6 | 197 | 8.5 | 1.25 (1.06–1.47) | 0.01 | 1.10 (0.92–1.32) | 0.28 | 0.11 |
| Cardiovascular death | 272 | 3.3 | 87 | 3.5 | 1.03 (0.81–1.31) | 0.81 | 0.87 (0.67–1.14) | 0.33 | 0.32 |
| HF hospitalization | 247 | 3.1 | 118 | 5.0 | 1.55 (1.25–1.93) | <0.001 | 1.34 (1.05–1.70) | 0.02 | 0.28 |
| Outpatient HF | 70 | 0.9 | 26 | 1.1 | 1.20 (0.76–1.88) | 0.43 | 1.08 (0.67–1.76) | 0.75 | 0.16 |
| Cardiovascular death or HF hospitalization | 463 | 5.9 | 180 | 7.7 | 1.26 (1.06–1.50) | 0.01 | 1.10 (0.91–1.33) | 0.33 | 0.12 |
| HF hospitalization or outpatient HF | 301 | 3.9 | 137 | 5.9 | 1.48 (1.21–1.81) | <0.001 | 1.29 (1.04–1.61) | 0.02 | 0.20 |
| Cardiovascular death, nonfatal MI, or nonfatal stroke | 507 | 6.5 | 157 | 6.5 | 1.00 (0.83–1.19) | 0.98 | 0.92 (0.75–1.12) | 0.39 | 0.79 |
| All‐cause death | 344 | 4.2 | 111 | 4.4 | 1.05 (0.84–1.29) | 0.69 | 0.83 (0.66–1.06) | 0.14 | 0.26 |
| Composite coronary end point‡ | 578 | 7.8 | 155 | 6.7 | 0.85 (0.71–1.01) | 0.07 | 0.83 (0.68–1.00) | 0.05 | 0.66 |
| Investigator‐reported outcomes | |||||||||
| Primary composite outcome | 688 | 9.2 | 271 | 12.4 | 1.30 (1.13–1.49) | <0.001 | 1.18 (1.01–1.38) | 0.03 | 0.31 |
| Cardiovascular death | 252 | 3.1 | 82 | 3.3 | 1.05 (0.82–134) | 0.71 | 0.90 (0.68–1.19) | 0.45 | 0.86 |
| HF hospitalization | 365 | 4.7 | 172 | 7.6 | 1.54 (1.29–1.85) | <0.001 | 1.34 (1.10–1.64) | 0.004 | 0.24 |
| Outpatient HF | 195 | 2.5 | 76 | 3.2 | 1.26 (0.96–1.64) | 0.09 | 1.19 (0.89–1.58) | 0.23 | 0.39 |
HF indicates heart failure; HR, hazard ratio; and MI, myocardial infarction.
Models adjusted for age, race, geographic region, number of risk‐augmenting factors, baseline left ventricular ejection fraction, intravenous treatment for pulmonary congestion, history of MI, hypertension, diabetes, current tobacco use, estimated glomerular filtration rate, type of index MI, ST‐segment–elevation MI without reperfusion, percutaneous coronary intervention, Killip class, and treatment assignment.
P for interaction: interaction between sex and treatment, adjusted for percutaneous coronary intervention and geographic region, stratified by type of MI.
Coronary composite end point: death from coronary heart disease, nonfatal MI, hospitalization for angina, or coronary revascularization.
Figure 1. The cumulative incidence for the primary composite outcome according to sex and treatment assignment in PARADISE‐MI.
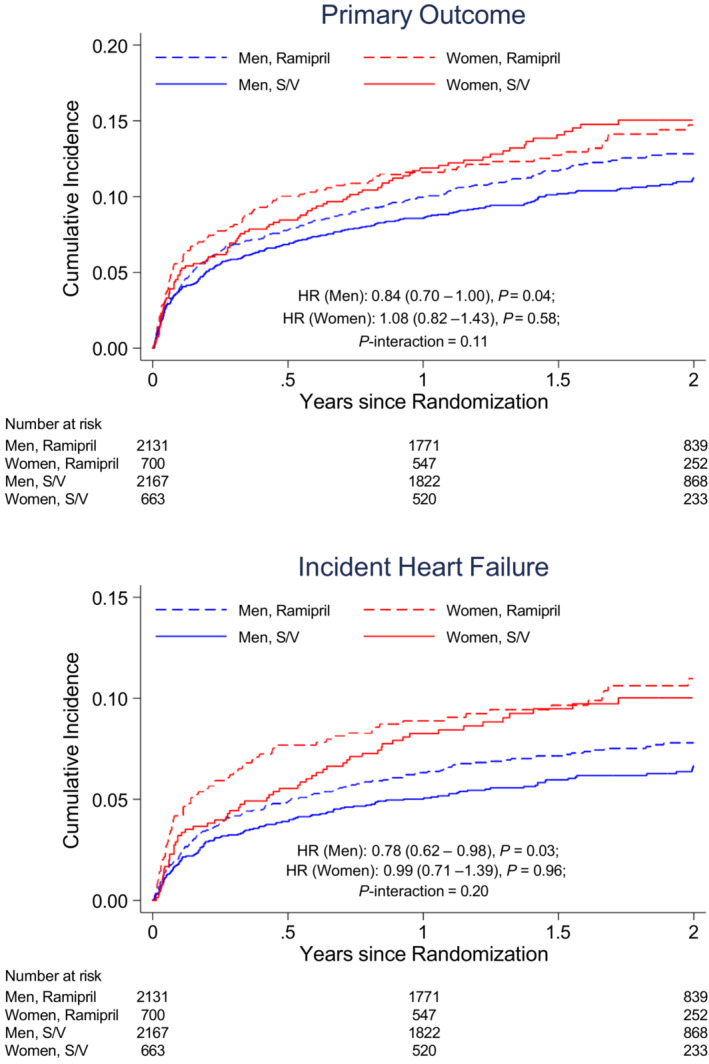
The HR (reference=ramipril) is adjusted for percutaneous coronary intervention and geographic region, stratified by type of myocardial infarction. HR indicates hazard ratio; PARADISE‐MI, Prospective ARNI (Angiotensin Receptor–Neprilysin Inhibitor) Versus ACE (Angiotensin‐Converting Enzyme) Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After Myocardial Infarction; and S/V, sacubitril/valsartan.
