Abstract
Background
Chronic disease, such as heart failure, influences cellular metabolism and shapes circulating metabolites. The relationships between key energy metabolites and chronic diseases in aging are not well understood. This study aims to determine the relationship between main components of energy metabolism with all‐cause mortality and incident heart failure.
Methods and Results
We analyzed the association between plasma metabolite levels with all‐cause mortality and incident heart failure among US older adults in the CHS (Cardiovascular Health Study). We followed 1758 participants without heart failure at baseline with hazard ratios (HRs) of analyte levels and metabolic profiles characterized by high levels of ketone bodies for all‐cause mortality and incident heart failure. Multivariable Cox analyses revealed a dose‐response relationship of 50% increase in all‐cause mortality between lowest and highest quintiles of ketone body concentrations (HR, 1.5 [95% CI, 1.0–1.9]; P=0.007). Ketone body levels remained associated with incident heart failure after adjusting for cardiovascular disease confounders (HR, 1.2 [95% CI, 1.0–1.3]; P=0.02). Using K‐means cluster analysis, we identified a cluster with higher levels of ketone bodies, citrate, interleukin‐6, and B‐type natriuretic peptide but lower levels of pyruvate, body mass index, and estimated glomerular filtration rate. The cluster with elevated ketone body levels was associated with higher all‐cause mortality (HR, 1.7 [95% CI, 1.1–2.7]; P=0.01).
Conclusions
Higher concentrations of ketone bodies predict incident heart failure and all‐cause mortality in an older US population, independent of metabolic and cardiovascular confounders. This association suggests a potentially important relationship between ketone body metabolism and aging.
Keywords: aging, heart failure, ketone bodies, metabolism, mortality
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- CHS
Cardiovascular Health Study
- CoA
coenzyme A
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- NMR
nuclear magnetic resonance
Clinical Perspective.
What Is New?
In this prospective cohort study of older US participants without heart failure at baseline, higher concentrations of plasma ketone body levels, measured by nuclear magnetic resonance spectroscopy, are associated with all‐cause mortality and incident heart failure.
Under K‐means clustering analysis, a cluster with elevated concentrations of plasma ketone bodies, characterized by higher interleukin‐6 and B‐type natriuretic peptide levels, but lower estimated glomerular filtration rate, body mass index, and pyruvate levels, was uniquely associated with all‐cause mortality.
What Are the Clinical Implications?
The relationship between aging and ketone body metabolism has not yet been established.
These findings suggest that ketone body metabolism predicts all‐cause mortality and incidence of heart failure among older US adults.
Participants with higher levels of plasma ketone bodies have a unique clinical profile and association with all‐cause mortality that can represent a key relationship to study further among older adults.
Carbohydrates and fatty acids are 2 main fuel sources for mammalian cells under normal conditions. The metabolism of these fuel sources converges on the tricarboxylic acid cycle in the mitochondria. Cells regulate the metabolic pathways in response to substrate availability and metabolic needs, which are also under neural and hormonal regulation. Ketone bodies, pyruvate, and citrate are pivotal products of these metabolic pathways. Ketone bodies are primarily produced by liver mitochondria when the production of acetyl–coenzyme A (CoA), derived from fatty acid β‐oxidation, exceeds its transformation to citrate. 1 Pyruvate, an alternative source for acetyl‐CoA, is produced from glycolysis in most cells. 2
Multiple studies have sought to use these metabolites as biomarkers for onset and progression of chronic diseases involving highly metabolically active organs. 3 , 4 Among these diseases, heart failure has been the most studied. Growing evidence has suggested extensive cardiac remodeling and altered metabolism that precede progressive pathologic adaptations in heart failure. 5 , 6 , 7 , 8 , 9 , 10 , 11 In animal models, a prominent shift from fatty acid oxidation to glucose metabolism has been identified as a maladaptive mechanism that contributes to heart failure progression. 12 Experimental and cross‐sectional studies demonstrated a shift in energy metabolism from β‐oxidation in the resting state to an adaptive increase in myocardial ketone oxidation in the failing heart with concomitant upregulation of ketogenesis. 13 , 14 Furthermore, proteomic studies have demonstrated a parallel progressive derangement in fatty acid metabolism with downregulation of proteins and enzymes in fatty acid transport and use. 15 The metabolic dysfunctions in aging and heart failure may alter fatty acid and glucose‐derived metabolites. The ability to capture and understand these changes may improve disease diagnosis and risk prognostication. 13 , 14 , 15 , 16 , 17 , 18
How mitochondrial energy metabolism interacts with aging and chronic diseases remains elusive. On the one hand, aging and chronic diseases impact mitochondrial metabolism. On the other hand, altered mitochondrial metabolism could cause end organ disease. Herein, we investigated the relationships between circulating ketone bodies, pyruvate, and citrate, 3 by‐products of energy metabolism, and all‐cause mortality and incident heart failure among older adults without index heart failure in the CHS (Cardiovascular Health Study).
METHODS
Study Population
CHS is a prospective observational cohort study among adults aged ≥65 years. 19 , 20 CHS recruited Medicare‐eligible noninstitutionalized individuals in 4 representative US communities. 19 The first 5201 eligible participants were recruited in 1989 to 1990, comprising 57% women and 95% White individuals. An additional 687 Black participants were recruited in 1992 to 1993. The study design, rationale, methods of measurement, and assessment quality have been described previously. 19 , 20 , 21 The institutional review committee of each participating center approved the study, and all participants provided informed written consent. All participants answered standardized questionnaires assessing medical history at enrollment and underwent an extensive clinical and laboratory examination. Baseline fasting EDTA‐plasma samples from a subset of participants (n=1850) were used for nuclear magnetic resonance (NMR) spectroscopic analysis. 22 Sampling weights were calculated for each subcohort participant to allow the estimation of associations that represent the entire cohort. Participants free from clinical cardiovascular disease at baseline were selected for the study, including 1622 White and 228 Black participants. 23
Measurement of Metabolites
A total 1850 archived baseline EDTA plasma samples from the CHS were analyzed by NMR spectroscopy at LipoScience (now Laboratory Corporation of America Holdings) in 2000. The NMR spectra were subsequently reanalyzed using newly validated algorithms to calculate concentrations for total ketone bodies (β‐hydroxybutyrate, acetoacetate, and acetone), pyruvate, and citrate in μmol/L, as described previuosly. 22 , 24 , 25 , 26 Each metabolite level was either log transformed or categorized using quintiles for final analyses.
