Abstract
Background
The interrelationships between left atrial appendage (LAA) dimensions and device following implantation are unknown. We aimed to analyze the impact of Watchman device implantation on LAA dimensions following its percutaneous closure and potential predictors of remodeling.
Methods and Results
All consecutive LAA closure procedures performed at 2 centers between November 2017 and December 2020 were included in the WATCH‐DUAL (Watchman 2.5 Versus Watchman FLX in a Dual‐Center Left Atrial Appendage Closure Cohort) registry. This study included patients who had pre‐ and postintervention computed tomography scan analysis. The LAA and device dimensions were measured in a centralized core lab by 3‐dimensional computed tomography scan reconstruction methods, focusing on the device landing zone. This analysis included 104 patients (age, 76.0 [range, 72.0–83.0] years; 72% men; 53% Watchman FLX; 47% Watchman 2.5). The baseline characteristics were comparable between Watchman 2.5 and Watchman FLX groups, except for the higher use of oversizing in the latter group. The median delay for computed tomography control was 49 (range, 43–64) days. The landing zone area (median, 446 [range, 363–523] versus 290 [222–366] mm2; P<0.001) and minimal diameter (median, 23.0 [range, 20.7–24.8] versus 16.7 [14.7–19.4] mm; P<0.001) significantly increased after implantation. The absolute (median, 157 [range, 98–220] versus 85 [18–148] mm2, P<0.001) and relative (median, 50% [range, 32%–79%] versus 26% [4%–50%]; P<0.001) increases in landing zone area were more pronounced in patients with oversized device. Baseline LAA dimensions were smaller, landing zone eccentricity larger, and oversized device more frequent in patients with significant overexpansion compared with the others.
Conclusions
LAA dimensions increased at the site of the Watchman prosthesis after implantation, suggesting a local positive remodeling after the procedure. This phenomenon was more pronounced in the case of oversized devices.
Keywords: atrial fibrillation, CT scan, left atrial appendage occlusion
Subject Categories: Catheter-Based Coronary and Valvular Interventions
Nonstandard Abbreviations and Acronyms
- AF
atrial fibrillation
- CT
computed tomography
- LAA
left atrial appendage
- LAAC
left atrial appendage closure
- LZ
landing zone
- NCDR LAAO
National Cardiovascular Data Registry Left Atrial Appendage Registry
- PDL
peridevice leak
- TEE
transesophageal echocardiography
- WATCH‐DUAL
Watchman 2.5 Versus Watchman FLX in a Dual‐Center Left Atrial Appendage Closure Cohort
- WMFLX
Watchman FLX
- WM2.5
Watchman 2.5
Research Perspective.
What Is New?
These data indicate that the left atrial appendage undergoes remodeling after its percutaneous closure, with an increase in appendage dimensions and a decrease in device compression at the site of implantation.
This phenomenon is observed with both generations of Watchman occluder and appears to be more pronounced in the case of oversized devices.
What Question Should Be Addressed Next?
The impact of left atrial appendage remodeling on peridevice leak appearance and long‐term clinical follow‐up remains to be assessed.
Percutaneous left atrial appendage closure (LAAC) has emerged as a valid option for prevention of thromboembolic events in patients with nonvalvular atrial fibrillation (AF) and contraindications for oral anticoagulation. 1 The Watchman 2.5 device (WM2.5; Boston Scientific, Marlborough, MA) was historically the first prosthesis that was evaluated, but newer devices have been developed since then. 2 Randomized studies and large international registries reported high procedural success rates, with low complication risk and favorable clinical efficacy outcomes. 3 , 4 , 5 , 6
Although the procedure is becoming more and more accepted, 6 the data regarding conformability, compression, and device‐related left atrial appendage (LAA) remodeling are scarce. Moreover, the interrelationships between prosthesis and LAA dimensions following implantation are unknown. Whereas some works investigated the impact of percutaneous LAAC on left atrial volume, 7 , 8 , 9 , 10 very few studies focused on LAA. 11 Indeed, potential subsequent LAA remodeling and enlargement might increase the risk of late peridevice leak and favor residual patency following closure. 12 Finally, the impact of device oversizing on LAA subsequent dimensions also remains poorly understood. 13
The aim of the present study was to analyze the impact of percutaneous LAAC on LAA dimensions and potential remodeling following device implantation.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patient Selection
This study included patients with AF treated with percutaneous LAAC enrolled in the WATCH‐DUAL (Watchman 2.5 Versus Watchman FLX in a Dual‐Center Left Atrial Appendage Closure Cohort) registry. The WATCH‐DUAL study was a dual‐center observational cohort including all the LAAC procedures consecutively performed and prospectively collected in local registries at the Bern University Hospital, Bern, Switzerland, and Institut Mutualiste Montsouris, Paris, France, between November 2017 and April 2021. The current analysis included only patients who had both pre‐ and postprocedural computed tomography (CT) scan analysis. The inclusion criteria for patients undergoing LAAC were (1) paroxysmal or persistent/permanent nonvalvular AF with high embolic risk; (2) formal and definitive contraindication to oral anticoagulation therapy or recurrent ischemic event in patients under oral anticoagulation therapy. Exclusion criteria were severe renal failure (creatinine clearance <30 mL/min), pregnancy, age <18 years, inability to consent, LAA too small or too large for percutaneous closure with WM2.5/Watchman FLX (WMFLX) device.