Secondary Outcomes and Components of the Primary Composite Outcome
Women and men had a similar incidence of cardiovascular death, outpatient HF, all‐cause death, composite coronary events, and the composite of cardiovascular death, nonfatal MI, or nonfatal stroke (Table 2). Women had a higher rate of HF‐related outcomes than men. Women had a higher incidence of CEC‐adjudicated HF hospitalization (5.0 per 100 person‐years in women versus 3.1 per 100 person‐years in men; unadjusted HR, 1.55 [95% CI, 1.25–1.93]; P<0.001; Figure 2), as well as a higher incidence of investigator‐reported HF hospitalization (7.6 per 100 person‐years in women versus 4.7 per 100 person‐years in men; unadjusted HR, 1.54 [95% CI, 1.29–1.85]; P<0.001). Similarly, in crude analyses, women were more likely to experience cardiovascular death or HF hospitalization (HR, 1.26 [95% CI, 1.06–1.50]; P=0.01) and HF hospitalization or outpatient HF (HR, 1.48 [95% CI, 1.21–1.81]; P<0.001). Women and men had similar incidence rates of all‐cause death and cardiovascular death (Figure 3). After adjusting for baseline characteristics, women were still 34% more likely to have a CEC‐adjudicated HF hospitalization and 29% more likely to have a CEC‐adjudicated HF hospitalization or outpatient HF (Table 2). Similar differences were seen in investigator‐reported HF hospitalizations (HR, 1.34 [95% CI, 1.10–1.64]; P=0.004). The proportional hazards assumption was found to be significantly violated for some adjusted models. Sensitivity analyses were conducted using an alternative model constructed to address these violations and produced consistent results (Table S1).
Figure 2. The cumulative incidence for incident heart failure (heart failure hospitalization or outpatient heart failure) and heart failure hospitalization according to sex and treatment assignment in PARADISE‐MI.
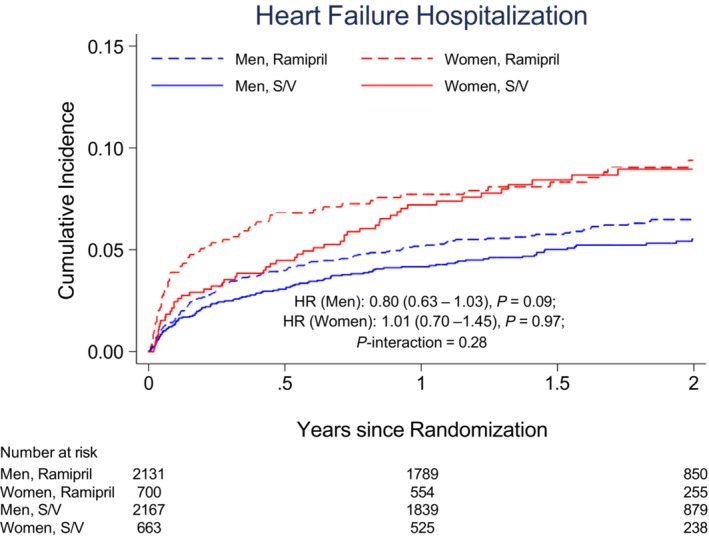
The HR (reference=ramipril) is adjusted for percutaneous coronary intervention and geographic region, stratified by type of myocardial infarction. HR indicates hazard ratio; PARADISE‐MI, Prospective ARNI (Angiotensin Receptor–Neprilysin Inhibitor) Versus ACE (Angiotensin‐Converting Enzyme) Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After Myocardial Infarction; and S/V, sacubitril/valsartan.
Figure 3. The cumulative incidence for all‐cause and cardiovascular death, according to sex and treatment assignment in PARADISE‐MI.
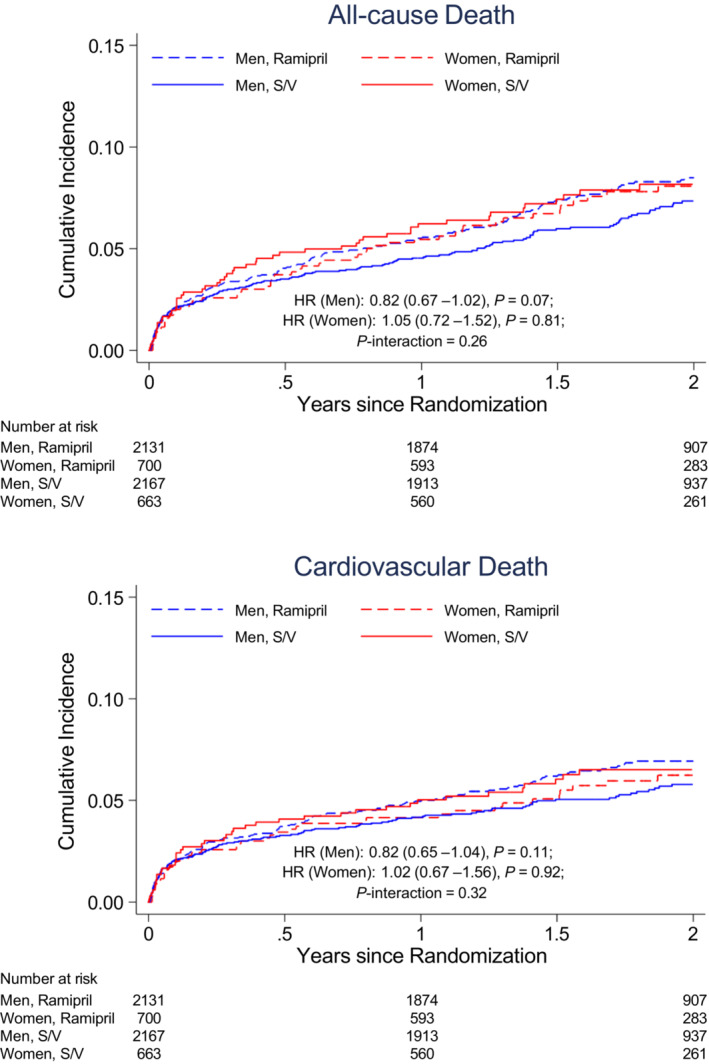
The HR (reference=ramipril) is adjusted for percutaneous coronary intervention and geographic region, stratified by type of myocardial infarction. HR indicates hazard ratio; PARADISE‐MI, Prospective ARNI (Angiotensin Receptor–Neprilysin Inhibitor) Versus ACE (Angiotensin‐Converting Enzyme) Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After Myocardial Infarction; and S/V, sacubitril/valsartan.