Mortality and Cardiovascular Outcomes
All‐cause mortality and incident congestive heart failure are the primary outcomes. All cases of heart failure and death were adjudicated by the CHS Adjudication Committee. 21 , 27 Heart failure with reduced ejection fraction (HFrEF) was defined by ejection fraction <45%, and heart failure with preserved ejection fraction (HFpEF) was defined by ejection fraction ≥45%. Participants were followed up every 6 months, with confirmation of death by reviews of obituaries, health records, death certificates, and the Healthcare Financing Administration health care use database. Follow‐up was completed in 100% of participants through interviews of contacts and proxies if the participants were unavailable for appropriate documentation of event. 28
Covariates
We selected covariates based on their possible links with both energy metabolism and outcome (mortality and heart failure). Baseline variables used for statistical analyses included age, sex, site of recruitment, race (White, Black, or other), education level, body mass index (BMI), smoking status, alcohol consumption (number of drinks per week), and diagnosis of diabetes or hypertension. Medication use for hypertension, hyperlipidemia, and estrogen replacement was also included. We included baseline levels of fasting triglycerides, cholesterol, insulin, and glucose, and estimated glomerular filtration rate calculated from cystatin C. Fasting status was dichotomized using 8 hours as cutoff, and physical activity was determined on the basis of total kilocalories consumed daily. We also extracted dietary constituents from the 1989 to 1990 questionnaire, including daily consumption in grams of carbohydrates, protein, saturated fat, oleic acid, and linoleic acid. In clustering analyses, we also included additional NMR‐derived biomarkers, including lipoprotein subclass concentration and sizes, and glycoprotein acetylation, a marker for inflammation. 26 , 29
Statistical Analysis
We first analyzed the interrelationships of ketone body, pyruvate, and citrate levels using Pearson correlation coefficients. We used K‐means cluster analysis to identify the phenotypes associated with total ketone body, pyruvate, and citrate profiles. Covariates included NMR‐derived lipoprotein parameters (very low‐density lipoprotein, low‐density lipoprotein, and high‐density lipoprotein particle concentrations and sizes), an NMR‐derived marker of inflammation (glycoprotein acetylation), as well as BMI, estimated glomerular filtration rate, and laboratory measurements reflecting metabolic and inflammatory phenotypes (insulin, glucose, B‐type natriuretic peptide, CRP [C‐reactive protein], interleukin‐6, and fibrinogen). To facilitate comparison across different variables, all variables were converted to their Z‐score, calculated as the difference between the value and the mean divided by the SD. After testing with serial increments of cluster numbers, we selected 4 cluster models for further analysis. Characteristics of the study population were described as prevalence estimates for categorical variables and weighted means for continuous variables. P values were calculated by using the linear trend estimation method.
Cox proportional hazard models were used to calculate hazard ratios (HRs) for all‐cause mortality and incident adjudicated heart failure in relation to log‐transformed continuous levels, quintiles of ketone bodies, pyruvate, and citrate, or clusters. We verified Cox proportional hazard assumptions were met before modeling. Survival time was coded as years from baseline to death or adjudicated heart failure. We censored the analysis to 20 years to ensure proportional hazard assumptions were satisfied in baseline models for the 3 main metabolites in the analysis. Model 1 included age in its continuous and quadratic form, sex, race, and clinic site. Model 2 adjusted for the following anthropometric measurements and questionnaire data: BMI, BMI2, education level, smoking status, alcohol consumption pattern per week, kilocalories in daily physical activities, and fasting status. Model 3 further adjusted for comorbidities and laboratory values, including diagnosis of diabetes or hypertension, triglyceride and cholesterol levels, and medication use for hypertension, hyperlipidemia, and postmenopausal estrogen therapy. As a sensitivity analysis, we first adjusted model 3 for reported dietary consumption of carbohydrates, protein, oleic acid, linoleic acid, and saturated fat. Second, we compared the HR of both log‐transformed and quintiles of ketone bodies as predictors of all‐cause mortality and incident heart failure using the Lunn‐McNeil competing risk models. 30 , 31
We next constructed Cox proportional hazard regression models to estimate the HRs of death and incident heart failure for K‐means clusters using cluster 1 (healthy) as a reference. Further analyses examined the HRs for specific causes of death, including cardiovascular (coronary heart disease, stroke, or other atherosclerotic disease), cancer, dementia, infection (pneumonia, sepsis, or other infection), respiratory, trauma (including fractures), and other causes (liver disease, gastrointestinal disease, renal failure, amyotrophic lateral sclerosis, Parkinson disease, bladder disease, metabolic conditions, amyloid, failure to thrive, myelodysplastic syndrome, and other musculoskeletal diseases).
All analyses were conducted in Stata version 16.1 (Stata Corp LLC, College Station, TX) and R (R Foundation, Vienna, Austria), with the sampling weights for NMR measurements to reconstitute the estimate corresponding to the entire CHS cohort. The data that support the findings of this study are available from the corresponding author on reasonable request.
RESULTS
Of the 1850 participants, 1758 had complete metabolite measurements by NMR spectroscopy. Of the participants, 59.3% were women, and 16.6% were Black individuals. The mean age of the participants at baseline was 72.7 years. Mean (SD) levels of total ketone bodies, pyruvate, and citrate were 204.1 (176.4), 55.4 (32.1), and 150.1 (31.5) μmol/L, respectively. The distribution of comorbidities, anthropometric measurements, and main laboratory values within each quintile of ketone body levels is shown in Table 1. The 92 participants excluded on the basis of incomplete metabolite measurements did not demonstrate statistically significant differences in baseline demographics from the final cohort. Participants in higher ketone body quintiles were more likely to be older, have metabolic comorbidities (diabetes, hypertension, or hyperlipidemia), have higher weekly alcohol consumption, and have higher levels of B‐type natriuretic peptide and citrate. On the contrary, the level of ketone bodies had an inverse relationship with triglyceride and pyruvate levels.
Table 1.
