Abstract
Background
There is little evidence about the prognostic role of mitral regurgitation (MR) in patients with low‐flow, low‐gradient aortic stenosis undergoing transcatheter aortic valve replacement (TAVR). The aim of this study was to assess the prevalence and outcome implications of MR severity in patients with low‐flow, low‐gradient aortic stenosis undergoing TAVR, and to evaluate whether MR improvement after TAVR could influence clinical outcome.
Methods and Results
This study included consecutive patients with low‐flow, low‐gradient aortic stenosis undergoing TAVR at 2 Italian high‐volume centers. The study population was categorized according to the baseline MR severity and to the presence of MR improvement at discharge. The primary outcome was the composite of all‐cause death and hospitalization for worsening heart failure up to 1 year. The study included 268 patients; 57 (21%) patients showed MR >2+. Patients with MR >2+ showed a lower 1‐year survival free from the primary outcome (P<0.001), all‐cause death (P<0.001), and heart failure hospitalization (P<0.001) compared with patients with MR ≤2+. At multivariable analysis, baseline MR >2+ was an independent predictor of the primary outcome (P<0.001). Among patients with baseline MR >2+, MR improvement was reported in 24 (44%) cases after TAVR. The persistence of MR was associated with a significantly reduced survival free from the primary outcome, all‐cause death, and heart failure hospitalization up to 1 year.
Conclusions
In this study, the presence of moderately severe to severe MR in patients with low‐flow, low‐gradient aortic stenosis undergoing TAVR portends a worse clinical outcome at 1 year. TAVR may improve MR severity in nearly half of the patients, resulting in a potential outcome benefit after discharge.
Keywords: clinical outcome, mitral insufficiency, transcatheter aortic valve implantation
Subject Categories: Valvular Heart Disease, Catheter-Based Coronary and Valvular Interventions, Heart Failure
Nonstandard Abbreviations and Acronyms
- AS
aortic stenosis
- AVA
aortic valve area
- EuroSCORE
European System for Cardiac Operative Risk Evaluation
- LFLG‐AS
low‐flow, low‐gradient aortic stenosis
- MR
mitral regurgitation
- sPAP
systolic pulmonary artery pressure
- SVi
stroke volume index
- TAVR
transcatheter aortic valve replacement
- VARC
Valve Academic Research Consortium
Clinical Perspective.
What Is New?
In this real‐world study including 268 patients with low‐flow, low‐gradient aortic stenosis undergoing transcatheter aortic valve replacement, the coexistence of moderately severe to severe mitral regurgitation at admission was reported in 21% of cases and was associated with a poor 1‐year outcome.
Transcatheter aortic valve replacement improved mitral regurgitation severity in nearly half of cases; in these patients, compared with those with persistently moderately severe to severe mitral regurgitation, there was a significant outcome benefit up to 1 year.
What Are the Clinical Implications?
Mitral regurgitation should be carefully and systematically assessed, both at admission and before discharge, in patients with low‐flow, low‐gradient aortic stenosis undergoing transcatheter aortic valve replacement in order to implement patients' prognostic stratification and clinical management.
Severe aortic stenosis (AS) is the most common valvular heart disease in Western countries due to the increasingly aging general population. 1 , 2 Severe low‐flow, low‐gradient AS (LFLG‐AS) is echocardiographically defined by an aortic valve area (AVA) <1 cm2 or an indexed AVA ≤0.6 cm2/m2, a mean transaortic gradient <40 mm Hg, and a stroke volume index <36 mL/m2. This is classified according to left ventricular ejection fraction (LVEF) values in classical (<50%) and paradoxical (≥50%) forms. 3
Patients with severe LFLG‐AS have a very high surgical risk, 4 , 5 , 6 especially those with classical forms. 6
Transcatheter aortic valve replacement (TAVR) is a less invasive and safe procedure than surgical aortic valve replacement and is recommended for symptomatic patients with AS at high risk for surgery. 3 The risk–benefit ratio of patients with LFLG‐AS may be less favorable than in the high‐gradient forms and depends on a proper patient selection by the local Heart Team. 7 , 8 , 9 Prior studies have investigated the conditions associated with poor outcome after TAVR in order to optimize procedural candidacy and patient management. 8 , 10 , 11 However, poor evidence is available in the very‐high‐risk scenario of LFLG‐AS.
Moderate‐to‐severe mitral regurgitation (MR) is reported in ≈22% of patients with high gradient severe AS and has been associated with higher risk of mortality after TAVR. 12 Also, the persistence of MR after TAVR is reported in more than half of cases and may mitigate the intended benefit of TAVR or be a critical determinant of patients' long‐term outcome. 13 , 14
Owing to the paucity of data, moderate‐to‐severe MR may be underestimated in patients with LFLG‐AS and its prognostic significance is still controversial. 15 , 16 Moreover, the potential benefit of MR improvement after TAVR has not yet been established.
The aim of this study was to determine the prevalence and prognostic implications of MR severity in patients with LFLG‐AS undergoing TAVR, and to evaluate whether the improvement of MR after the procedure could influence 1‐year outcome in this very‐high‐risk population.
METHODS
Study Population
This was an observational study including all symptomatic consecutive patients with severe LFLG‐AS undergoing TAVR from 2017 to 2022 at 2 Italian high‐volume centers (Salerno and Castel Volturno).
LFLG‐AS was defined as AVA <1 cm2 or an indexed AVA ≤0.6 cm2/m2, mean transaortic gradient <40 mm Hg, and stroke volume index <36 mL/m2 according with current guidelines. 3 LFLG‐AS was classified as classical if LVEF was <50% and paradoxical in patients with preserved LVEF (≥50%). Dobutamine stress echocardiography was systematically performed in patients with LVEF <50% in order or exclude pseudo‐severe AS. All patients with paradoxical LFLG‐AS underwent calcium volume assessment by computed tomography in order to confirm the severity of AS.
Patients were accepted as candidate to TAVR after a Heart Team decision‐making process developed on the basis of comprehensive surgical risk (European System for Cardiac Operative Risk Evaluation II), clinical, echocardiographic, and computed tomography assessment. Demographic, clinical, instrumental, and procedural data were prospectively collected by using an electronic case report form. Patients with recent history of myocardial infarction (<90 days before TAVR procedure) or endocarditis were excluded from this analysis. Also, patients with prior mitral valve surgery or transcatheter repair were excluded.
The study population was divided into 2 groups according to the baseline MR severity: ≤2+ (moderate or less than moderate) and>2+ (moderately severe or severe). MR was reassessed after TAVR before discharge in each patient; among patients with baseline MR >2+, the improvement after TAVR was defined by a reduction to ≤2+ at hospital discharge.
All participants provided written informed consent. The study complies with the principles of the Declaration of Helsinki. In order to minimize the possibility of unintentionally sharing information that can be used to re‐identify private information, a subset of the data generated for this study is available from the corresponding author upon reasonable request.