Total (First and Recurrent) Events
For the CEC‐adjudicated composite of cardiovascular death and total cardiovascular events, there was a trend toward a higher event rate in women, compared with men (13.7 per 100 person‐years in women versus 11.4 per 100 person‐years in men; rate ratio [RR], 1.22 [95% CI, 0.99–1.50]; P=0.06). The difference was attenuated after adjusting for baseline characteristics (RR, 1.05 [95% CI, 0.86–1.29]; P=0.64). When considering investigator‐reported outcomes, women were more likely to experience cardiovascular death or total HF events (unadjusted RR, 1.46 [95% CI, 1.21–1.76]; P<0.001; adjusted RR, 1.21 [95% CI, 1.00–1.47]; P=0.04).
Women were more likely to have total HF hospitalizations and total HF events, both CEC adjudicated and investigator reported (Table 3). After adjusting for baseline characteristics, women were still 39% more likely to experience CEC‐adjudicated total HF hospitalizations, and they were 31% more likely to experience CEC‐adjudicated total HF hospitalizations and outpatient episodes of HF (Table 3). There were similar differences in investigator‐reported outcomes: women were 47% more likely to have HF hospitalizations and 37% more likely to have HF hospitalizations and outpatient HF compared with men (Table 3).
Table 3.
Total (First and Recurrent) Events in Women Versus Men
| Men (N=4298) | Women (N=1363) | Unadjusted outcomes | Adjusted outcomes* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | No. of events | Event rate (per 100 person‐years) | No. of events | Event rate (per 100 person‐years) | Rate ratio (95% CI) (reference=men) | P value | Rate ratio (95% CI) (reference=men) | P value | P for interaction value† |
| Clinical events committee–adjudicated outcomes | |||||||||
| Cardiovascular death and total cardiovascular events (HHF, MI, and CVA) | 930 | 11.4 | 343 | 13.7 | 1.22 (0.99–1.50) | 0.06 | 1.05 (0.86–1.29) | 0.64 | 0.40 |
| Cardiovascular death and total HF events‡ | 710 | 8.7 | 281 | 11.2 | 1.30 (1.03–1.64) | 0.03 | 1.08 (0.86–1.36) | 0.50 | 0.22 |
| Total HF hospitalizations | 360 | 4.4 | 166 | 6.6 | 1.54 (1.17–2.02) | 0.002 | 1.39 (1.05–1.84) | 0.02 | 0.37 |
| Total outpatient HF events | 78 | 1.0 | 28 | 1.1 | 1.17 (0.72–1.88) | 0.53 | 1.03 (0.61–1.72) | 0.92 | 0.23 |
| Total HF events‡ | 438 | 5.3 | 194 | 7.8 | 1.48 (1.51–1.89) | 0.002 | 1.31 (1.01–1.69) | 0.04 | 0.22 |
| Investigator‐reported outcomes | |||||||||
| Cardiovascular death and total HF events‡ | 1023 | 12.5 | 451 | 18.0 | 1.46 (1.21–1.76) | <0.001 | 1.21 (1.00–1.47) | 0.04 | 0.22 |
| Total HF hospitalizations | 557 | 6.8 | 285 | 11.4 | 1.72 (1.37–2.17) | <0.001 | 1.47 (1.16–1.86) | 0.001 | 0.23 |
| Total HF events‡ | 770 | 9.4 | 368 | 14.7 | 1.59 (1.31–1.93) | <0.001 | 1.37 (1.13–1.67) | 0.002 | 0.13 |
CVA indicates cerebrovascular accident; HF, heart failure; HHF, hospitalization for HF; and MI, myocardial infarction.
Models adjusted for age, race, geographic region, number of risk‐augmenting factors, baseline left ventricular ejection fraction, intravenous treatment for pulmonary congestion, history of MI, hypertension, diabetes, current tobacco use, estimated glomerular filtration rate, type of index MI, ST‐segment–elevation MI without reperfusion, percutaneous coronary intervention, Killip class, and treatment assignment.
P for interaction: interaction between sex and treatment, adjusted for percutaneous coronary intervention and geographic region, stratified by type of MI.
Total HF events: HF hospitalizations and outpatient episodes of HF.