Participant Characteristics, According to Quintiles of Ketone Bodies
| Characteristic | Ketone body quintiles* | P value† | ||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| (N=352) | (N=352) | (N=351) | (N=352) | (N=351) | ||
| Age, y | 71.0 (4.5) | 71.5 (4.6) | 72.9 (5.3) | 72.8 (5.6) | 74.3 (6.4) | <0.001 |
| Sex | 0.5 | |||||
| Women | 214 (60.8) | 211 (59.9) | 200 (57.0) | 217 (61.6) | 201 (57.3) | |
| Men | 138 (39.2) | 141 (40.1) | 151 (43.0) | 135 (38.4) | 150 (42.7) | |
| Race | <0.001 | |||||
| White | 314 (89.2) | 290 (82.4) | 297 (84.6) | 291 (82.7) | 267 (76.1) | |
| Black | 37 (10.5) | 61 (17.3) | 53 (15.1) | 58 (16.5) | 82 (23.4) | |
| Other | 1 (0.3) | 1 (0.3) | 1 (0.3) | 3 (0.9) | 2 (0.6) | |
| Education level | 0.1 | |||||
| No school | 0 (0) | 0 (0) | 2 (1) | 0 (0) | 0 (0) | |
| High school or less | 185 (53) | 194 (55) | 188 (54) | 213 (61) | 195 (56) | |
| College | 125 (36) | 118 (34) | 117 (34) | 111 (32) | 119 (34) | |
| Graduate or professional | 41 (12) | 40 (11) | 42 (12) | 28 (8) | 34 (10) | |
| Smoking status | 0.6 | |||||
| Never smoked | 180 (51.1) | 150 (42.6) | 171 (48.7) | 174 (49.4) | 168 (47.9) | |
| Former smoker | 127 (36.1) | 161 (45.7) | 127 (36.2) | 141 (40.1) | 150 (42.7) | |
| Current smoker | 45 (12.8) | 41 (11.6) | 53 (15.1) | 37 (10.5) | 33 (9.4) | |
| Alcohol consumption, no. of drinks/wk | 1.9 (4.6) | 2.5 (5.6) | 2.2 (5.6) | 2.6 (6.2) | 3.4 (8.2) | 0.4 |
| Body mass index, kg/m2 | 25.5 (3.9) | 26.3 (4.1) | 26.8 (4.6) | 27.3 (5.0) | 26.0 (4.8) | 0.06 |
| eGFR, mL/min per 1.73 m2 | 81.8 (19.3) | 79.7 (18.6) | 79.3 (18.3) | 79.2 (21.5) | 77.8 (18.7) | 0.2 |
| Hypertension | 120 (34.1) | 150 (42.6) | 142 (40.5) | 147 (41.9) | 171 (48.7) | 0.001 |
| Hyperlipidemia | 225 (63.9) | 260 (73.9) | 268 (76.4) | 247 (70.2) | 225 (64.1) | 0.6 |
| Diabetes | 29 (8.4) | 45 (12.8) | 54 (15.5) | 64 (18.4) | 65 (18.5) | <0.001 |
| Hypertension medication | 113 (32.5) | 147 (41.8) | 145 (41.4) | 142 (40.5) | 152 (43.4) | 0.01 |
| Lipid‐lowering agents | 17 (4.9) | 15 (4.3) | 26 (7.4) | 12 (3.4) | 11 (3.1) | 0.2 |
| Estrogen replacement | 28 (8.0) | 25 (7.1) | 21 (6.0) | 38 (10.8) | 26 (7.4) | 0.5 |
| Triglycerides, mg/dL | 132 (58) | 141 (64) | 153 (75) | 133 (61) | 116 (65) | <0.001 |
| Cholesterol, mg/dL | 202 (46) | 208 (34) | 218 (33) | 198 (30) | 211 (46) | 0.4 |
| Fasting glucose, mg/dL | 101 (21) | 106 (34) | 110 (31) | 111 (34) | 118 (59) | <0.001 |
| Fasting insulin, μU/mL | 15.0 (10.9) | 16.8 (25.2) | 18.2 (27.2) | 16.3 (13.6) | 18.1 (37.0) | 0.1 |
| NT‐proBNP, pg/dL | 148 (227) | 174 (243) | 169 (232) | 230 (421) | 261 (470) | <0.001 |
| Ketone bodies, μmol/L | 77.2 (14.8) | 113.7 (9.4) | 151.3 (12.9) | 217.1 (26.1) | 461.7 (247.2) | <0.001 |
| Pyruvate, μmol/L | 59.5 (28.3) | 60.4 (31.0) | 60.8 (35.1) | 53.6 (31.5) | 42.6 (30.7) | <0.001 |
| Citrate, μmol/L | 140.8 (27.9) | 141.0 (27.2) | 147.8 (33.2) | 153.1 (27.8) | 167.8 (32.9) | <0.001 |
| Deaths | 294 (83.5) | 310 (88.1) | 309 (88.0) | 315 (89.5) | 319 (90.9) | 0.003 |
| Incident CHF | 119 (33.8) | 123 (34.9) | 130 (37.0) | 133 (38.0) | 132 (37.6) | 0.1 |
Data are presented mean (SD) for continuous variables and number (percentage) of participants for categorical variables. CHF indicates congestive heart failure; eGFR, estimated glomerular filtration rate; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Participants with complete nuclear magnetic resonance spectroscopy were subdivided on the basis of quintiles of ketone body levels.
P value calculated by linear trend methods.
Ketone Body and Pyruvate Levels Capture Distinct Metabolic Phenotypes
A cross‐sectional analysis revealed that total ketone body was correlated positively with citrate levels (R=0.32; P<0.001) and inversely with pyruvate levels (R=−0.12; P<0.001; Figure S1). To understand the relationship among these 3 metabolites in the context of various metabolic phenotypes, we performed hypothesis‐free clustering analysis by including a variety of metabolic, inflammatory, and chronic disease biomarkers. Using a stepwise approach, a 4‐cluster solution was found that best represented the phenotypes seen in the observed population: healthy (n=359), high ketone bodies (n=65), chronic inflammation (n=287), and metabolic syndrome (n=250; Figure 1). Cluster A (healthy) was characterized by a healthy lipid profile, normal glucose metabolism, lower BMI, low levels of inflammatory biomarkers, and normal cardiorenal function (Table S1). This cluster was also associated with lower mean total ketone body, pyruvate, and citric acid levels. Cluster B (high ketone bodies) had the fewest number of participants. These individuals had high ketone body and citrate concentrations, but low pyruvate levels, with Z‐scores of 2.5 (1.3), 1.0 (1.0), and –0.7 (–0.7), respectively. This cluster also exhibited distinctly higher levels of B‐type natriuretic peptide and interleukin‐6; and lower estimated glomerular filtration rate and BMI. Cluster C (chronic inflammation) was characterized by a combination of hypercholesterolemia markers and more consistent elevation of inflammatory markers, including fibrinogen, interleukin‐6, and glycoprotein acetylation. Cluster D (metabolic syndrome) was led mostly by metabolic syndrome markers, including higher levels of very low‐density lipoprotein, triglyceride, and pyruvate, with lower levels of ketone bodies.