Echocardiographic Evaluation
Comprehensive transthoracic echocardiography was systematically performed with commercially available equipment (GE Vivid E9 and E95; GE Medical Systems, Milwaukee, WI) at baseline and after TAVR before discharge by experienced echocardiographers. Four‐chamber and 2‐chamber apical cine‐loops were used to calculate LVEF according to the biplane Simpson's method. 17
The severity of AS was based on a multiparametric approach according to current guideline recommendations including the following: peak transaortic flow velocity, mean transvalvular pressure gradient, and AVA using the continuity equation. Stroke volume index was calculated using left ventricular outflow tract diameter, obtained from the parasternal long‐axis section, and left ventricular outflow tract velocity time integral, both indexed to body surface area. 18
MR was graded as no/trivial (0), mild (1+), moderate (2+), moderately severe (3+), and severe (4+) using an integrative multiparametric approach including qualitative, semiquantitative, and quantitative measures according with current recommendations. 19 MR degree after TAVR was evaluated before discharge and MR improvement was defined by a reduction from >2+ to ≤2+.
For the assessment of right ventricular (RV) systolic function, tricuspid annular plane excursion was measured by aligning the M‐mode linear cursor to the lateral tricuspid annulus in the apical 4‐chamber. RV dysfunction was defined in our register as tricuspid annular plane excursion <17 mm. 17
Systolic pulmonary artery pressure was derived from the tricuspid regurgitant jet velocity using systolic trans‐tricuspid pressure gradient calculated by the modified Bernoulli equation with the addition of estimated mean right atrial pressure derived from inferior vena cava dimension and inspiratory distensibility. 17
Computed Tomography Assessment and TAVR Procedure
Patients with severe LFLG‐AS eligible for TAVR underwent a systematic preoperative work‐up including contrast‐enhanced computed tomography imaging of the chest, abdomen, and ileo‐femoral axes using 64‐row or higher scans with heart cycle synchronization.
Planning of the procedure, including arterial access, approach, device type and size, and pre‐ and postdilatation were all at the operator's discretion. Nonfemoral access was reserved for patients with complex ileo‐femoral anatomy and peripheral arterial disease not amenable to conventional arterial access. 20
Clinical Outcome
In‐hospital and 1‐year clinical outcomes were defined based on the Valve Academic Research Consortium 3 statement. 21 Valve Academic Research Consortium 3 definition of bleeding and transfusions is summarized in Table S1.
The primary outcome of this study was the composite of all‐cause death and hospitalization for worsening heart failure (HF) up to 1 year. The secondary outcomes were the single components of the primary outcome, cardiac and noncardiac death.
Clinical follow‐up was systematically performed during the hospitalization, at 1 month after TAVR and then every 6 months in HF outpatient clinics by clinicians not directly involved in the TAVR program. In some cases, information was obtained by telephone interview with the treating physicians or next of kin.
Statistical Analysis
The normal distribution of continuous parameters was tested with the Kolmogorov–Smirnov test. Normally distributed variables were expressed as mean±SD and compared using the Student t test; variables with a skewed distribution were reported as median and interquartile range and were compared with the Mann–Whitney U test. Categorical variables were reported as numbers and percentages and compared using the Fisher exact test.
Survival free from the study outcomes was estimated by the Kaplan–Meier method, and the log‐rank test was used for comparison between groups. First, we tested the effect of baseline MR severity (≤2+ versus MR >2+) on the study outcomes; then, among patients with baseline MR >2+, we tested the effect of MR improvement versus the lack of improvement after TAVR. The proportional hazard assumption was verified by visually inspecting the log–log plots and with the Schoenfeld residuals test. Violation of the proportional hazard assumption was addressed by using landmark survival models.
A sensitivity analysis was performed to test the study results in subgroups with classical or paradoxical LFLG‐AS forms. Also, the association between MR severity and the study outcomes was tested in subgroups stratified for MR degree 0/1+, 2+, or >2+.
A competing risk analysis was also conducted to estimate the risk of HF rehospitalization taking into account the competing risk of death.
The association between baseline MR >2+ and the study outcomes was assessed using the Cox proportional hazard regression model and reported as unadjusted and adjusted hazard ratio (HR) with their 95% CI. We used the propensity score weighting technique to account for potential selection bias between groups. The propensity score model was developed using a parsimonious approach and incorporating, beyond age, all the baseline covariates significantly associated with exposure at univariable regression analysis (EuroSCORE II, New York Heart Association class, paradoxical/classical form, baseline LVEF, RV dysfunction, implantation of self‐expandable/balloon‐expandable prosthesis, and predischarge LVEF). After weighting, a standardized mean difference below 0.10, which reflects an optimal balance for all covariates included in the propensity score model, was achieved (Figure S1).
A multivariable stepwise Cox regression model was performed to identify a set of independent predictors for the risk of the primary outcome at 1 year. In order to limit the risk of overfitting, only variables statistically significant (P<0.05) at univariable analysis were entered into the model.
The rate of missing baseline values, if any, is shown in Table S2. Missing data were handled using multiple imputations with the method of chained equations. Twenty imputed data sets were generated and combined using Rubin's rules. 22
For all test, a P value <0.05 was considered statistically significant. Analysis was performed by using SPSS software version 25.0 (SPSS Inc., Chicago, IL) and R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The study included 268 patients (median 81 years, 53% women); of them, 57 (21.3%) patients showed MR >2+ at hospital admission (Table 1). The study selection process is shown in Figure S2.
Table 1.