Safety Events
Irrespective of treatment assignment, women were more likely to have serious adverse events (44.6% versus 38.7%; P<0.001) and adverse events (86.1% versus 81.5%; P<0.001). Women had similar rates of drug discontinuation attributable to adverse events compared with men (14.5% versus 12.5%; P=0.07), with no significant sex‐by‐treatment interaction (Table 4 and Figure 4). Women were more likely to have cough (14.7% versus 9.9%; P<0.001), hepatotoxicity (6.7% versus 4.8%; P=0.008), and renal impairment (15.8% versus 10.2%; P<0.001; Table 4) than men. After adjusting for baseline eGFR, women were still more likely to have serious adverse events (odds ratio [OR], 1.21 [95% CI, 1.07–1.38]; P=0.003), adverse events (OR, 1.37 [95% CI, 1.14–1.64]; P=0.001), and renal impairment (OR, 1.34 [95% CI, 1.11–1.61]; P=0.002). Women were more likely to have elevated liver enzymes; however, the event rate was low for both men and women. Both women and men were more likely to have hypotension when randomized to sacubitril/valsartan and less likely to have cough when compared with those randomized to ramipril (Table 4). Differences in treatment‐related adverse events, serious adverse events, or drug discontinuation attributable to adverse events between sacubitril/valsartan and ramipril did not differ by sex (P for interaction >0.05 for all adverse events of interest; Table 4).
Table 4.
Adherence, Tolerability, and Safety in Men and Women
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | All | Sacubitril‐ valsartan (n=2167) | Ramipril (n=2131) | All | Sacubitril‐ valsartan (n=663) | Ramipril (n=700) | Interaction P value* (sex × treatment) | P value (women vs men) |
| Drug discontinuation because of adverse events | 539 (12.5) | 266 (12.3) | 273 (12.8) | 197 (14.5) | 91 (13.7) | 106 (15.1) | 0.71 | 0.07 |
| Serious adverse event | 1664 (38.7) | 856 (39.5) | 808 (37.9) | 608 (44.6) | 290 (43.7) | 318 (45.4) | 0.28 | <0.001 |
| Adverse event | 3503 (81.5) | 1778 (82.0) | 1725 (80.9) | 1174 (86.1) | 574 (86.6) | 600 (85.7) | 0.10 | <0.001 |
| Adverse events of interest | ||||||||
| Hypotension | 1067 (24.8) | 607 (28.0) | 460 (21.6) | 355 (26.0) | 195 (29.4) | 160 (22.9) | 0.97 | 0.37 |
| Cough | 426 (9.9) | 184 (8.5) | 242 (11.4) | 200 (14.7) | 71 (10.7) | 129 (18.4) | 0.10 | <0.001 |
| Angioedema | 21 (0.5) | 9 (0.4) | 12 (0.6) | 10 (0.7) | 5 (0.8) | 5 (0.7) | 0.64 | 0.29 |
| Hepatotoxicity | 208 (4.8) | 92 (4.2) | 116 (5.4) | 91 (6.7) | 40 (6.0) | 51 (7.3) | 0.82 | 0.008 |
| Hyperkalemia | 456 (10.6) | 231 (10.7) | 225 (10.6) | 130 (9.5) | 70 (10.6) | 60 (8.6) | 0.30 | 0.26 |
| Cognitive impairment | 82 (1.9) | 40 (1.8) | 42 (2.0) | 32 (2.3) | 14 (2.1) | 18 (2.6) | 0.75 | 0.31 |
| Hypersensitivity | 451 (10.5) | 240 (11.1) | 211 (9.9) | 167 (12.3) | 82 (12.4) | 85 (12.1) | 0.59 | 0.07 |
| Risk of malignancy | 118 (2.7) | 61 (2.8) | 57 (2.7) | 38 (2.8) | 24 (3.6) | 14 (2.0) | 0.15 | 0.93 |
| Renal impairment | 440 (10.2) | 221 (10.2) | 219 (10.3) | 215 (15.8) | 108 (16.3) | 107 (15.3) | 0.64 | <0.001 |
| Statin drug‐drug interaction | 169 (3.9) | 75 (3.5) | 94 (4.4) | 66 (4.8) | 31 (4.7) | 35 (5.0) | 0.54 | 0.14 |
| Laboratory abnormalities | ||||||||
| Elevated serum creatinine level | ||||||||
| ≥2.0 mg/dL | 263 (6.1) | 129 (6.0) | 134 (6.3) | 70 (5.1) | 33 (5.0) | 37 (5.3) | 0.99 | 0.18 |
| ≥2.5 mg/dL | 112 (2.6) | 53 (2.4) | 59 (2.8) | 20 (1.5) | 14 (2.1) | 6 (0.9) | 0.048 | 0.02 |
| ≥3.0 mg/dL | 46 (1.1) | 16 (0.7) | 30 (1.4) | 11 (0.8) | 7 (1.1) | 4 (0.6) | 0.07 | 0.40 |
| Elevated serum potassium level | ||||||||
| ≥5.5 mmol/L | 570 (13.3) | 301 (13.9) | 269 (12.6) | 194 (14.2) | 102 (15.4) | 92 (13.1) | 0.68 | 0.36 |
| ≥6.0 mmol/L | 146 (3.4) | 69 (3.2) | 77 (3.6) | 41 (3.0) | 23 (3.5) | 18 (2.6) | 0.22 | 0.48 |
| Elevated aspartate aminotransferase level | ||||||||
| >3× Upper limit of reference range | 29 (0.7) | 13 (0.6) | 16 (0.8) | 21 (1.5) | 10 (1.5) | 11 (1.6) | 0.75 | 0.003 |
| >5× Upper limit of reference range | 11 (0.3) | 5 (0.2) | 6 (0.3) | 10 (0.7) | 3 (0.5) | 7 (1.0) | 0.52 | 0.01 |
| Elevated alanine aminotransferase level | ||||||||
| >3× Upper limit of reference range | 50 (1.2) | 23 (1.1) | 27 (1.3) | 20 (1.5) | 9 (1.4) | 11 (1.6) | 0.95 | 0.38 |
| >5× Upper limit of reference range | 12 (0.3) | 7 (0.3) | 5 (0.2) | 11 (0.8) | 4 (0.6) | 7 (1.0) | 0.34 | 0.01 |
Data are given as number (percentage) unless otherwise indicated.
P for interaction: adjusted for percutaneous coronary intervention and region, stratified by type of myocardial infarction.
Figure 4. The cumulative incidence of study drug discontinuation because of adverse events, according to sex and treatment assignment in PARADISE‐MI.