Figure 1. Clustering analysis of metabolic phenotypes.
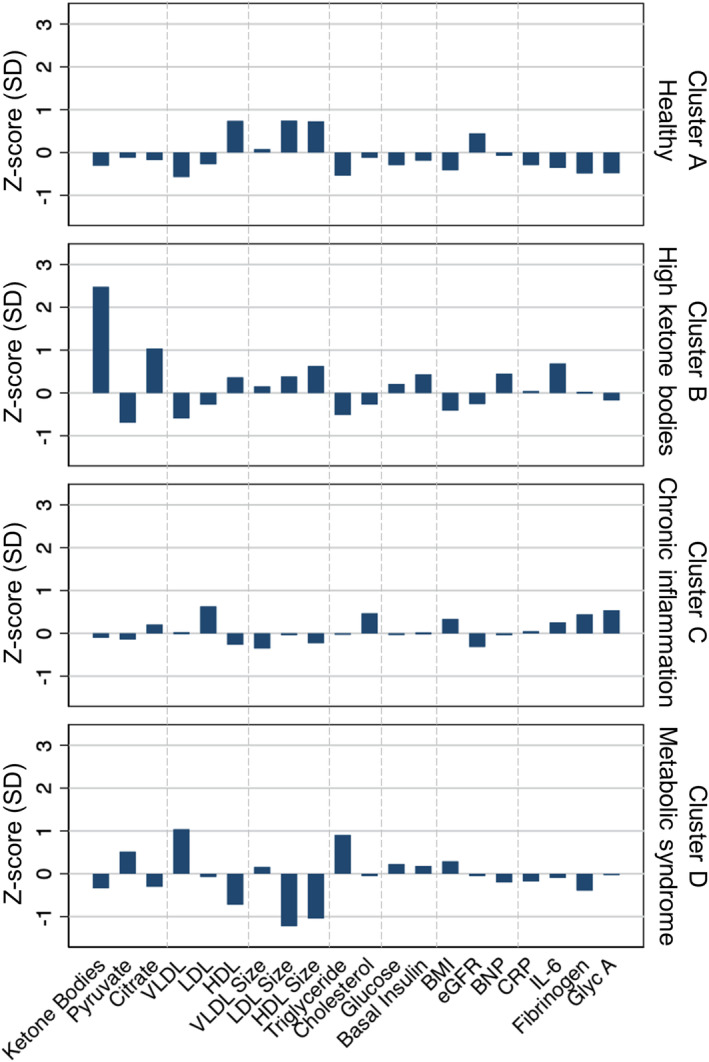
Data are presented as Z‐score, calculated by the difference from the mean divided by the SD. Four clusters are identified using K‐means clustering analysis of ketone bodies, pyruvate, and citrate, along with biomarkers of metabolic and inflammatory phenotypes, including very low‐density lipoprotein (VLDL), low‐density lipoprotein (LDL), and high‐density lipoprotein (HDL) particle concentrations and sizes, levels of triglyceride, total cholesterol, glucose, basal insulin, BNP (B‐type natriuretic peptide), CRP (C‐reactive protein), IL‐6 (interleukin‐6), fibrinogen, and glycoprotein acetylation (Glyc A), as well as body mass index (BMI) and estimated glomerular filtration rate (eGFR). Probable cluster phenotypes are assigned on the basis of biomarker profiles: cluster A, healthy; cluster B, high ketone bodies; cluster C, chronic inflammation; and cluster D, metabolic syndrome.
Ketone Body Levels Predict All‐Cause Mortality
We examined how ketone body, pyruvate, and citrate levels were associated with mortality in CHS. Quintiles with higher ketone bodies and citrate were associated with higher all‐cause mortality, as shown by Kaplan‐Meier plots censored at 20 years (Figure 2). In comparison, no apparent relationship was noted between pyruvate levels and mortality.
Figure 2. Kaplan‐Meier survival analysis for ketone bodies, pyruvate, and citrate in quintiles (Qs).
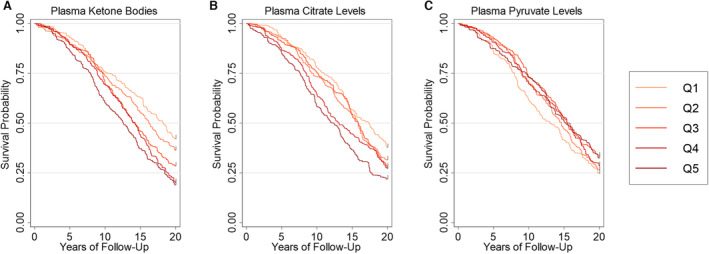
Kaplan‐Meier survival analyses are shown for ketone bodies (A), pyruvate (B), and citrate (C), comparing quintiles over 20 years (Q1, light orange; Q5, dark red).
Multivariate Cox analysis was used to estimate the HR for metabolites that met the proportional hazard assumption (ketone bodies and citrate). Log‐transformed ketone body and citrate levels were independently associated with an increased likelihood of death in the fully adjusted models. The risk of all‐cause mortality increase incrementally across ketone body quintiles, with a 50% increase between the fifth and first quintiles after adjusting for demographics (Table 2). The association with mortality was maintained after adjusting for cardiac and metabolic risk factors. All 3 individual ketone body constituents (3‐hydroxybutyrate, acetoacetate, and acetone) demonstrated similar relationships with all‐cause mortality (Table S2). When testing the predictive value of all 3 metabolic biomarkers under 1 model, only total ketone bodies maintained a statistically significant association with all‐cause mortality after adjusting for confounders.
Table 2.