Baseline Demographic and Clinical Features
| All (N=268) | MR ≤2+ (N=211) | MR >2+ (N=57) | P value | |
|---|---|---|---|---|
| Age, y | 81 (77–85) | 81 (77–85) | 82 (78–87) | 0.192 |
| Sex, female, N (%) | 142 (53.0) | 111 (52.6) | 31 (54.4) | 0.881 |
| BSA, m2 | 1.8±0.2 | 1.8±0.2 | 1.8±0.2 | 0.286 |
| Diabetes, N (%) | 90 (33.6) | 72 (34.1) | 18 (31.6) | 0.755 |
| Hypertension, N (%) | 257 (95.9) | 203 (96.2) | 54 (94.7) | 0.706 |
| Hypercholesterolemia, N (%) | 228 (85.1) | 182 (86.3) | 46 (80.7) | 0.300 |
| Smoking, N (%) | 19 (10.2) | 17 (11.6) | 2 (5) | 0.374 |
| COPD, N (%) | 91 (34.0) | 75 (35.5) | 16 (28.1) | 0.345 |
| eGFR, mL/min | 57 (43–74) | 57 (43–74) | 56 (41–74) | 0.455 |
| CCS, N (%) | 104 (38.8) | 81 (38.4) | 23 (40.4) | 0.878 |
| Prior MI, N (%) | 51 (19.0) | 44 (20.9) | 7 (12.3) | 0.183 |
| Prior PCI, N (%) | 89 (33.2) | 68 (32.2) | 21 (36.8) | 0.529 |
| Prior CABG, N (%) | 42 (15.7) | 35 (16.6) | 7 (12.3) | 0.540 |
| Prior stroke/TIA, N (%) | 18 (6.7) | 16 (7.6) | 2 (3.5) | 0.379 |
| Peripheral arteriopathy, N (%) | 74 (27.6) | 60 (34.1) | 14 (31.1) | 0.860 |
| Atrial fibrillation/flutter, N (%) | 79 (29.5) | 58 (27.5) | 21 (36.8) | 0.191 |
| Pacemaker, N (%) | 36 (13.4) | 29 (13.7) | 7 (12.3) | >0.99 |
| ICD/CRT‐D, N (%) | 20 (7.5) | 15 (7.1) | 5 (8.8) | 0.776 |
| EuroSCORE II, % | 3.3 (2.0–7.3) | 3.0 (1.9–5.7) | 5.1 (3.1–10.7) | <0.001 |
| NYHA | ||||
| NYHA I, N (%) | 1 (0.4) | 1 (0.5) | 0 (0) | 0.001 |
| NYHA II, N (%) | 156 (58.2) | 136 (64.5) | 20 (35.1) | |
| NYHA III, N (%) | 93 (34.7) | 62 (29.4) | 31 (54.4) | |
| NYHA IV, N (%) | 18 (6.7) | 12 (5.7) | 6 (10.5) | |
| Baseline echocardiography | ||||
| LVEF, % | 50 (38–55) | 50 (40–55) | 40 (33–55) | 0.006 |
| Average AVG, mm Hg | 32 (26–35) | 32 (26–36) | 31 (25–35) | 0.319 |
| Aortic valve area, cm2 | 0.7±0.1 | 0.7±0.1 | 0.7±0.2 | 0.386 |
| Aortic valve area index, cm2/m2 | 0.4±0.1 | 0.4±0.1 | 0.4±0.1 | 0.122 |
| Paradoxical LFLG, N (%) | 155 (57.8) | 130 (61.6) | 25 (43.9) | 0.023 |
| Classical LFLG, N (%) | 113 (42.2) | 81 (38.4) | 32 (56.1) | 0.023 |
| Moderate‐to‐severe AR, N (%) | 22 (8.2) | 18 (8.5) | 4 (7) | >0.99 |
| BAV, N (%) | 6 (2.2) | 5 (2.4) | 1 (1.8) | 1.000 |
| MR 0–1+, N (%) | 123 (45.9) | 123 (58.3) | … | … |
| MR 2+, N (%) | 88 (32.8) | 88 (41.7) | … | … |
| MR 3+, N (%) | 50 (18.7) | … | 50 (87.7) | … |
| MR 4+, N (%) | 7 (2.6) | … | 7 (12.3) | … |
| sPAP, mm Hg | 45 (35–50) | 45 (35–50) | 45 (37–56) | 0.197 |
| Right ventricular dysfunction, N (%) | 50 (22.4) | 31 (17.9) | 19 (38) | 0.004 |
| Procedural features | ||||
| Transfemoral approach, N (%) | 253 (94.4) | 201 (95.3) | 52 (91.2) | 0.325 |
| Pacing, N (%) | 246 (91.8) | 196 (92.9) | 50 (87.7) | 0.273 |
| Predilatation, N (%) | 135 (50.4) | 112 (53.1) | 23 (40.4) | 0.101 |
| Balloon‐expandable prosthesis, N (%) | 37 (13.8) | 22 (10.4) | 15 (26.3) | 0.004 |
| Self‐expandable prosthesis, N (%) | 231 (86.2) | 189 (89.6) | 42 (73.7) | 0.004 |
| Postdilatation, N (%) | 86 (32.1) | 74 (35.2) | 12 (21.1) | 0.054 |
| Contrast dose, mL | 80 (70–100) | 80 (70–100) | 90 (80–100) | 0.566 |
| Prosthesis size, mm | ||||
| 23 | 22 (8.2) | 14 (6.6) | 8 (14) | 0.093 |
| 25 | 28 (10.4) | 22 (10.4) | 6 (10.5) | |
| 26 | 53 (19.8) | 39 (18.5) | 14 (24.6) | |
| 27 | 49 (18.3) | 41 (19.4) | 8 (14.0) | |
| 29 | 102 (38.1) | 86 (40.8) | 16 (28.2) | |
| 31 | 1 (0.4) | 0 | 1 (1.8) | |
| 34 | 13 (4.9) | 9 (4.3) | 4 (7.0) | |
| Predischarge echocardiography | ||||
| LVEF, % | 51 (40–55) | 55 (40–55) | 49 (35–55) | 0.032 |
| Average AVG, mm Hg | 6 (4–8) | 6 (4–8) | 6 (4–8) | 0.749 |
| Residual moderate‐to‐severe AR, N (%) | 1 (0.4) | 0 | 1 (1.8) | 0.216 |
| sPAP, mm Hg | 40 (35–50) | 40 (35–47) | 45 (35–55) | 0.175 |
Continuous normally distributed variables are expressed as mean±SD. Categorical variables are expressed as N (%). Continuous non‐normally distributed variables are expressed as median (interquartile range). AR indicates aortic regurgitation; AVG, aortic valve gradient; BAV, bicuspid aortic valve; BSA, body surface area; CABG, coronary artery bypass graft surgery; CCS, chronic coronary syndrome; COPD, chronic obstructive pulmonary disease; CRT‐D, cardiac resynchronization therapy‐defibrillator; eGFR, estimated glomerular filtration rate; EuroSCORE II, European System for Cardiac Operative Risk Evaluation; ICD, Implantable cardioverter‐defibrillator; LFLG, left ventricular ejection fraction; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MR, mitral regurgitation; NYHA, New York Heart Association functional class; PCI, percutaneous coronary intervention; sPAP, systolic pulmonary artery pressure; and TIA, transient ischemic attack.
Patients with MR >2+ showed a significantly higher New York Heart Association class at admission (P=0.001) and a higher surgical risk profile according to European System for Cardiac Operative Risk Evaluation II (P<0.001) compared with those with MR ≤2+. Patients with MR >2+ also showed lower LVEF at baseline (P=0.006) and higher prevalence of classical LFLG‐AS form (P=0.023). The proportion of RV dysfunction was also higher among patients with MR >2+ compared with those with MR ≤2+ (P=0.004).
The list of medications at baseline and before discharge is summarized in Tables S3 and S4. Medications at admission did not significantly differ between groups. Before discharge, the reported prescription of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers was more frequent in patients with MR >2+ compared with those with MR ≤2+.
The rate of adverse events was substantially low during the hospitalization and did not differ between groups (Table 2). Death was reported in 2 (0.7%) patients, vascular complications in 8 (3%), and permanent pacemaker implantation in 23 (8.5%).
Table 2.
In‐Hospital Adverse Events
| All (N=268) | MR ≤2+ (N=211) | MR >2+ (N=57) | P value | |
|---|---|---|---|---|
| All‐cause mortality, N (%) | 2 (0.7) | 1 (0.5) | 1 (1.7) | 0.381 |
| Myocardial infarction, N (%) | 3 (1.1) | 3 (1.4) | 0 | >0.99 |
| Stroke, N (%) | 4 (1.4) | 3 (1.4) | 1 (1.7) | >0.99 |
| Bleeding and transfusion, N (%) | ||||
| Type 1 | 2 (0.7) | 2 (0.9) | 0 | >0.99 |
| Type 2 | 1 (0.4) | 0 | 1 (1.8) | 0.148 |
| Type 3–4 | 0 | 0 | 0 | |
| Vascular complication, N (%) | ||||
| Minor | 6 (2.2) | 5 (2.4) | 1 (1.8) | >0.99 |
| Major | 2 (0.7) | 1 (0.5) | 1 (1.8) | 0.279 |
| Annulus rupture/aortic perforation, N (%) | 1 (0.4) | 1 (0.5) | 0 | >0.99 |
| Valve migration/embolization, N (%) | 3 (1.1) | 3 (1.4) | 0 | >0.99 |
| Cardiogenic shock, N (%) | 2 (0.7) | 2 (0.9) | 0 | >0.99 |
| Conversion to open surgery, N (%) | 2 (0.7) | 2 (0.9) | 0 | >0.99 |
| Ventricular arrhythmia, N (%) | 4 (1.4) | 3 (1.4) | 1 (1.8) | >0.99 |
| Permanent pacemaker implantation, N (%) | 23 (8.5) | 18 (8.5) | 5 (8.7) | |
| Procedural success, N (%) | 264 (98.5) | 208 (98.6) | 56 (98.2) | >0.99 |
MR indicates mitral regurgitation.
