Principal to optimizing outcomes after transcatheter aortic valve replacement (TAVR) is understanding each patient's individualized procedural risks and taking steps to maximize their likelihood of a good long‐term result in the years after treatment. This includes appropriate patient selection, proper valve choice, implantation strategies to minimize pacemaker dependency and maximize the success of future valve‐in‐valve procedures if needed, and often how to co‐manage significant concomitant valve disease. Patients with low‐flow, low‐gradient (LFLG) severe aortic valve stenosis (AS) represent a particularly high‐risk patient group, because they either have significant left ventricular (LV) systolic or diastolic dysfunction that impairs stroke volume, as well as a fixed outflow obstruction from AS, all of which combine to negatively affect prognosis. Mitral regurgitation (MR) is especially prevalent in LFLG severe AS; it is seen in upwards of one‐third of patients 1 , 2 , 3 and portends worse outcomes, 2 yet data to guide the management of patients with LFLG severe AS and at least moderate MR are scarce. A heart team approach is particularly important in these patients, as management strategies can be complex, ranging from surgical AVR+surgical mitral valve repair or mitral valve replacement in appropriate surgical candidates, TAVR with plans for transcatheter edge‐to‐edge mitral valve repair (TEER) or transcatheter mitral valve replacement staged at a later date, or medical management of MR in patients not TEER or transcatheter mitral valve replacement candidates or whose MR improves after TAVR (Figure 1). Key to deciding which approach is best involves understanding the mechanism of MR and estimating whether the MR might improve after TAVR. While studies suggest that MR may improve to some degree in about half of patients after TAVR, 4 , 5 there are limited data on incidence of MR improvement post‐TAVR in patients specifically with LFLG AS, and even less on outcomes in patients who have an improvement in MR or persistent MR after TAVR. This information would be especially important to guide decision making of whether to pursue catheter‐based MV interventions after TAVR, as residual MR may be an attractive target for TEER or transcatheter mitral valve replacement in this patient population.
Figure 1. Strategy for management of concomitant severe AS and MR.
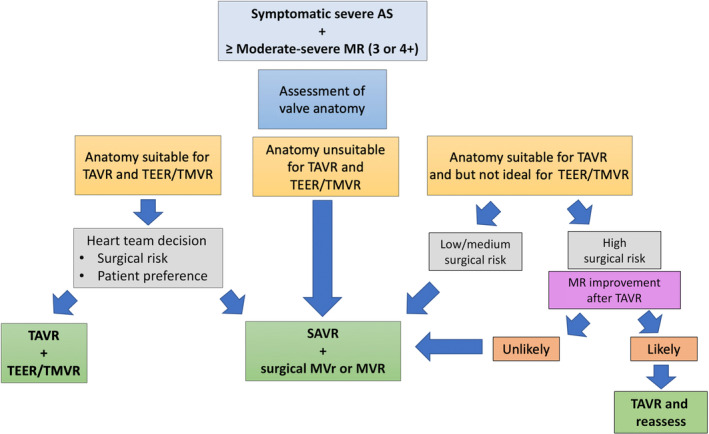
The strategy should be discussed within the heart team and is based on the patient's surgical risk, response to TAVR, and anatomic candidacy for percutaneous MV therapies. Testing may vary per institution. AS indicates aortic stenosis; MR, mitral regurgitation; MVR, mitral valve replacement; MVr, mitral valve repair; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement; TEER, transcatheter edge‐to‐edge mitral valve repair; and TMVR, transcatheter mitral valve replacement.