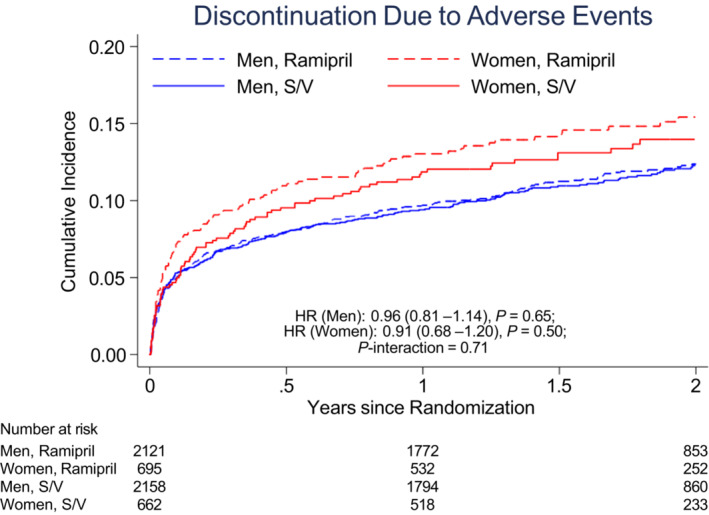
The HR (reference=ramipril) is adjusted for baseline estimated glomerular filtration rate. HR indicates hazard ratio; PARADISE‐MI, Prospective ARNI (Angiotensin Receptor–Neprilysin Inhibitor) Versus ACE (Angiotensin‐Converting Enzyme) Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After Myocardial Infarction; and S/V, sacubitril/valsartan.
Discussion
Our analyses identified significant differences in baseline clinical characteristics in men and women following high‐risk MI. There were no significant differences in all‐cause death or cardiovascular death between men and women. However, women were more likely to have HF‐related events, in particular HF hospitalizations. Women were also more likely to experience drug‐related serious adverse events.
Consistent with prior studies, women in PARADISE‐MI were older than men, and they had a higher prevalence of comorbidities, such as hypertension and diabetes, but a lower prevalence of prior MI. 13 , 14 , 15 Estrogen has been shown to have a cardioprotective effect, and as a result, women tend to be older when they develop cardiovascular disease. 14 , 15 After menopause, women have similar risks of developing cardiovascular disease compared with their male counterparts. Because women tend to be older, they likely have a higher burden of comorbidities and cardiovascular risk factors.
Analysis of the INTERHEART study shows that the same risk factors appear to contribute to higher population‐attributable risk in women compared with men. 16 For example, diabetes is associated with 19.1% population‐attributable risk in women, compared with 10.1% in men, and hypertension is associated with 35.8% and 19.5% population‐attributable risk in women and men, respectively. Consistent with other studies, women in PARADISE‐MI also had higher Killip class when they presented with their qualifying MI. 17 These epidemiologic differences may contribute to different outcomes in men and women with acute coronary syndromes (ACSs).
Significant advances in the treatment of acute MI have been made for both men and women. Indeed, the mortality rate following ACS has steadily decreased in the past several decades. 1 Some studies suggest that women have similar or lower mortality following ACS after adjusting for comorbidities and severity of angiographically documented disease. 3 , 18 , 19 Others show that women are treated less aggressively when presenting with ACS, and they have higher in‐hospital, 1‐year, and 5‐year mortality, with some differences persisting even after adjusting for baseline differences. 1 , 13 , 20 , 21 , 22 However, in PARADISE‐MI, no significant differences were detected in all‐cause death or cardiovascular death between men and women, both in crude and adjusted analyses over 2.5 to 3 years.
In addition to mortality, nonfatal events, such as recurrent coronary events and HF events, are also important outcomes following acute MI. In PARADISE‐MI, men were more likely to have prior MI and coronary interventions. However, we did not find any significant differences in adjusted and unadjusted composite coronary end points following the index MI. In comparison, in prior studies, women were more likely to have other nonfatal complications after MI, such as stroke, HF, serious bleeding after PCI, and reinfarction. 5 , 23 In a recent analysis of patients from the ISACS‐TC (The International Survey of Acute Coronary Syndromes in Transitional Countries) registry, women were 34% more likely to develop de novo HF following STEMI, and those who developed HF were 29% more likely to die within 30 days. 24 Our analyses showed that women were 29% more likely to have first HF hospitalization or outpatient HF and 34% more likely to have first HF hospitalization alone, even after adjusting for baseline differences. For total HF events, women were 39% more likely to have HF hospitalizations and 31% more likely to have total HF events (HF hospitalization and outpatient HF). This was comparable to the VALIANT trial, where women were 36% more likely to be hospitalized for HF after adjusting for baseline differences. 6 When considering total events (first and recurrent), women were 48% more likely to have total HF events; however, this difference dissipates after adjusting for baseline differences.
These differences in outcomes are likely multifactorial, attributable to both underlying sex‐based differences in the pathophysiological features and sex‐related differences in the treatment of ACS. Women have smaller epicardial coronary arteries and are less likely to have obstructive coronary artery disease; the role of microvasculature seems to be more prominent in coronary perfusion. 25 Furthermore, after MI, women and men undergo left ventricular remodeling differently. Postmortem studies suggest that men have a higher apoptotic rate after acute MI and are more likely to undergo ventricular dilation. 26 Although it may be detrimental in the long‐term, initial dilation in the immediate phase may be beneficial in maintaining stroke volume and cardiac output. 26 In response to volume overload, women are more prone to develop concentric remodeling, whereas men undergo eccentric remodeling. 26 Men tend to develop HF with reduced ejection fraction, whereas women tend to develop HF with preserved ejection fraction. These differences in pathophysiological features may account for some difference in outcomes.