The rate of adverse events at 1 year according to baseline MR severity is summarized in Table S5. Overall, the primary outcome was reported in 55 (20.5%) patients; all‐cause death was reported in 29 (10.8%) patients, and HF hospitalization in 34 (12.7%). At 1 year, patients with MR >2+ showed a significantly higher percentage of the primary outcome (P<0.001) and of all‐cause mortality (P<0.001), which was related to cardiovascular cause in the majority of cases, and of HF hospitalization (P<0.001).
The survival free from the primary outcome was significantly lower in patients with MR >2+ compared with those with MR ≤2+ (log‐rank <0.001; Figure 1). Survival free from all‐cause death and HF hospitalization was also significantly lower in patients with MR >2+ (log‐rank <0.001). This result was consistent among the prespecified subgroups with classical and paradoxical LFLG‐AS forms (Figure S3) and in the analysis stratified for MR degree 0/1+, 2+, or >2+ (Figure S4).
Figure 1. Survival free from the primary outcome (A), all‐cause death (B), and HF hospitalization (C) in patients with MR ≤2+ vs those with MR >2+.
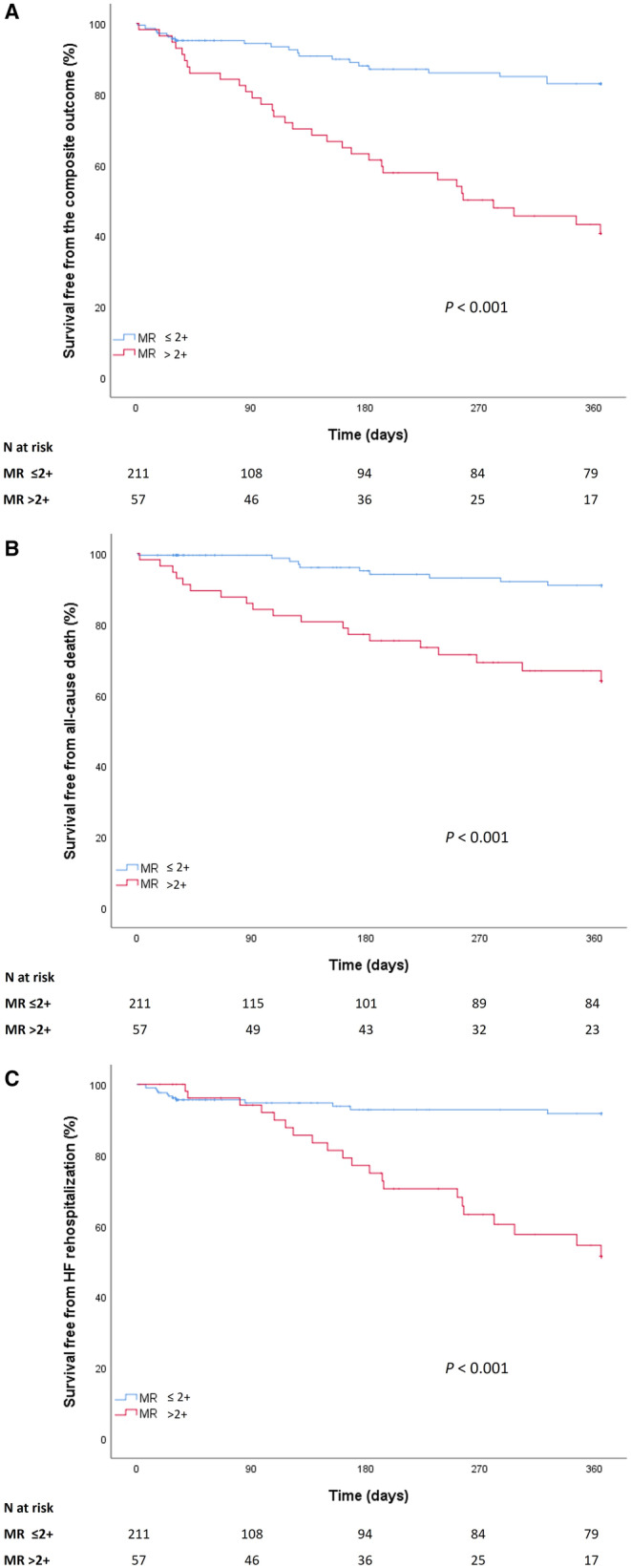
HF indicates heart failure; and MR, mitral regurgitation.
The violation of the proportional hazard assumption for HF hospitalization was addressed using a landmark survival analysis with time splitting at 3 months, when the curves intersect. The survival free from HF hospitalization was comparable between groups up to 3 months (log‐rank=0.937), but lower in patients with MR >2+ compared with those with MR ≤2+ from 3 to 12 months (log‐rank <0.001; Figure S5).
The competing risk analysis confirmed a significantly lower survival free from HF rehospitalization in patients with MR >2+ compared with those with MR ≤2+ (log‐rank <0.001) with death being considered as a competing risk event (Figure S6).
The association between MR >2+ and the primary outcome was confirmed by the unadjusted and propensity score weighting adjusted Cox regression analysis (Table 3). Of note, the higher mortality risk observed in patients with MR >2+ was largely related to cardiac cause.
Table 3.
Unadjusted and Adjusted Cox Regression Analysis for the Association Between Baseline MR >2+ and the Study Outcomes
| Unadjusted | |||
|---|---|---|---|
| HR | 95% CI | P value | |
| Primary outcome | 4.15 | 2.42–7.12 | <0.001 |
| All‐cause death | 5.13 | 2.38–11.06 | <0.001 |
| HF rehospitalization | 4.93 | 2.46–9.88 | <0.001 |
| Cardiac death | 9.09 | 3.32–24.89 | <0.001 |
| Noncardiac death | 1.43 | 0.34–5.98 | 0.624 |
| Adjusted* | |||
|---|---|---|---|
| aHR | 95% CI | P value | |
| Primary outcome | 3.29 | 1.86–5.83 | <0.001 |
| All‐cause death | 4.68 | 2.02–10.85 | <0.001 |
| HF rehospitalization | 3.99 | 1.93–8.23 | <0.001 |
| Cardiac death | 10.45 | 3.64–29.96 | <0.001 |
| Noncardiac death | 0.78 | 0.17–3.45 | 0.738 |
aHR indicates adjusted hazard ratio; HF, heart failure; and HR, hazard ratio.