In this issue of the Journal of the American Heart Association (JAHA), Ferruzzi et al present a study that aims to address these questions. 6 In this retrospective, observational study of 268 patients with LFLG‐AS treated with TAVR at 2 Italian centers between 2017 and 2022, patients were assessed for survival and heart failure (HF) rehospitalization at 1 year, stratified by degree of baseline MR and improvement in MR after TAVR. The key findings are that in this population of patients with LFLG severe AS, at least moderate–severe MR defined as MR >2+ (in other words, MR 3 or 4+) was relatively common, seen in 57 (21%) of patients before TAVR. The presence of at least moderate–severe MR >2+ before TAVR was highly associated with the primary end point of death+HF rehospitalization at 1 year (56.1% versus 10.9%, P<0.001), as well as all secondary end points including all‐cause death (33.3% versus 4.7%, P<0.001), cardiac death (28.1% versus 2.4%, P<0.001), and HF rehospitalization (36.8% versus 6.2%, P<0.001). Encouragingly, improvement in MR from moderate–severe to moderate or less (MR ≤2+) was frequent, seen in 24 (44%) after TAVR, and improvement in MR associated strongly with less all‐cause mortality (16.7% versus 42.8%, P=0.044) and HF rehospitalization (20.8% versus 50.0%, P=0.030). After multivariable adjustment, persistence of moderate–severe MR >2+ remained a strong independent predictor of composite death + HF rehospitalization at 1 year (hazard ratio, 3.02 [95% CI, 1.65–5.56], P<0.001), and on Kaplan–Meier analysis, HF‐rehospitalization‐free survival was better in patients who had MR improvement after TAVR versus those with persistent moderate–severe MR.
The authors should be lauded for performing a contemporary, timely analysis that uses advanced statistical techniques including propensity score adjustment and consideration of competing risks to tackle a clinical question that has proven difficult to study. However, it is important to note that their ability to adjust for confounders may be compromised due to the relatively small sample size, despite using propensity score weighting. That said, the recognition that the hazards of mortality and HF rehospitalization are not proportional over time after TAVR is an important finding. Though not particularly highlighted by the authors, the landmark analysis of outcomes stratified by baseline MR is particularly useful because it reveals that HF rehospitalization was no different within the first 3 months after TAVR, regardless of MR severity pre‐procedure (log‐rank P=0.937). This is reassuring for providers, because it suggests that risks of the TAVR procedure are not meaningfully impacted by baseline MR in patients with LFLG AS, and that TAVR is likely no less safe in the LFLG AS population compared with others.
Along those lines, this study highlights that patients with LFLG severe AS and concomitant MV disease are a complex patient population at high risk for HF rehospitalization 3 to 12 months post‐TAVR even despite a good TAVR result. This reinforces the fact that our responsibilities in managing our patients should not stop when we insert the valve. Although we appreciate that it was not the authors' main focus, there was a missed opportunity to improve the quality of HF management post‐TAVR in this study. In support of this point, only 31% of patients were on a β‐blocker, 47% were on an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, and rates of mineralocorticoid receptor antagonist/angiotensin receptor neprilysin inhibitor/sodium‐glucose cotransporter‐2 inhibitor use were not reported; moreover, rates did not improve significantly post‐TAVR. While the purpose of this study was to reflect real‐world clinical practice, and longitudinal changes in medications were not reported, the high rates of HF rehospitalization are a sobering reminder that patients with LFLG AS and concomitant MR are an especially high‐risk patient population, and as such deserve maximal guideline‐directed medical therapy even post‐TAVR. 7
While it is attractive to stratify patients into categories of risk based on pre‐TAVR MR severity, the limitations of this approach must be acknowledged. While this study does attempt to assess improvement in MR after TAVR, the authors asses this only by discharge echocardiogram. Because it may take time for the LV to remodel and LV function and dimensions to improve, MR improvement may be better assessed by echocardiography at 60, 90, or 180 days than at discharge. While the authors may be identifying the subset of patients who are quick responders with an immediate improvement in MR after TAVR, this does not rule out the possibility that some patients had an improvement in MR after discharge because it was not captured in this study. Indeed, it is feasible that MR persistence at 1 year is more important than an improvement in MR at hospital discharge, because it is not clear whether the improvement in MR seen at discharge will be long‐lasting. Ideally, a larger, prospective study with serial echocardiography during longitudinal follow‐up, read by a single core‐laboratory, with close attention to guideline‐directed medical therapy pre‐ and post‐TAVR would allow for a better understanding of the trajectory of patients with LFLG severe AS and concomitant MR post‐TAVR.