Multivariable Cox Models for Population Quintiles and All‐Cause Mortality
| Variable | Model 1 HR (95% CI)* | P value | Model 2 HR (95% CI)† | P value | Model 3 HR (95% CI)‡ | P value | |
|---|---|---|---|---|---|---|---|
| Ketone bodies | Log ketone | 1.2 (1.1–1.3) | <0.001 | 1.2 (1.1–1.3) | <0.001 | 1.2 (1.1–1.3) | 0.001 |
| Quintiles: quintile 1 | Reference | Reference | Reference | ||||
| Quintile 2 | 1.2 (0.9–1.5) | 0.3 | 1.2 (0.9–1.6) | 0.1 | 1.1 (0.8–1.5) | 0.4 | |
| Quintile 3 | 1.3 (1.0–1.7) | 0.03 | 1.3 (1.0–1.7) | 0.07 | 1.2 (0.9–1.6) | 0.2 | |
| Quintile 4 | 1.5 (1.2–1.9) | 0.002 | 1.4 (1.1–1.9) | 0.01 | 1.4 (1.0–1.9) | 0.02 | |
| Quintile 5 | 1.5 (1.2–1.9) | 0.002 | 1.6 (1.3–2.1) | <0.001 | 1.5 (1.1–2.0) | 0.007 | |
| Pyruvate | Log pyruvate | 1.0 (0.9–1.1) | 0.7 | 1.0 (0.9–1.1) | 0.5 | 1.0 (0.9–1.1) | 0.5 |
| Quintiles: quintile 1 | Reference | Reference | Reference | ||||
| Quintile 2 | 1.0 (0.8–1.3) | 0.8 | 0.9 (0.7–1.2) | 0.4 | 0.9 (0.7–1.2) | 0.5 | |
| Quintile 3 | 0.9 (0.7–1.2) | 0.4 | 0.9 (0.7–1.1) | 0.3 | 0.9 (0.6–1.2) | 0.2 | |
| Quintile 4 | 1.0 (0.8–1.3) | 0.8 | 0.9 (0.7–1.2) | 0.4 | 1.0 (0.7–1.3) | 0.7 | |
| Quintile 5 | 1.0 (0.7–1.3) | 0.7 | 0.9 (0.7–1.2) | 0.3 | 0.8 (0.6–1.1) | 0.1 | |
| Citrate | Log citrate | 1.1 (1.0–1.3) | 0.005 | 1.2 (1.1–1.3) | 0.002 | 1.1 (1.0–1.2) | 0.04 |
| Quintiles: quintile 1 | Reference | Reference | Reference | ||||
| Quintile 2 | 1.3 (1.0–1.7) | 0.06 | 1.3 (1.0–1.8) | 0.06 | 1.4 (1.0–2.0) | 0.02 | |
| Quintile 3 | 1.2 (1.0–1.6) | 0.1 | 1.3 (1.0–1.8) | 0.04 | 1.3 (1.0–1.9) | 0.07 | |
| Quintile 4 | 1.3 (1.0–1.8) | 0.05 | 1.4 (1.0–2.0) | 0.02 | 1.4 (1.0–1.9) | 0.08 | |
| Quintile 5 | 1.5 (1.2–1.9) | 0.002 | 1.6 (1.2–2.1) | 0.001 | 1.5 (1.1–2.1) | 0.01 |
HR indicates hazard ratio.
Model 1 is adjusted for age, age2, sex, race, and clinic site.
Model 2 is adjusted for age, sex, clinic site, race, education level, kilocalories of physical activity per week, body mass index, body mass index2, smoking status, alcoholic drinks per week, and fasting >8 hours.
Model 3 is adjusted for covariates in model 2 and for diagnosis of hypertension, cholesterol level, triglycerides level, diagnosis of diabetes, estimated glomerular filtration rate, hypertension medication use, lipid‐lowering medication use, and estrogen use.
We next compared all‐cause mortality among the 4 clusters of metabolic phenotypes in CHS (Figure S2 and Table S3). Cluster B (high ketone bodies) was associated with a significantly higher all‐cause mortality compared with clusters A, C, and D. In a Cox proportional hazard model, cluster B had a 2.0‐fold increase in the HR of death (95% CI, 1.3–3.1; P=0.003) when adjusting for metabolic components and dietary components.
A total of 835 participants had an adjudicated cause of death (Table S4). Cox proportional hazard regression models were constructed for common causes of death (Table S5). Under model 2, participants in the high ketone bodies cluster had >2‐fold increase in the risk of dying from cardiovascular causes (HR, 2.1 [95% CI, 1.1–4.2]; P=0.03). No significant associations between any of the clusters with the other causes of death were observed.
Ketone Body Levels Predict Incidence of Heart Failure
Given the link between levels of ketone bodies and mortality from cardiovascular diseases, we examined how ketone body levels predict incidence of heart failure. In multivariate regression models, log‐transformed ketone body levels were associated with a 20% increase in the HR of incident heart failure (95% CI, 1.0–1.3; P=0.02) when adjusted for demographics (model 1) and simple health history (model 2) (Table S6). The inclusion of comorbidities relevant to cardiovascular diseases (model 3) only slightly weakened the relationship between ketone body levels and incident heart failure. In comparison, levels of pyruvate or citrate did not demonstrate similar associations with heart failure. When competing for the risk of mortality, the subdistribution HR of incidental heart failure is no longer significant. In subgroup analyses, the relationship between ketone body levels and incident heart failure was the most pronounced in individuals who were younger, with lower BMI (Tables S7 and S8).
There were no significant differences in the association between ketone bodies for either incident heart failure or all‐cause mortality using the Lunn‐McNeil competing‐risk models for all‐cause mortality (Table S9). Among the 4 clusters of metabolic phenotypes, cluster B (high ketone bodies) demonstrated a significantly higher rate of heart failure, as shown in Kaplan‐Meier analysis (Figure 3 and Table S10), whereas clusters A, C, and D showed similar rates.
Figure 3. Kaplan‐Meier analysis for incident heart failure by metabolic phenotypes.
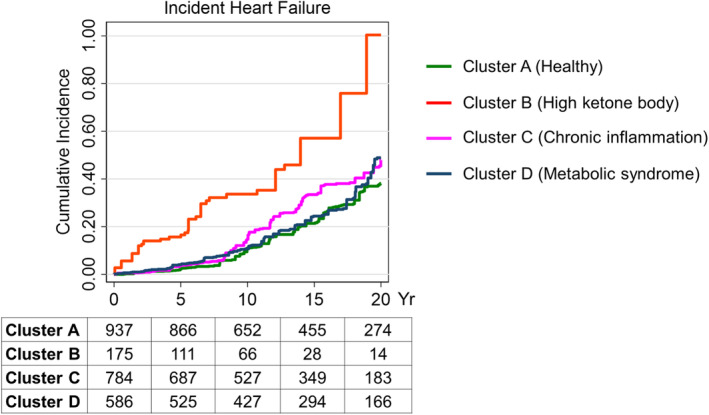
Kaplan‐Meier analysis for the incidence of heart failure is shown for each phenotype cluster (cluster A, healthy; cluster B, high ketone bodies; cluster C, chronic inflammation; and cluster D, metabolic syndrome) over 20 years. At‐risk table is shown at the bottom at 5‐year intervals.