In addition to sex‐based differences in pathophysiological features, studies have shown that women are treated differently when they present with acute MI. In the ISACS‐TC registry, women had a longer delay before arrival at the hospital (median, 270 minutes for women versus 240 minutes for men) because of the lag between first medical contact and hospital presentation. 27 Women are less likely to receive reperfusion therapy when they present with MI, 5 , 28 , 29 and they have a longer door‐to‐balloon time when presenting with STEMI. 28 , 30 Women are also less likely to receive aggressive medical management 5 attributable to less treatment initiation (not treatment adherence). 31 Consistent with prior studies, in PARADISE‐MI, fewer women than men received reperfusion therapy (including PCI) within 24 hours after presenting with STEMI.
Our analysis also showed a higher incidence of drug‐related adverse events in women. In the primary analysis, participants randomized to receive sacubitril/valsartan had a higher incidence of hypotension and a lower incidence of cough than those assigned to ramipril. 12 In our analysis, women had more serious adverse events and adverse events, mostly driven by more cough, hepatotoxicity, and renal impairment regardless of study drug assigned. Given women's and men's differences in physiological features, body composition, and hormonal changes, it is possible that sacubitril/valsartan and ramipril have different pharmacokinetics in women and men. 32 It has been well established that women and men have different ACEi‐related adverse events, women are 2 to 3 times more likely to report cough when taking an ACEi, 32 , 33 , 34 and women are more likely to have spontaneously reported adverse drug events. 35 On the other hand, it has not been well established whether women have more adverse events with sacubitril/valsartan. 36 Indeed, we did not find sex to be a modifier for study drug–related adverse events. Further studies are needed to assess the different adverse event profile in men versus women.
Our study findings should be interpreted with the following limitations. First, our subgroup analysis of PARADISE‐MI included a population who was highly selected for left ventricular dysfunction, therefore limiting the generalizability of our findings. In particular, although the observed differences in demographics between men and women and disparities in receiving invasive management for acute MI were consistent with prior studies, additional large and current epidemiologic studies are needed. Second, among the 5661 participants, only 24% (1363) were women, likely underpowered to detect differences in women receiving ramipril versus sacubitril/valsartan.
In conclusion, in contemporary patients with acute MI and with reduced LVEF, pulmonary congestion, or both, women were older and had more comorbidities compared with men. Women received less aggressive treatment, were more likely to experience adverse events, and had a higher rate of HF during follow‐up. Sex did not modify the treatment effects of sacubitril/valsartan.
Sources of Funding
PARADISE‐MI (Prospective ARNI [Angiotensin Receptor–Neprilysin Inhibitor] Versus ACE [Angiotensin‐Converting Enzyme] Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After Myocardial Infarction) was funded by Novartis. Dr X. Wang is supported by a T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute (T32 HL094301) and by the Scott Schoen and Nancy Adams First.In.Women Cardiovascular Fellowship, Mary Horrigan Connors Center for Women's Health and Gender Biology at Brigham and Women's Hospital. Dr McMurray is supported by a British Heart Foundation Centre of Research Excellence grant RE/18/6/34217.
Disclosures
Dr Cikes reports research grants and clinical study contracts to institution from Novartis, Abbott, Pfizer, and CorVia; personal fees and nonfinancial support from Pfizer, Bayer, Boehringer Ingelheim, AstraZeneca, Novartis, Swixx, Abiomed, Amicus, Amgen, NovoNordisk, Medtronic, GE Healthcare, Teva Pharmaceutical Industries, and Krka Pharma. Dr Mehran reports institutional research payments from Abbott, Abiomed, Alleviant Medical, AM‐Pharma, Applied Therapeutics, Arena, AstraZeneca, BAIM, Bayer, Beth Israel Deaconess, Biosensors, Biotronik, Boston Scientific, Bristol‐Myers Squibb, CardiaWave, CellAegis, CeloNova, CERC, Chiesi, Concept Medical, CSL Behring, Cytosorbents, DSI, Duke University, Element Science, Faraday, Humacyte, Idorsia, Insel Gruppe AG, Magenta, Medtronic, Novartis, OrbusNeich, Philips, RenalPro, Vivasure, and Zoll; personal fees from Cine‐Med Research and WebMD; consulting fees paid to the institution from Abbott, Janssen, Medtronic, and Novartis; equity <1% in Applied Therapeutics, Elixir Medical, STEL, and CONTROLRAD (spouse); Scientific Advisory Board for American Medical Association, American College of Cardiology (Board of Trustees member), Society for Cardiovascular Angiography & Interventions (Women in Innovations Committee member), and JAMA Associate Editor; and faculty CRF (no fee). Dr East receives consulting fees from Novartis. Dr Mody receives institutional grants from AstraZeneca, National Institutes of Health (NIH), LivaNova USA, Inc, and Novartis; and consulting fees from SCPharmaceuticals. Dr Y. Wang is employed by Novartis. Dr Lewis receives institutional grant from Novartis; and consulting fees from Amgen and Merck. Dr Claggett reported receiving consulting fees from Boehringer Ingelheim, Cardurion, Corvia, Cytokinetics, Intellia, and Novartis outside of the submitted work. He has received payments through Glasgow University from work on clinical trials, consulting, and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardurion, Cytokinetics, Dal‐Cor, GSK, Ionis, KBP Biosciences, Novartis, Pfizer, and Theracos; and personal lecture fees from Corpus, Abbott, Hikma, Sun Pharmaceuticals, Medscape/Heart.Org, Radcliffe Cardiology, Servier Director, and Global Clinical Trial Partners. Dr Granger receives institution grant and consulting fees from Novartis. Dr Pfeffer receives research support from Novartis; he serves as a consultant for AstraZeneca, Corvidia, DalCor, GlaxoSmithKline, Jazz, MyoKardia, Novartis, Novo Nordisk, Roche, Sanofi, Servier, and Takeda; and has equity in DalCor. Dr Solomon has received research grants from Alnylam, AstraZeneca, Bellerophon, Bayer, BMS, Cytokinetics, Eidos, GSK, Ionis, Lilly, MyoKardia, NIH/National Heart, Lung, and Blood Institute, Novartis, NovoNordisk, Respicardia, Sanofi‐Pasteur, Theracos, US2.AI, and has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boeringer‐Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi‐Sankyo, GSK, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi‐Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, and Akros. The remaining authors have no disclosures to report.