List of variables entered in the propensity score weighting model: age, EuroSCORE II, NYHA class, paradoxical/classical form, baseline LVEF, RV dysfunction, implantation of self‐expandable/balloon‐expandable prosthesis, and predischarge LVEF.
All variables statistically significant at univariable Cox regression analysis for the primary outcome (hypertension, European System for Cardiac Operative Risk Evaluation II, New York Heart Association class, MR >2+, RV dysfunction, balloon‐expandable/self‐expandable prosthesis, and LVEF predischarge) were entered into the multivariable model. Poorer New York Heart Association class (hazard ratio [HR], 1.85 [95% CI, 1.18–1.91]), MR >2+ (HR, 3.02 [95% CI, 1.65–5.56]), and RV dysfunction (HR, 2.18 [95% CI, 1.21–3.94]) were the only independent predictors of the primary outcome (Table 4).
Table 4.
Predictors of the Composite Outcome
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age, y | 1.02 | 0.97–1.07 | 0.559 | |||
| Sex, female, N (%) | 1.10 | 0.65–1.88 | 0.718 | |||
| BSA, m2 | 0.83 | 0.17–3.94 | 0.813 | |||
| Diabetes, N (%) | 1.46 | 0.85–2.51 | 0.169 | |||
| Hypertension, N (%) | 0.34 | 0.14–0.85 | 0.022* | … | ||
| Hypercholesterolemia, N (%) | 0.56 | 0.30–1.05 | 0.069 | |||
| Smoking, N (%) | 2.02 | 0.94–4.34 | 0.073 | |||
| COPD, N (%) | 0.85 | 0.49–1.50 | 0.584 | |||
| eGFR, mL/min | 0.99 | 0.97–1.00 | 0.063 | |||
| CCS, N (%) | 1.21 | 0.71–2.08 | 0.483 | |||
| Prior MI, N (%) | 1.00 | 0.50–1.99 | 0.999 | |||
| Prior PCI, N (%) | 1.45 | 0.85–2.49 | 0.175 | |||
| Prior CABG, N (%) | 1.47 | 0.76–2.85 | 0.253 | |||
| Prior stroke/TIA, N (%) | 1.08 | 0.39–2.99 | 0.881 | |||
| Peripheral arteriopathy, N (%) | 0.32 | 0.74–2.35 | 0.346 | |||
| Atrial fibrillation/flutter, N (%) | 1.36 | 0.79–2.33 | 0.274 | |||
| Pacemaker, N (%) | 0.95 | 0.41–2.22 | 0.907 | |||
| ICD/CRT‐D, N (%) | 1.45 | 0.58–3.64 | 0.432 | |||
| EuroSCORE II, % | 1.03 | 1.01–1.05 | 0.015* | … | ||
| NYHA class | 2.33 | 1.57–3.48 | <0.001* | 1.85 | 1.18–2.91 | 0.007* |
| Baseline echocardiography | ||||||
| LVEF, % | 0.98 | 0.96–1.00 | 0.110 | |||
| Average AVG, mm Hg | 0.98 | 0.94–1.03 | 0.438 | |||
| Aortic valve area, cm2 | 0.58 | 0.09–3.72 | 0.568 | |||
| Aortic valve area index, cm2/m2 | 0.50 | 0.02–11.64 | 0.665 | |||
| Paradoxical LFLG, N (%) | 0.66 | 0.39–1.13 | 0.128 | |||
| Classical LFLG, N (%) | 1.51 | 0.89–2.57 | 0.128 | |||
| Moderate‐to‐severe AR, N (%) | 1.68 | 0.75–3.76 | 0.212 | |||
| MR >2+ | 4.15 | 2.42–7.12 | <0.001* | 3.02 | 1.65–5.56 | <0.001* |
| BAV, N (%) | 2.00 | 0.49–8.23 | 0.335 | |||
| sPAP, mm Hg | 1.00 | 0.98–1.03 | 0.917 | |||
| Right ventricular dysfunction, N (%) | 2.60 | 1.48–4.58 | 0.001* | 2.18 | 1.21–3.94 | 0.010* |
| Procedural features | ||||||
| Transfemoral approach, N (%) | 0.32 | 1.13–0.74 | 0.008 | |||
| Pacing, N (%) | 0.61 | 0.28–1.34 | 0.218 | |||
| Predilatation, N (%) | 0.61 | 0.36–1.04 | 0.071 | |||
| Balloon‐expandable prosthesis, N (%) | 2.22 | 1.23–4.03 | 0.008* | … | ||
| Self‐expandable prosthesis, N (%) | 0.45 | 0.25–0.82 | 0.008* | … | ||
| Prosthesis size <23 mm, N (%) | 1.54 | 0.73–3.26 | 0.261 | |||
| Postdilatation, N (%) | 0.88 | 0.49–1.55 | 0.650 | |||
| Predischarge echocardiography | ||||||
| LVEF, % | 0.98 | 0.95–0.99 | 0.041* | … | ||
| Average AVG, mm Hg | 0.98 | 0.90–1.07 | 0.693 | |||
| sPAP, mm Hg | 1.02 | 0.99–1.04 | 0.187 | |||
AVG indicates aortic valve gradient; AR, aortic regurgitation; BAV, bicuspid aortic valve; BSA, body surface area; CABG, coronary artery bypass graft surgery; CCS, chronic coronary syndrome; COPD, chronic obstructive pulmonary disease; CRT‐D, cardiac resynchronization therapy‐defibrillator; eGFR, estimated glomerular filtration rate; EuroSCORE, European System for Cardiac Operative Risk Evaluation; HR, hazard ratio; ICD, implantable cardioverter‐defibrillator; LFLG, low flow, low gradient; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MR, mitral regurgitation; NYHA, New York Heart Association functional class; PCI, percutaneous coronary intervention; sPAP, systolic pulmonary artery pressure; and TIA, transient ischemic attack.
P <0.05 was considered statistically significant.
At discharge, of the 56 patients with baseline MR >2+ (1 patient died during the TAVR procedure), a significant MR improvement was reported in 24 (44%) patients (Table S6). MR improvement was reported in patients with both classical (14, 58.3%) and paradoxical (10, 41.7%) LFLG‐AS forms.
The absence of MR improvement after TAVR was associated with a higher percentage of the primary outcome (P=0.030), all‐cause death (P=0.044), and HF hospitalization (P=0.030) at 1 year (Table S7). The 1‐year survival free from the primary outcome was significantly higher in patients with MR improvement than in those without (log‐rank=0.009), and both groups had a significantly lower survival free from the primary outcome when compared with patients with baseline MR <2+ (Figure 2). One‐year survival free from the all‐cause death (log‐rank=0.036) and HF hospitalization (log‐rank=0.005) was consistently higher in patients with MR improvement than in those without (Figure 2).
Figure 2. Survival free from the primary outcome (A), all‐cause death (B), and HF hospitalization (C) in patients with MR ≤2+ and in patients with MR >2+ with or without significant improvement after TAVR.