Finally, we examined whether ketone body levels predict HFrEF and HFpEF. Kaplan‐Meier analysis of ketone body quintiles showed that the fourth and fifth quintiles were associated with significantly higher incidence of HFrEF, but not HFpEF (Figure S3A and S3B). In multivariable Cox models, log‐transformed ketone bodies were significantly associated with the incidence of HFrEF in all models (Table S11). No association was observed with HFpEF. In analysis for quintiles, the fourth and fifth quintiles showed significant associations with the incidence of HFrEF in models 1 and 2, whereas the association was weakened when the analysis was adjusted for a comprehensive health history (model 3).
DISCUSSION
In this population‐based cohort study of older adults, baseline plasma ketone body levels were prospectively associated with all‐cause mortality and incident heart failure. The levels of ketone bodies and pyruvate were inversely correlated with each other, whereas both have a strong correlation with citrate. Using clustering analysis, we found differential levels of ketone bodies, pyruvate, and citrate were associated with unique patient phenotypes. Individuals with this metabolic profile were at a significantly higher risk of developing heart failure and death compared with their counterparts, including those with features of metabolic syndrome or chronic inflammation.
The concentration of circulating ketone bodies is determined by the rate balance between their production and consumption. A high level of ketone bodies results from either an increase in their production or a decrease in their consumption. It is well known that certain diets are more ketogenic than others, although ketogenic diets have often been associated with health benefits. 32 Meanwhile, caloric restriction with subsequent ketogenesis has been implicated in longevity in animal models, including nematodes, fruit flies, mice, and primates. 33 , 34 , 35 There seems to be a lack of evidence to indicate diet‐ or calorie restriction–induced ketogenesis is harmful. In our study, the relationship between ketone bodies and overall mortality was not mitigated by the adjustment of individual dietary components (carbohydrates, protein, and fat), which further suggests that the mechanism underlying the current observation is not mainly driven by diet.
In humans, ketone bodies are produced predominantly in the liver from fatty acid oxidation–derived acetyl‐CoA. Ketone body levels in healthy adults will oscillate by a circadian rhythm between 100 and 250 μmol/L. 36 Meanwhile, ketone body levels will increase to ≈1 mmol/L after prolonged exercise or 24 hours of fasting. 37 , 38 In CHS, individuals in the highest quintile had a mean ketone body level of 462 μmol/L, suggesting a potentially altered mitochondrial fuel metabolism in the setting of aging. Classically, ketone body production is considered as a spillover, in which acetyl‐CoA exceeds tricarboxylic acid cycle activity. In cellular senescence, respiratory chain enzyme activities decrease with a parallel increase to reactive oxygen species production as a result of decreased mitochondrial pyruvate dehydrogenase; thus, they may favor the use of acetyl‐CoA from fatty acid β‐oxidation, leading to the production of ketone bodies as a by‐product. 39 , 40 , 41 , 42 , 43 , 44 Studies interrogating the mitochondrial fuel source in aging and dissecting ketone body production and consumption are needed to further understand this relationship.
Alternatively, high levels of ketone body could result from decreased ketone body consumption or clearance, which could include the following: (1) decreased extrahepatic ketone body catabolism, (2) decreased lipogenesis or sterol synthesis, or (3) decreased excretion in the urine. In fact, we observe a lower glomerular filtration rate in the cluster with elevated ketone bodies, supporting this possibility. However, usually only a small fraction of ketone bodies is detected in the urine. Ketone bodies are predominantly used in the brain, skeletal muscle, and heart. Their use is dependent on succinyl‐CoA:3‐oxoacid‐CoA transferase, an enzyme expressed in the extrahepatic tissue. 45 It is conceivable that decreased peripheral tissue consumption associated with mitochondrial dysfunction or functional decline of succinyl‐CoA:3‐oxoacid‐CoA transferase during aging could contribute to the elevation level of plasma ketone bodies.
Our observation of an association between elevated plasma ketone bodies and mortality is supported by observations in the PREVEND (Prevention of Renal and Vascular Endstage Disease Intervention), where authors found a similar link. 46 The prospective association between ketone body and incidental heart failure has not been reported previously. Myocardium is one of the most active consumers of ketone bodies. Several studies have indicated that heart disease, including heart failure and myocardial infarction, is associated with an increase in circulating ketone bodies. 14 , 17 , 46 , 47 , 48 , 49 Thus, missed diagnosis of heart failure at baseline could contribute to our observation, but it is unlikely to be a common cause of ketone body elevation in our study. Furthermore, animal models and human studies have shown that myocardium in heart failure favors ketone body consumption over other fuel sources. 50 , 51 Our observation indicates that a high level of ketone bodies could be a biomarker of subclinical heart failure or could even contribute to heart failure. An increase in plasma ketone bodies can occur in the setting of increased sympathetic tone, which promotes lipolysis and fatty acid metabolism and exacerbates heart failure. 48 Under physiological conditions, myocardium uses ketone bodies in proportion to their delivery at the cost of fatty acid or glucose oxidization. 52 , 53 This raised the concern that long‐term exposure to a high level of ketone bodies could reduce metabolic flexibility of the heart and impair myocardial function.
Limitations
The results should be interpreted in the context of the CHS design. First, CHS mainly comprises older White and Black Americans, limiting the generalizability of these findings to other races. Second, the metabolites were measured on the basis of peripheral blood samples, and their levels could be impacted by multiple organs aside from the heart and any relationship should be inferred with caution. An understanding of the mechanism and causality will likely require measuring a more comprehensive panel of metabolites and ideally metabolites from the heart that may acquire invasive techniques. Third, the phenotype captured by the cluster analysis is dictated by the available baseline data. CHS is a study designed 32 years ago, and some covariates, such as transaminases, are lacking, although our results include a wide variety of biochemical markers. Further studies in other populations could help to further refine the link between clinical outcomes and ketone body levels.
CONCLUSIONS
In this cohort study, we present the first evidence that higher concentrations of ketone bodies predict incidence of heart failure and mortality in older adults. The prospective association is independent from conventional metabolic and cardiovascular confounders. Compared with individuals with metabolic syndrome or elevated inflammatory biomarkers, individuals with high levels of ketone bodies have a distinctive clinical phenotype and are at a significantly higher risk for mortality, especially mortality from cardiovascular diseases. Further investigation is warranted to determine the interplay between the heart and other organs in ketone body metabolism during aging and heart failure.