This study contributes to our understanding because it confirms that at least moderate–severe MR is common in patients with LFLG severe AS and suggests it should not be ignored. Specifically, 21% of patients had >2+ MR at baseline, and while 44% improved to ≤2+ MR after TAVR, those who did not improve had particularly poor outcomes including more cardiac death and high rates of HF rehospitalization within 1 year. These data are consistent with data from studies of high‐flow AS including analysis from the PARTNER (Placement of Aortic Transcatheter Valve) trial, which show that nonresponsiveness of MR to TAVR is a poor prognostic sign, 5 as well as data from the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) trial that indicates persistent MR leads to worse outcomes after TEER. 8
However, there remain several unanswered questions, including why some patients with LFLG severe AS and moderate–severe MR have a MR benefit and others do not. Here we can hypothesize some mechanisms, which likely differ based on each individual patient's LV and MV pathology (Figure 2). In patients with “classical” LFLG severe AS with reduced LV ejection fraction (LVEF), patients with AS‐induced LV dysfunction may have improvement in LVEF, leading to improved LV dimensions, less MV annular dilation, and reduced functional MR. In both classical LFLG severe AS and paradoxical LFLG severe AS with preserved LVEF, alleviation of fixed LV afterload due to AS may improve LV dimensions, and reduce functional MR or degree of degenerative MR as well. Conversely, it is possible that in patients who do not have an improvement in MR, this could be due to a failure of improvement in LVEF or dimensions, possibly because their LV dysfunction with or without dilation was due to another cause (ie, ischemic cardiomyopathy), or out of proportion to their degree of AS. It is likewise unknown whether patients with degree of degenerative MR or atrial MR may still benefit from a reduction in LV dimensions with or without relief of fixed LV afterload. Similarly, mitral annular calcification may be an important predictor of lack of improvement of MR post‐TAVR, 9 possibly due to rigidity of the annulus, which may not respond to changes in LV dimensions even with improvement in LV function. Despite the strengths of the current study, it did not stratify patients by MR mechanism, mitral annular calcification severity, or provide longitudinal data on LVEF or LV dimensions, all of which would have been helpful and lead to an even more informative analysis.
Figure 2. Unanswered questions in TAVR for LFLG Severe AS and MR.
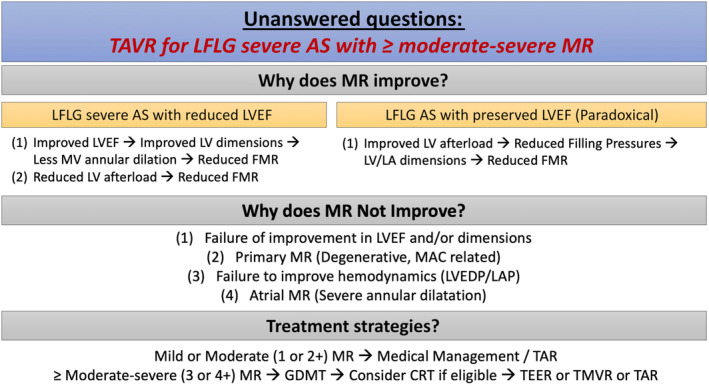
AS indicates aortic stenosis; CRT, cardiac resynchronization therapy; FMR, functional mitral regurgitation; GDMT, guideline‐directed medical therapy; LA, left atrial; LAP, left atrial pressure; LFLG, low‐flow, low‐gradient; LV, left ventricular; LVEDP, left ventricular end diastolic pressure; LVEF, left ventricular ejection fraction; MAC, mitral annular calcification; MR, mitral regurgitation; MV, mitral valve; TAR, transcatheter annular remodeling; TAVR, transcatheter aortic valve replacement; TEER, transcatheter edge‐to‐edge mitral valve repair; and TMVR, transcatheter mitral valve replacement.