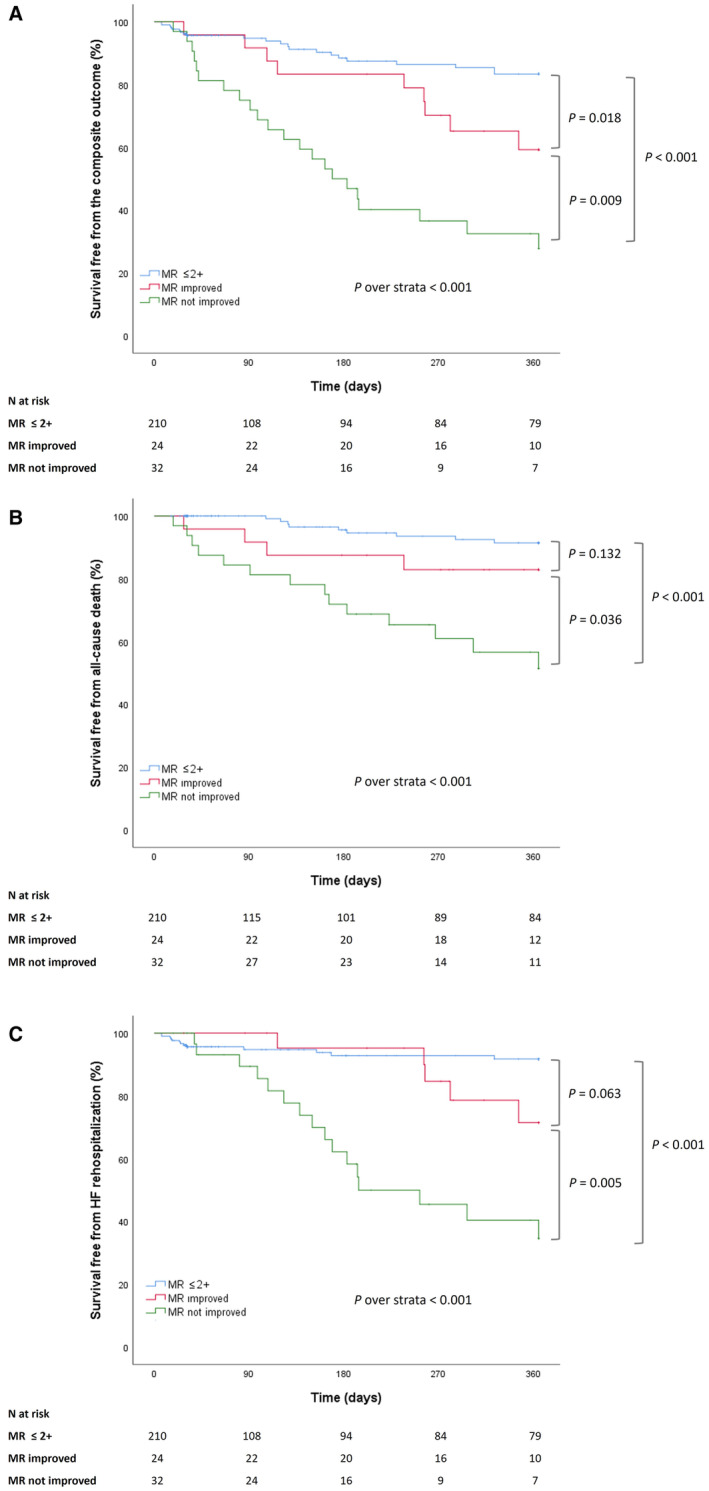
HF indicates heart failure; MR, mitral regurgitation; and TAVR, transcatheter aortic valve replacement.
DISCUSSION
The main findings of this real‐world multicenter study on LFLG‐AS undergoing TAVR can be summarized as follows: (1) moderately severe to severe MR was reported in ≈1 out of 5 patients; (2) MR severity at admission was independently associated with a higher risk of mortality and HF hospitalization at 1 year; and (3) at discharge, a significant MR improvement was reported in 44% of patients with baseline MR >2+; in these patients there was a significant outcome benefit at 1 year compared with those with persistently severe MR.
In this TAVR population, MR >2+ was reported in ≈20% of cases. This prevalence was higher than previously reported in patients with high‐gradient severe AS undergoing TAVR, 14 , 23 but consistent with previous observational studies on LFLG‐AS. 15 , 16
Although the prevalence of severe MR is substantially high in this particular TAVR population, there are few studies evaluating its prognostic impact on long‐term clinical outcome. In our study, coexistent MR >2+ before TAVR identified patients with LFLG‐AS with a significantly higher risk of adverse events at 1 year.
A previous single‐center study by O'Sullivan and colleagues investigated the prognostic role of MR severity in 113 patients with classical LFLG‐AS undergoing TAVR with older‐generation devices implanted from 2007 to 2012; consistently with our study, the authors reported a significantly higher 1‐year mortality in patients with moderate or severe MR compared with those with mild MR. 15
More recently, a substudy from the TOPAS‐TAVI multicenter registry did not report an association between baseline moderate or severe MR and poorer outcomes in patients with classical LFLG‐AS undergoing TAVR. 16 This discrepancy with our results could be partially explained using a 3‐class MR grading (mild, moderate, and severe) in the TOPAS‐TAVI (True or Pseudo‐Severe Aortic Stenosis‐Transcatheter Aortic Valve Implantation) registry, and by the comparison of patients with mild versus those with moderate/severe MR. Conversely, our registry adopted a 4‐class MR classification, which may provide more granularity in the grading of the severity of MR. 19 We evaluated the prognostic role of moderately severe to severe (3+ and 4+, respectively) MR, considering these patients as the most exposed to the hemodynamic consequences of MR, 14 versus the presence of mild or moderate MR (1+ and 2+, respectively). Our results also emphasize the importance of MR severity assessment regardless of baseline LVEF, since the primary results were consistent in both classical and paradoxical LFLG‐AS subgroups.
The reduction of MR severity after TAVR may be related to multiple adaptative mechanisms following the prosthetic valve implantation, including the reduction of mitral leaflet tethering and the decrease in the global left ventricular hemodynamic afterload. 24 In the present study, the improvement of MR was reported in 44% of patients with LFLG‐AS with baseline MR >2+ after TAVR. This result was consistent with previous studies, which reported MR improvement in 50% to 60% of patients with AS after TAVR. 12 , 25 Although related to mechanisms potentially different, the improvement of MR after TAVR was reported both in patients with classical and paradoxical LFLG‐AS forms. In patients with classical LFLG‐AS, it is mainly explained by a reduction in the mitral leaflet tethering area. 24 Conversely, the improvement of MR in patients with paradoxical form is predominantly related to a reduction of hemodynamic afterload. This positive global left ventricular hemodynamic improvement could be associated with a reduction of left ventricular diastolic dysfunction, which could be another possible reason for the improvement of MR. 24
The potential outcome benefit of MR improvement after TAVR, albeit theoretically plausible, has long been debated. The first evidence of a survival benefit comes from studies on TAVR in patients with high gradient severe AS, which demonstrated a better long‐term survival in patients with postprocedural MR improvement compared with those with persistently moderate‐to‐severe MR. 26 , 27
Furthermore, in a substudy from the PARTNER (Placement of Aortic Transcatheter Valve) trial, the persistence of low stroke volume with residual moderate‐to‐severe MR after TAVR at discharge was associated with poorer 1‐year outcome compared with those with mild MR. 28
Several unfavorable structural and hemodynamic consequences of persistent moderate‐to‐severe MR may attenuate the intended benefit of TAVR, particularly in patients with LFLG‐AS. Ben‐Assa and colleagues reported that the persistence of moderate or severe MR after TAVR resulted in a larger ventricular diameter, left atrial volume, and worse RV function and hemodynamic status up to 6 months. 29 More recently, in the LFLG‐AS setting, those with persistent moderate‐to‐severe MR experienced a lower improvement in terms of LVEF and left ventricular diameters compared with patients with MR improvement after TAVR. 16 These structural and hemodynamic changes could explain the higher mortality of patients with persistent moderately severe or severe MR after TAVR and, along with the persistently high pulmonary pressure, the higher rate of hospitalizations for worsening HF.