Supporting information
Table S1
This article was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028942
For Sources of Funding and Disclosures, see page 13.
References
- 1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2021 update. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 2. Wenger NK. Women and coronary heart disease: a century after Herrick: understudied, underdiagnosed, and undertreated. Circulation. 2012;126:604–611. doi: 10.1161/CIRCULATIONAHA.111.086892 [DOI] [PubMed] [Google Scholar]
- 3. Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW, Simes RJ, White HD, Van de Werf F, Topol EJ, et al. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302:874–882. doi: 10.1001/jama.2009.1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jneid H, Fonarow GC, Cannon CP, Hernandez AF, Palacios IF, Maree AO, Wells Q, Bozkurt B, LaBresh KA, Liang L, et al. Sex differences in medical care and early death after acute myocardial infarction. Circulation. 2008;118:2803–2810. doi: 10.1161/CIRCULATIONAHA.108.789800 [DOI] [PubMed] [Google Scholar]
- 5. Blomkalns AL, Chen AY, Hochman JS, Peterson ED, Trynosky K, Diercks DB, Brogan GX Jr, Boden WE, Roe MT, Ohman EM, et al. Gender disparities in the diagnosis and treatment of non‐ST‐segment elevation acute coronary syndromes: large‐scale observations from the CRUSADE (can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the American College of Cardiology/American Heart Association guidelines) National Quality Improvement Initiative. J Am Coll Cardiol. 2005;45:832–837. doi: 10.1016/j.jacc.2004.11.055 [DOI] [PubMed] [Google Scholar]
- 6. Lam CS, McEntegart M, Claggett B, Liu J, Skali H, Lewis E, Køber L, Rouleau J, Velazquez E, Califf R, et al. Sex differences in clinical characteristics and outcomes after myocardial infarction: insights from the valsartan in acute myocardial infarction trial (VALIANT). Eur J Heart Fail. 2015;17:301–312. doi: 10.1002/ejhf.238 [DOI] [PubMed] [Google Scholar]
- 7. Jering KS, Claggett B, Pfeffer MA, Granger C, Køber L, Lewis EF, Maggioni AP, Mann D, McMurray JJV, Rouleau J‐L, et al. Prospective ARNI vs. ACE inhibitor trial to determine superiority in reducing heart failure events after myocardial infarction (PARADISE‐MI): design and baseline characteristics. Eur J Heart Fail. 2021;23:1040–1048. doi: 10.1002/ejhf.2191 [DOI] [PubMed] [Google Scholar]
- 8. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 9. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, et al. Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655 [DOI] [PubMed] [Google Scholar]
- 10. McMurray JJV, Jackson AM, Lam CSP, Redfield MM, Anand IS, Ge J, Lefkowitz MP, Maggioni AP, Martinez F, Packer M, et al. Effects of sacubitril‐valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction: insights from PARAGON‐HF. Circulation. 2020;141:338–351. doi: 10.1161/CIRCULATIONAHA.119.044491 [DOI] [PubMed] [Google Scholar]
- 11. Novartis Position on Clinical Study Transparency–Clinical Study Registration, Results Reporting and Data Sharing 2016 . Accessed March 30, 2023. https://www.novartis.com/sites/www.novartis.com/files/clinical‐trial‐data‐transparency.pdf
- 12. Pfeffer MA, Claggett B, Lewis EF, Granger CB, Køber L, Maggioni AP, Mann DL, McMurray JJV, Rouleau J‐L, Solomon SD, et al. Angiotensin receptor–neprilysin inhibition in acute myocardial infarction. N Engl J Med. 2021;385:1845–1855. doi: 10.1056/NEJMoa2104508 [DOI] [PubMed] [Google Scholar]
- 13. Aggarwal NR, Patel HN, Mehta LS, Sanghani RM, Lundberg GP, Lewis SJ, Mendelson MA, Wood MJ, Volgman AS, Mieres JH. Sex differences in ischemic heart disease: advances, obstacles, and next steps. Circ Cardiovasc Qual Outcomes. 2018;11:e004437. doi: 10.1161/CIRCOUTCOMES.117.004437 [DOI] [PubMed] [Google Scholar]
- 14. Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, et al. Insights from the NHLBI‐sponsored Women's Ischemia Syndrome Evaluation (WISE) study: part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender‐optimized diagnostic strategies. J Am Coll Cardiol. 2006;47:S4–s20. doi: 10.1016/j.jacc.2005.01.072 [DOI] [PubMed] [Google Scholar]
- 15. Garcia M, Mulvagh SL, Bairey Merz CN, Buring JE, Manson JE. Cardiovascular disease in women. Circ Res. 2016;118:1273–1293. doi: 10.1161/CIRCRESAHA.116.307547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 17. Radovanovic D, Erne P, Urban P, Bertel O, Rickli H, Gaspoz JM. Gender differences in management and outcomes in patients with acute coronary syndromes: results on 20,290 patients from the AMIS plus registry. Heart. 2007;93:1369–1375. doi: 10.1136/hrt.2006.106781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarma Amy A, Braunwald E, Cannon Christopher P, Guo J, Im K, Antman Elliott M, Gibson CM, Newby LK, Giugliano Robert P, Morrow David A, et al. Outcomes of women compared with men after non–ST‐segment elevation acute coronary syndromes. J Am Coll Cardiol. 2019;74:3013–3022. doi: 10.1016/j.jacc.2019.09.065 [DOI] [PubMed] [Google Scholar]
- 19. Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG, Flather MD, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345–2353. doi: 10.1001/archinte.163.19.2345 [DOI] [PubMed] [Google Scholar]