Sources of Funding
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and 75N92021D00006 and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by R01AG023629 and K24AG065525 from the National Institute on Aging and K08DK115883 from the National Institute of Diabetes and Digestive and Kidney Diseases. A full list of principal CHS (Cardiovascular Health Study) investigators and institutions can be found at CHS‐NHLBI.org.
Disclosures
None.
Supporting information
Tables S1–S11
Figures S1–S3
Acknowledgments
Author contributions: Drs Niezen, Jiang, and Mukamal were involved in data analysis; and Drs Niezen, Connelly, Hirsch, Jiang, and Mukamal contributed to the conception of the study and were involved in providing the initial draft of the manuscript. All authors were involved in the revision of the manuscript.
This article was sent to Yen‐Hung Lin, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.029960
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Zhenghui Gordon Jiang, Email: zgjiang@bidmc.harvard.edu.
Kenneth J. Mukamal, Email: kmukamal@bidmc.harvard.edu.
References
- 1. Puchalska P, Crawford PA. Multi‐dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25:262–284. doi: 10.1016/j.cmet.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci. 2014;71:2577–2604. doi: 10.1007/s00018-013-1539-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larach DB, Kofke WA, Le Roux P. Potential non‐hypoxic/ischemic causes of increased cerebral interstitial fluid lactate/pyruvate ratio: a review of available literature. Neurocrit Care. 2011;15:609–622. doi: 10.1007/s12028-011-9517-8 [DOI] [PubMed] [Google Scholar]
- 4. Bullo M, Papandreou C, Garcia‐Gavilan J, Ruiz‐Canela M, Li J, Guasch‐Ferre M, Toledo E, Clish C, Corella D, Estruch R, et al. Tricarboxylic acid cycle related‐ metabolites and risk of atrial fibrillation and heart failure. Metabolism. 2021;125:154915. doi: 10.1016/j.metabol.2021.154915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 6. Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11:e004873. doi: 10.1161/CIRCHEARTFAILURE.117.004873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tromp J, Paniagua SMA, Lau ES, Allen NB, Blaha MJ, Gansevoort RT, Hillege HL, Lee DE, Levy D, Vasan RS, et al. Age dependent associations of risk factors with heart failure: pooled population based cohort study. BMJ. 2021;372:n461. doi: 10.1136/bmj.n461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng Y, Yu B, Alexander D, Manolio TA, Aguilar D, Coresh J, Heiss G, Boerwinkle E, Nettleton JA. Associations between metabolomic compounds and incident heart failure among African Americans: the ARIC study. Am J Epidemiol. 2013;178:534–542. doi: 10.1093/aje/kwt004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Selvaraj S, Kelly DP, Margulies KB. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation. 2020;141:1800–1812. doi: 10.1161/CIRCULATIONAHA.119.045033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Jong KA, Lopaschuk GD. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can J Cardiol. 2017;33:860–871. doi: 10.1016/j.cjca.2017.03.009 [DOI] [PubMed] [Google Scholar]
- 11. Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ Res. 2021;128:1487–1513. doi: 10.1161/CIRCRESAHA.121.318241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tran DH, Wang ZV. Glucose metabolism in cardiac hypertrophy and heart failure. J Am Heart Assoc. 2019;8:e012673. doi: 10.1161/JAHA.119.012673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304:H1060–H1076. doi: 10.1152/ajpheart.00646.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flores‐Guerrero JL, Westenbrink BD, Connelly MA, Otvos JD, Groothof D, Shalaurova I, Garcia E, Navis G, de Boer RA, Bakker SJL, et al. Association of beta‐hydroxybutyrate with development of heart failure: sex differences in a Dutch population cohort. Eur J Clin Invest. 2021;51:e13468. doi: 10.1111/eci.13468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009;81:412–419. doi: 10.1093/cvr/cvn301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hunter WG, Kelly JP, McGarrah RW III, Kraus WE, Shah SH. Metabolic dysfunction in heart failure: diagnostic, prognostic, and pathophysiologic insights from metabolomic profiling. Curr Heart Fail Rep. 2016;13:119–131. doi: 10.1007/s11897-016-0289-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stryeck S, Gastrager M, Degoricija V, Trbusic M, Potocnjak I, Radulovic B, Pregartner G, Berghold A, Madl T, Frank S. Serum concentrations of citrate, tyrosine, 2‐ and 3‐ hydroxybutyrate are associated with increased 3‐month mortality in acute heart failure patients. Sci Rep. 2019;9:6743. doi: 10.1038/s41598-019-42937-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanai E, Frantz S. Pathophysiology of heart failure. Compr Physiol. 2015;6:187–214. doi: 10.1002/cphy.c140055 [DOI] [PubMed] [Google Scholar]
- 19. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-W [DOI] [PubMed] [Google Scholar]
- 20. Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the cardiovascular health study. Clin Chem. 1995;41:264–270. doi: 10.1093/clinchem/41.2.264 [DOI] [PubMed] [Google Scholar]
- 21. Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8 [DOI] [PubMed] [Google Scholar]
- 22. Kuller L, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, Siscovick D, Freedman DS, Kronmal R. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol. 2002;22:1175–1180. doi: 10.1161/01.atv.0000022015.97341.3a [DOI] [PubMed] [Google Scholar]
- 23. Mukamal KJ, Mackey RH, Kuller LH, Tracy RP, Kronmal RA, Mittleman MA, Siscovick DS. Alcohol consumption and lipoprotein subclasses in older adults. J Clin Endocrinol Metab. 2007;92:2559–2566. doi: 10.1210/jc.2006-2422 [DOI] [PubMed] [Google Scholar]
- 24. Garcia E, Shalaurova I, Matyus SP, Oskardmay DN, Otvos JD, Dullaart RPF, Connelly MA. Ketone bodies are mildly elevated in subjects with type 2 diabetes mellitus and are inversely associated with insulin resistance as measured by the lipoprotein insulin resistance index. J Clin Med. 2020;9(2):321. doi: 10.3390/jcm9020321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia E, Connelly MA, Matyus SP, Otvos JD, Shalaurova I. High‐throughput nuclear magnetic resonance measurement of citrate in serum and plasma in the clinical laboratory. Pract Lab Med. 2021;25:e00213. doi: 10.1016/j.plabm.2021.e00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Otvos JD, Shalaurova I, Wolak‐Dinsmore J, Connelly MA, Mackey RH, Stein JH, Tracy RP. GlycA: a composite nuclear magnetic resonance biomarker of systemic inflammation. Clin Chem. 2015;61:714–723. doi: 10.1373/clinchem.2014.232918 [DOI] [PubMed] [Google Scholar]