Our study suggests that, in patients with LFLG‐AS undergoing TAVR, the assessment of concomitant MR is of utmost importance not only before the procedure but also after TAVR to identify patients with improvement versus those with persistence of severe MR. Patients with persistent moderately severe or severe MR should be carefully monitored after discharge and should receive guideline‐directed medical therapy for HF, including transcatheter mitral valve replacement, when judged appropriate by the local Heart Team. 13 , 30 However, further studies are warranted to establish the best clinical and therapeutic management of this LFLG‐AS population at persistently high risk despite TAVR treatment.
Study Limitations
Our results should be interpreted considering some limitations. The first limitation is the relatively small sample size of the study population. However, this study focuses on a very particular AS population, which represents only a minority of patients undergoing TAVR, and the population size is consistent with previous multicenter registry‐based studies. 8 Also, we did not perform a formal calculation of the sample size, which may be underpowered for the analysis of MR improvement after TAVR as well as for the assessment of in‐hospital outcomes.
The echocardiographic data were prospectively collected on site at the participating centers, and no centralized analysis was performed in a core echocardiographic laboratory.
Another limitation of our registry concerns the absence of information on the cause of MR; however, the presence or the persistence of significant MR in patients with AS undergoing TAVR has been demonstrated to be independent from the MR cause, and was not associated with patient outcome. 12 , 16
Although this real‐world registry does not adopt a blinding strategy during follow‐up, in the present study we focused on the hard clinical endpoint (death and HF rehospitalization), which is poorly susceptible to subjectivity or bias.
The preferential use of self‐expandable prosthesis in this population might limit the generalizability of the results to patients with LFLG‐AS undergoing TAVR with balloon‐expandable prosthesis.
Eventually, information on echocardiographic changes during follow‐up was not systematically collected in our registry. However, this study was not conceived with evaluating echocardiographic changes after TAVR, but rather to investigate whether baseline MR severity, and MR improvement after TAVR (assessed before discharge), were associated with the risk of adverse events up to 1 year.
CONCLUSIONS
This study showed that the presence of moderately severe or severe MR in patients with LFLG‐AS undergoing TAVR portends a worse clinical outcome up to 1 year. TAVR may improve MR severity in nearly half of the patients, resulting in a potential outcome benefit after discharge.
Our study suggests that both the presence and the persistence of moderately severe or severe MR are of utmost importance for patient prognostic stratification, and should be carefully and systematically assessed in the very high‐risk clinical scenario of LFLG‐AS undergoing TAVR.
Sources of Funding
None.
Disclosures
Giuseppe Biondi‐Zoccai has consulted for Amarin, Balmed, Cardionovum, Crannmedical, Endocore Lab, Eukon, Innovheart, Guidotti, Meditrial, Microport, Opsens Medical, Replycare, Teleflex, and Terumo. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S7
Figures S1–S6
This manuscript was sent to Amgad Mentias, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.029553
For Sources of Funding and Disclosures, see page 12.
See Editorial by Reed and Kapadia.
References
- 1. Iung B, Delgado V, Rosenhek R, Price S, Prendergast B, Wendler O, De Bonis M, Tribouilloy C, Evangelista A, Bogachev‐Prokophiev A, et al. Contemporary presentation and management of valvular heart disease: the EURObservational Research Programme Valvular Heart Disease II Survey. Circulation. 2019;140:1156–1169. doi: 10.1161/circulationaha.119.041080 [DOI] [PubMed] [Google Scholar]
- 2. Owens DS, Bartz TM, Buzkova P, Massera D, Biggs ML, Carlson SD, Psaty BM, Sotoodehnia N, Gottdiener JS, Kizer JR. Cumulative burden of clinically significant aortic stenosis in community‐dwelling older adults. Heart. 2021;107:1493–1502. doi: 10.1136/heartjnl-2021-319025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923 [DOI] [PubMed] [Google Scholar]
- 4. Clavel MA, Magne J, Pibarot P. Low‐gradient aortic stenosis. Eur Heart J. 2016;37:2645–2657. doi: 10.1093/eurheartj/ehw096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pibarot P, Dumesnil JG. Low‐flow, low‐gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1845–1853. doi: 10.1016/j.jacc.2012.06.051 [DOI] [PubMed] [Google Scholar]
- 6. Clavel MA, Berthelot‐Richer M, Le Ven F, Capoulade R, Dahou A, Dumesnil JG, Mathieu P, Pibarot P. Impact of classic and paradoxical low flow on survival after aortic valve replacement for severe aortic stenosis. J Am Coll Cardiol. 2015;65:645–653. doi: 10.1016/j.jacc.2014.11.047 [DOI] [PubMed] [Google Scholar]
- 7. Lauten A, Figulla HR, Möllmann H, Holzhey D, Kötting J, Beckmann A, Veit C, Cremer J, Kuck KH, Lange R, et al. TAVI for low‐flow, low‐gradient severe aortic stenosis with preserved or reduced ejection fraction: a subgroup analysis from the German Aortic Valve Registry (GARY). EuroIntervention. 2014;10:850–859. doi: 10.4244/eijv10i7a145 [DOI] [PubMed] [Google Scholar]
- 8. Ribeiro HB, Lerakis S, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, Windecker S, Enriquez‐Sarano M, Cheema AN, Nombela‐Franco L, et al. Transcatheter aortic valve replacement in patients with low‐flow, low‐gradient aortic stenosis: the TOPAS‐TAVI registry. J Am Coll Cardiol. 2018;71:1297–1308. doi: 10.1016/j.jacc.2018.01.054 [DOI] [PubMed] [Google Scholar]
- 9. Steffen J, Reißig N, Andreae D, Beckmann M, Haum M, Fischer J, Theiss H, Braun D, Orban M, Rizas K, et al. TAVI in patients with low‐flow low‐gradient aortic stenosis‐short‐term and long‐term outcomes. Clin Res Cardiol. 2022;111:1325–1335. doi: 10.1007/s00392-022-02011-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ludwig S, Goßling A, Waldschmidt L, Linder M, Bhadra OD, Voigtländer L, Schäfer A, Deuschl F, Schirmer J, Reichenspurner H, et al. TAVR for low‐flow, low‐gradient aortic stenosis: prognostic impact of aortic valve calcification. Am Heart J. 2020;225:138–148. doi: 10.1016/j.ahj.2020.03.013 [DOI] [PubMed] [Google Scholar]