Perhaps the most important practical questions that remain are whether addressing residual MR post‐TAVR in patients with LFLG can modify their longer‐term risk, and how best to do so. Encouragingly, the current study suggests that residual MR is not just a marker of worse prognosis, but that modifying the MR may benefit these patients, since there was an improvement in outcomes in patients whose MR improved as well. This supports the premise that residual MR should be targeted aggressively with guideline‐directed medical therapy (as mentioned), cardiac resynchronization therapy in patients with a left bundle branch block or pacemaker dependency, and then with transcatheter interventions if ≥3+ MR persists and they are appropriate anatomic candidates (Figures 1 and 2). The current study also supports the generalization of data from patients with high‐flow AS and from studies including COAPT that suggest the risk of death, HF rehospitalization, and quality of life can all be significantly improved with TEER in appropriate patients. 10 , 11 , 12 Likewise, transcatheter annular remodeling via percutaneous mitral annuloplasty achieved through tightening the coronary sinus may be a complementary option to TEER and transcatheter mitral valve replacement in certain patients. 13 The ongoing The Carillon Mitral Contour System® in Treating Heart Failure With at Least Mild FMR trial stands poised to address the safety and efficacy of transcatheter annular remodeling with the CARILLON Mitral Contour System in patients with persistent HF symptoms and at least mild MR, LVEF ≤50%, and LV dilation (NCT03142152).
The evaluation of patients with polyvalvular disease including LFLG severe AS and MR is often complex, though it can be simplified with a methodical, stepwise approach to evaluation and treatment. If a patient is not a surgical candidate, treatment typically starts with catheter‐based intervention of AS because it is usually the dominant valve lesion, and longitudinal assessment of residual MR with serial assessment of risk and benefit of additional therapies. The current study is consistent with others and has a valuable message: that concomitant MR is common in patients with LFLG severe AS, improves in nearly half of patients after TAVR, but that lack of improvement indicates high risk of death and HF hospitalization within the following year. Whether treatment of the MR will modify this risk deserves future study, but in the absence of additional evidence, catheter‐based intervention of residual MR after TAVR appears to be an important target to improve outcomes in this patient population.
Disclosures
None.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
This manuscript was sent to Amgad Mentias, MD, Associate Editor, for editorial decision and final disposition.
For Disclosures, see page 4.
See article by Ferruzzi et al.
References
- 1. Freitas‐Ferraz AB, Lerakis S, Barbosa Ribeiro H, Gilard M, Cavalcande JL, Makkar R, Hermann HC, Windecker S, Enriquez‐Sarano M, Cheema AN, et al. Mitral regurgitation in low‐flow, low‐gradient aortic stenosis patients undergoing TAVR: insights from the TOPAS‐TAVI registry. JACC Cardiovasc Interv. 2020;13:567–579. doi: 10.1016/j.jcin.2019.11.042 [DOI] [PubMed] [Google Scholar]