- 27. Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9 [DOI] [PubMed] [Google Scholar]
- 28. Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, Robbins JA, Gardin JM. Risk factors for 5‐year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585 [DOI] [PubMed] [Google Scholar]
- 29. Mackey RH, Mora S, Bertoni AG, Wassel CL, Carnethon MR, Sibley CT, Goff DC Jr. Lipoprotein particles and incident type 2 diabetes in the multi‐ethnic study of atherosclerosis. Diabetes Care. 2015;38:628–636. doi: 10.2337/dc14-0645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–532. doi: 10.2307/2532940 [DOI] [PubMed] [Google Scholar]
- 31. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. doi: 10.1161/CIRCULATIONAHA.115.018614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kolb H, Kempf K, Rohling M, Lenzen‐Schulte M, Schloot NC, Martin S. Ketone bodies: from enemy to friend and guardian angel. BMC Med. 2021;19:313. doi: 10.1186/s12916-021-02185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4 [DOI] [PubMed] [Google Scholar]
- 34. Luong N, Davies CR, Wessells RJ, Graham SM, King MT, Veech R, Bodmer R, Oldham SM. Activated FOXO‐mediated insulin resistance is blocked by reduction of TOR activity. Cell Metab. 2006;4:133–142. doi: 10.1016/j.cmet.2006.05.013 [DOI] [PubMed] [Google Scholar]
- 35. Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wildenhoff KE, Johansen JP, Karstoft H, Yde H, Sorensen NS. Diurnal variations in the concentrations of blood acetoacetate and 3‐hydroxybutyrate. The ketone body peak around midnight and its relationship to free fatty acids, glycerol, insulin, growth hormone and glucose in serum and plasma. Acta Med Scand. 1974;195:25–28. doi: 10.1111/j.0954-6820.1974.tb08090.x [DOI] [PubMed] [Google Scholar]
- 37. Koeslag JH, Noakes TD, Sloan AW. Post‐exercise ketosis. J Physiol. 1980;301:79–90. doi: 10.1113/jphysiol.1980.sp013190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cahill GF Jr. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258 [DOI] [PubMed] [Google Scholar]
- 39. Nakai N, Sato Y, Oshida Y, Yoshimura A, Fujitsuka N, Sugiyama S, Shimomura Y. Effects of aging on the activities of pyruvate dehydrogenase complex and its kinase in rat heart. Life Sci. 1997;60:2309–2314. doi: 10.1016/s0024-3205(97)00286-5 [DOI] [PubMed] [Google Scholar]
- 40. Kaplon J, Zheng L, Meissl K, Chaneton B, Selivanov VA, Mackay G, van der Burg SH, Verdegaal EM, Cascante M, Shlomi T, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene‐induced senescence. Nature. 2013;498:109–112. doi: 10.1038/nature12154 [DOI] [PubMed] [Google Scholar]
- 41. Pinto A, Bonucci A, Maggi E, Corsi M, Businaro R. Anti‐oxidant and anti‐inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants (Basel). 2018;7(5):63. doi: 10.3390/antiox7050063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D'Agostino D, Planavsky N, Lupfer C, Kanneganti TD, et al. The ketone metabolite beta‐hydroxybutyrate blocks NLRP3 inflammasome‐mediated inflammatory disease. Nat Med. 2015;21:263–269. doi: 10.1038/nm.3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Faris MA, Kacimi S, Al‐Kurd RA, Fararjeh MA, Bustanji YK, Mohammad MK, Salem ML. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr Res. 2012;32:947–955. doi: 10.1016/j.nutres.2012.06.021 [DOI] [PubMed] [Google Scholar]
- 44. Bratic I, Trifunovic A. Mitochondrial energy metabolism and ageing. Biochim Biophys Acta. 2010;1797:961–967. doi: 10.1016/j.bbabio.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 45. Cotter DG, d'Avignon DA, Wentz AE, Weber ML, Crawford PA. Obligate role for ketone body oxidation in neonatal metabolic homeostasis. J Biol Chem. 2011;286:6902–6910. doi: 10.1074/jbc.M110.192369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Post A, Garcia E, van den Berg EH, Flores‐Guerrero JL, Gruppen EG, Groothof D, Westenbrink BD, Connelly MA, Bakker SJL, Dullaart RPF. Nonalcoholic fatty liver disease, circulating ketone bodies and all‐cause mortality in a general population‐based cohort. Eur J Clin Invest. 2021;51:e13627. doi: 10.1111/eci.13627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fischer K, Kettunen J, Wurtz P, Haller T, Havulinna AS, Kangas AJ, Soininen P, Esko T, Tammesoo ML, Magi R, et al. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all‐cause mortality: an observational study of 17,345 persons. PLoS Med. 2014;11:e1001606. doi: 10.1371/journal.pmed.1001606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Koning MLY, Westenbrink BD, Assa S, Garcia E, Connelly MA, van Veldhuisen DJ, Dullaart RPF, Lipsic E, van der Harst P. Association of circulating ketone bodies with functional outcomes after ST‐segment elevation myocardial infarction. J Am Coll Cardiol. 2021;78:1421–1432. doi: 10.1016/j.jacc.2021.07.054 [DOI] [PubMed] [Google Scholar]
- 49. Kang SM, Park JC, Shin MJ, Lee H, Oh J, Ryu DH, Hwang GS, Chung JH. 1H nuclear magnetic resonance based metabolic urinary profiling of patients with ischemic heart failure. Clin Biochem. 2011;44:293–299. doi: 10.1016/j.clinbiochem.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 50. Bedi KC Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133:706–716. doi: 10.1161/CIRCULATIONAHA.115.017545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Kruger M, Hoppel CL, et al. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133:698–705. doi: 10.1161/CIRCULATIONAHA.115.017355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hasselbaink DM, Glatz JF, Luiken JJ, Roemen TH, Van der Vusse GJ. Ketone bodies disturb fatty acid handling in isolated cardiomyocytes derived from control and diabetic rats. Biochem J. 2003;371:753–760. doi: 10.1042/BJ20021617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pelletier A, Tardif A, Gingras MH, Chiasson JL, Coderre L. Chronic exposure to ketone bodies impairs glucose uptake in adult cardiomyocytes in response to insulin but not vanadate: the role of PI3‐K. Mol Cell Biochem. 2007;296:97–108. doi: 10.1007/s11010-006-9303-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S11
Figures S1–S3