- 20. Ezekowitz JA, Savu A, Welsh RC, McAlister FA, Goodman SG, Kaul P. Is there a sex gap in surviving an acute coronary syndrome or subsequent development of heart failure? Circulation. 2020;142:2231–2239. doi: 10.1161/CIRCULATIONAHA.120.048015 [DOI] [PubMed] [Google Scholar]
- 21. Pancholy SB, Shantha GP, Patel T, Cheskin LJ. Sex differences in short‐term and long‐ term all‐cause mortality among patients with ST‐segment elevation myocardial infarction treated by primary percutaneous intervention: a meta‐analysis. JAMA Intern Med. 2014;174:1822–1830. doi: 10.1001/jamainternmed.2014.4762 [DOI] [PubMed] [Google Scholar]
- 22. Matetic A, Shamkhani W, Rashid M, Volgman AS, Van Spall HGC, Coutinho T, Mehta LS, Sharma G, Parwani P, Mohamed MO, et al. Trends of sex differences in clinical outcomes after myocardial infarction in the United States. CJC Open. 2021;3:S19–s27. doi: 10.1016/j.cjco.2021.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weaver WD, White HD, Wilcox RG, Aylward PE, Morris D, Guerci A, Ohman EM, Barbash GI, Betriu A, Sadowski Z, et al. Comparisons of characteristics and outcomes among women and men with acute myocardial infarction treated with thrombolytic therapy. GUSTO‐I Investigators. JAMA. 1996;275:777–782. doi: 10.1001/jama.1996.03530340041027 [DOI] [PubMed] [Google Scholar]
- 24. Cenko E, van der Schaar M, Yoon J, Manfrini O, Vasiljevic Z, Vavlukis M, Kedev S, Miličić D, Badimon L, Bugiardini R. Sex‐related differences in heart failure after ST‐segment elevation myocardial infarction. J Am Coll Cardiol. 2019;74:2379–2389. doi: 10.1016/j.jacc.2019.08.1047 [DOI] [PubMed] [Google Scholar]
- 25. Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, et al. Insights from the NHLBI‐sponsored Women's Ischemia Syndrome Evaluation (WISE) study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender‐based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–S29. doi: 10.1016/j.jacc.2004.12.084 [DOI] [PubMed] [Google Scholar]
- 26. Piro M, Della Bona R, Abbate A, Biasucci LM, Crea F. Sex‐related differences in myocardial remodeling. J Am Coll Cardiol. 2010;55:1057–1065. doi: 10.1016/j.jacc.2009.09.065 [DOI] [PubMed] [Google Scholar]
- 27. Bugiardini R, Ricci B, Cenko E, Vasiljevic Z, Kedev S, Davidovic G, Zdravkovic M, Miličić D, Dilic M, Manfrini O, et al. Delayed care and mortality among women and men with myocardial infarction. J Am Heart Assoc. 2017;6:6. doi: 10.1161/JAHA.117.005968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steingart RM, Packer M, Hamm P, Coglianese ME, Gersh B, Geltman EM, Sollano J, Katz S, Moyé L, Basta LL, et al. Sex differences in the management of coronary artery disease. N Engl J Med. 1991;325:226–230. doi: 10.1056/NEJM199107253250402 [DOI] [PubMed] [Google Scholar]
- 29. Ayanian JZ, Epstein AM. Differences in the use of procedures between women and men hospitalized for coronary heart disease. N Engl J Med. 1991;325:221–225. doi: 10.1056/NEJM199107253250401 [DOI] [PubMed] [Google Scholar]
- 30. Stehli J, Martin C, Brennan A, Dinh DT, Lefkovits J, Zaman S. Sex differences persist in time to presentation, revascularization, and mortality in myocardial infarction treated with percutaneous coronary intervention. J Am Heart Assoc. 2019;8:e012161. doi: 10.1161/JAHA.119.012161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smolina K, Ball L, Humphries KH, Khan N, Morgan SG. Sex disparities in post‐acute myocardial infarction pharmacologic treatment initiation and adherence: problem for young women. Circ Cardiovasc Qual Outcomes. 2015;8:586–592. doi: 10.1161/CIRCOUTCOMES.115.001987 [DOI] [PubMed] [Google Scholar]
- 32. Tamargo J, Rosano G, Walther T, Duarte J, Niessner A, Kaski JC, Ceconi C, Drexel H, Kjeldsen K, Savarese G, et al. Gender differences in the effects of cardiovascular drugs. Eur Heart J Cardiovasc Pharmacother. 2017;3:163–182. doi: 10.1093/ehjcvp/pvw042 [DOI] [PubMed] [Google Scholar]
- 33. Visser LE, Stricker BH, van der Velden J, Paes AH, Bakker A. Angiotensin converting enzyme inhibitor associated cough: a population‐based case‐control study. J Clin Epidemiol. 1995;48:851–857. doi: 10.1016/0895-4356(94)00231-E [DOI] [PubMed] [Google Scholar]
- 34. Os I, Bratland B, Dahlöf B, Gisholt K, Syvertsen JO, Tretli S. Female sex as an important determinant of lisinopril‐induced cough. Lancet. 1992;339:372. doi: 10.1016/0140-6736(92)91694-4 [DOI] [PubMed] [Google Scholar]
- 35. Rydberg DM, Mejyr S, Loikas D, Schenck‐Gustafsson K, von Euler M, Malmström RE. Sex differences in spontaneous reports on adverse drug events for common antihypertensive drugs. Eur J Clin Pharmacol. 2018;74:1165–1173. doi: 10.1007/s00228-018-2480-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gan L, Langenickel T, Petruck J, Kode K, Rajman I, Chandra P, Zhou W, Rebello S, Sunkara G. Effects of age and sex on the pharmacokinetics of LCZ696, an angiotensin receptor neprilysin inhibitor. J Clin Pharmacol. 2016;56:78–86. doi: 10.1002/jcph.571 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