- 2. Alushi B, Ensminger S, Herrmann E, Balaban U, Bauer T, Beckmann A, Cleiziffer S, Mollmann H, Walther T, Bekeredjian R, et al. Concomitant mitral regurgitation in patients with low‐gradient aortic stenosis: an analysis from the German Aortic Valve Registry. Clin Res Cardiol. 2022;111:1377–1386. doi: 10.1007/s00392-022-02067-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benfari G, Clavel MA, Nistri S, Maffeis C, Vassanelli C, Enriquez‐Sarano M, Rossi A. Concomitant mitral regurgitation and aortic stenosis: one step further to low‐flow preserved ejection fraction aortic stenosis. Eur Heart J Cardiovasc Imaging. 2018;19:569–573. doi: 10.1093/ehjci/jex172 [DOI] [PubMed] [Google Scholar]
- 4. Chiche O, Rodes‐Cabau J, Campelo‐Parada F, Freitas‐Ferraz AB, Regueiro A, Chamandi C, Rodriguez‐Gabella T, Cote M, DeLarochelliere R, Paradis J, et al. Significant mitral regurgitation in patients undergoing TAVR: mechanisms and imaging variables associated with improvement. Echocardiography. 2019;36:722–731. doi: 10.1111/echo.14303 [DOI] [PubMed] [Google Scholar]
- 5. Barbanti M, Webb JG, Hahn RT, Feldman T, Boone RH, Smith CR, Kodali S, Zajarias A, Thompson CR, Green P, et al. Impact of preoperative moderate/severe mitral regurgitation on 2‐year outcome after transcatheter and surgical aortic valve replacement: insight from the Placement of Aortic Transcatheter Valve (PARTNER) Trial Cohort a. Circulation. 2013;128:2776–2784. doi: 10.1161/CIRCULATIONAHA.113.003885 [DOI] [PubMed] [Google Scholar]
- 6. Ferruzzi G, Silverio A, Giordano A, Corcione N, Bellino M, Attisano T, Baldi C, Morello A, Biondi‐Zoccai G, Citro R, et al. Prognostic impact of mitral regurgitation before and after transcathter aortic valve replacement in patients with severe low‐flow, low‐gradient aortic stenosis. J Am Heart Assoc. 2023;12:e029553. doi: 10.1161/JAHA.123.029553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e35–e71. doi: 10.1161/CIR.0000000000000932 [DOI] [PubMed] [Google Scholar]
- 8. Kar S, Mack MJ, Lindenfeld J, Abraham WT, Asch FM, Weissman NJ, Enriquez‐Sarano M, Lim DS, Mischell JM, Whisenant BK, et al. Relationship between residual mitral regurgitation and clinical and quality‐of‐life outcomes after transcatheter and medical treatments in heart failure: COAPT trial. Circulation. 2021;144:426–437. doi: 10.1161/CIRCULATIONAHA.120.053061 [DOI] [PubMed] [Google Scholar]
- 9. Cortes C, Amat‐Santos IJ, Nombela‐Franco L, Munoz‐Garcia A, Gutierrez‐Ibanes E, Hernandez J, Cordoba‐Soriano JG, Jimenez‐Quevedo P, Hernandez‐Garcia JM, Gonzalez‐Mansilla A, et al. Mitral regurgitation after transcatheter aortic valve replacement: prognosis, imaging predictors, and potential management. JACC Cardiovasc Interv. 2016;9:1603–1614. doi: 10.1016/j.jcin.2016.05.025 [DOI] [PubMed] [Google Scholar]
- 10. Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355 [DOI] [PubMed] [Google Scholar]
- 11. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, et al. Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640 [DOI] [PubMed] [Google Scholar]
- 12. Kar S, von Bardeleben RS, Rottbauer W, Mahoney P, Price MJ, Grasso C, Williams M, Lurz P, Ahmed M, Hausleiter J, et al. Contemporary outcomes following transcatheter edge‐to‐edge repair: 1‐year results from the EXPAND study. JACC Cardiovasc Interv. 2023;16:589–602. doi: 10.1016/j.jcin.2023.01.010 [DOI] [PubMed] [Google Scholar]
- 13. Schofer J, Siminiak T, Haude M, Herrman JP, Vainer J, Wu JC, Levy WC, Mauri L, Feldman T, Kwong RY, et al. Percutaneous mitral annuloplasty for functional mitral regurgitation: results of the CARILLON Mitral Annuloplasty Device European Union Study. Circulation. 2009;120:326–333. doi: 10.1161/CIRCULATIONAHA.109.849885 [DOI] [PMC free article] [PubMed] [Google Scholar]