- 11. Puri R, Iung B, Cohen DJ, Rodés‐Cabau J. TAVI or No TAVI: identifying patients unlikely to benefit from transcatheter aortic valve implantation. Eur Heart J. 2016;37(28):2217–2225. doi: 10.1093/eurheartj/ehv756 [DOI] [PubMed] [Google Scholar]
- 12. Chakravarty T, Van Belle E, Jilaihawi H, Noheria A, Testa L, Bedogni F, Rück A, Barbanti M, Toggweiler S, Thomas M, et al. Meta‐analysis of the impact of mitral regurgitation on outcomes after transcatheter aortic valve implantation. Am J Cardiol. 2015;115:942–949. doi: 10.1016/j.amjcard.2015.01.022 [DOI] [PubMed] [Google Scholar]
- 13. Witberg G, Codner P, Landes U, Schwartzenberg S, Barbanti M, Valvo R, De Backer O, Ooms JF, Islas F, Marroquin L, et al. Effect of transcatheter aortic valve replacement on concomitant mitral regurgitation and its impact on mortality. JACC Cardiovasc Interv. 2021;14:1181–1192. doi: 10.1016/j.jcin.2021.02.030 [DOI] [PubMed] [Google Scholar]
- 14. Mauri V, Körber MI, Kuhn E, Schmidt T, Frerker C, Wahlers T, Rudolph TK, Baldus S, Adam M, Ten Freyhaus H. Prognosis of persistent mitral regurgitation in patients undergoing transcatheter aortic valve replacement. Clin Res Cardiol. 2020;109:1261–1270. doi: 10.1007/s00392-020-01618-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Sullivan CJ, Stortecky S, Bütikofer A, Heg D, Zanchin T, Huber C, Pilgrim T, Praz F, Buellesfeld L, Khattab AA, et al. Impact of mitral regurgitation on clinical outcomes of patients with low‐ejection fraction, low‐gradient severe aortic stenosis undergoing transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2015;8:e001895. doi: 10.1161/circinterventions.114.001895 [DOI] [PubMed] [Google Scholar]
- 16. Freitas‐Ferraz AB, Lerakis S, Barbosa Ribeiro H, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, Windecker S, Enriquez‐Sarano M, Cheema AN, et al. Mitral regurgitation in low‐flow, low‐gradient aortic stenosis patients undergoing TAVR: insights from the TOPAS‐TAVI registry. JACC Cardiovasc Interv. 2020;13:567–579. doi: 10.1016/j.jcin.2019.11.042 [DOI] [PubMed] [Google Scholar]
- 17. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 18. Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, Lancellotti P, LeFevre M, Miller F Jr, Otto CM. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–392. doi: 10.1016/j.echo.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 19. Lancellotti P, Pibarot P, Chambers J, La Canna G, Pepi M, Dulgheru R, Dweck M, Delgado V, Garbi M, Vannan MA, et al. Multi‐modality imaging assessment of native valvular regurgitation: an EACVI and ESC council of valvular heart disease position paper. Eur Heart J Cardiovasc Imaging. 2022;23:e171–e232. doi: 10.1093/ehjci/jeab253 [DOI] [PubMed] [Google Scholar]
- 20. Morello A, Corcione N, Ferraro P, Cimmino M, Pepe M, Cassese M, Frati G, Biondi‐Zoccai G, Giordano A. The best way to transcatheter aortic valve implantation: from standard to new approaches. Int J Cardiol. 2021;322:86–94. doi: 10.1016/j.ijcard.2020.08.036 [DOI] [PubMed] [Google Scholar]
- 21. Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, Bax JJ, Leipsic JA, Blanke P, Blackstone EH, et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. 2021;42:1825–1857. doi: 10.1093/eurheartj/ehaa799 [DOI] [PubMed] [Google Scholar]
- 22. Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nombela‐Franco L, Ribeiro HB, Urena M, Allende R, Amat‐Santos I, DeLarochellière R, Dumont E, Doyle D, DeLarochellière H, Laflamme J, et al. Significant mitral regurgitation left untreated at the time of aortic valve replacement: a comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J Am Coll Cardiol. 2014;63:2643–2658. doi: 10.1016/j.jacc.2014.02.573 [DOI] [PubMed] [Google Scholar]
- 24. Shibayama K, Harada K, Berdejo J, Mihara H, Tanaka J, Gurudevan SV, Siegel R, Jilaihawi H, Makkar RR, Shiota T. Effect of transcatheter aortic valve replacement on the mitral valve apparatus and mitral regurgitation: real‐time three‐dimensional transesophageal echocardiography study. Circ Cardiovasc Imaging. 2014;7:344–351. doi: 10.1161/circimaging.113.000942Nombela-Franco [DOI] [PubMed] [Google Scholar]
- 25. Nombela‐Franco L, Eltchaninoff H, Zahn R, Testa L, Leon MB, Trillo‐Nouche R, D'Onofrio A, Smith CR, Webb J, Bleiziffer S, et al. Clinical impact and evolution of mitral regurgitation following transcatheter aortic valve replacement: a meta‐analysis. Heart. 2015;101:1395–1405. doi: 10.1136/heartjnl-2014-307120 [DOI] [PubMed] [Google Scholar]
- 26. Mavromatis K, Thourani VH, Stebbins A, Vemulapalli S, Devireddy C, Guyton RA, Matsouaka R, Ghasemzadeh N, Block PC, Leshnower BG, et al. Transcatheter aortic valve replacement in patients with aortic stenosis and mitral regurgitation. Ann Thorac Surg. 2017;104:1977–1985. doi: 10.1016/j.athoracsur.2017.05.065 [DOI] [PubMed] [Google Scholar]
- 27. Feldt K, De Palma R, Bjursten H, Petursson P, Nielsen NE, Kellerth T, Jönsson A, Nilsson J, Rück A, Settergren M. Change in mitral regurgitation severity impacts survival after transcatheter aortic valve replacement. Int J Cardiol. 2019;294:32–36. doi: 10.1016/j.ijcard.2019.07.075 [DOI] [PubMed] [Google Scholar]
- 28. Anjan VY, Herrmann HC, Pibarot P, Stewart WJ, Kapadia S, Tuzcu EM, Babaliaros V, Thourani VH, Szeto WY, Bavaria JE, et al. Evaluation of flow after transcatheter aortic valve replacement in patients with low‐flow aortic stenosis: a secondary analysis of the PARTNER randomized clinical trial. JAMA Cardiol. 2016;1:584–592. doi: 10.1001/jamacardio.2016.0759 [DOI] [PubMed] [Google Scholar]
- 29. Ben‐Assa E, Biner S, Banai S, Arbel Y, Laufer‐Perl M, Kramarz J, Elmariah S, Inglessis I, Keren G, Finkelstein A, et al. Clinical impact of post procedural mitral regurgitation after transcatheter aortic valve replacement. Int J Cardiol. 2020;299:215–221. doi: 10.1016/j.ijcard.2019.07.092 [DOI] [PubMed] [Google Scholar]
- 30. Corcione N, Ferraro P, Finizio F, Giordano A. Transcatheter management of combined mitral and aortic disease: dynamic duo or double trouble? Minerva Cardiol Angiol. 2021;69:117–121. doi: 10.23736/s2724-5683.20.05193-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7
Figures S1–S6