For each patient, the medical history, demographics, comorbidities, clinical and laboratory data, and echocardiographic characteristics were recorded prospectively by patient interview and medical record review. The study was approved by the relevant ethics committee at each center before patient documentation and performed in accordance with the Declaration of Helsinki and its amendments. All patients gave informed consent before inclusion.
LAAC Procedure
All patients underwent WM2.5/WMFLX implantation according to the latest consensus. 14 The procedures have been extensively described elsewhere. 3 , 14 , 15 Briefly, transseptal puncture was performed through the right femoral vein access aiming at the posteroinferior zone, and the left upper pulmonary vein was then catheterized. The 14 French delivery sheath was inserted into the left atrium and advanced subsequently into the LAA. The LAA landing zone (as identified on preintervention CT scan) was measured by 2‐dimensional and real‐time 3‐dimensional transesophageal echocardiography (TEE) methods, 16 , 17 leading to the final choice of the prosthesis size on the basis of the manufacturer's recommendations.
In case 2 devices could be implanted, according to landing zone (LZ) dimensions and the manufacturer abacus (“normal” device or “oversized” device), the final choice was left to the discretion of the first operator (Figure S1). The degree of device oversizing was expressed as the following ratio: device diameter/LZ maximum diameter.
The device was placed in the appropriate position using a combination of TEE and fluoroscopy as recommended. The quality of the deployment (including device compression and stability) and the existence of potential leaks were controlled by 2‐dimensional and 3‐dimensional TEE. A compression rate >10% in all sections of the device on TEE was mandatory to process to prosthesis final release. In case this was not obtained, the device was repositioned in a different position or changed for a larger model.
All patients received 500 to 1500 mL saline infusion before intervention (in order to have a mean left atrial pressure >12 mm Hg) and 100 UI/kg heparin during the procedure. The ACT was monitored during the intervention. The postimplantation antithrombotic therapy was left at the discretion of the operators according to the indication for LAAC and baseline clinical characteristics.
CT Scan Procedures and Analysis
Both pre‐ and postimplantation CTs were performed according to the same protocol and therefore by using either a prospective high‐pitch flash mode or broad coverage single shot/step and shoot ECG‐gated CT acquisition technique typically at the end‐systolic phase. Higher tube voltages were selected to mitigate the potential artifacts related to the presence of the metallic device. Postprocedural CTs were generally performed between 6 and 8 weeks after LAAC in comparable hemodynamics and clinical conditions. Images were reconstructed using iterative reconstruction or filtered back‐projection at 0.75 mm slice width and 0.5 mm slice increment. The pre‐ and postimplantation CT scan images were centrally analyzed by a core lab (Inselspital, Bern) composed of 3 CT experts not involved in the procedure by using a 3‐dimensional reconstruction dedicated software (3mensio, Pie Medical, Netherlands) according to the latest consensus. 17 In the event of inconsistent adjudication, a consensus between all of them was required. The detailed methodology for CT analysis in the core lab and the intra‐/interobserver agreements have been extensively described elsewhere. 18 , 19 The different variables measured on LAA and devices for this subanalysis are illustrated in Figure 1. The LAA morphology classification (cauliflower, chicken‐wing, cactus, windsock) was based on the number of lobes and appendage angulation according to the consensus. The LAA LZ was defined as the cross section of the appendage that was perpendicular to its axis and connected the circumflex artery to a point 1 to 2 cm inside the LAA. 7 The appendage length was the distance between the LZ and the tip of the LAA.
Figure 1. CT scan analysis methodology.
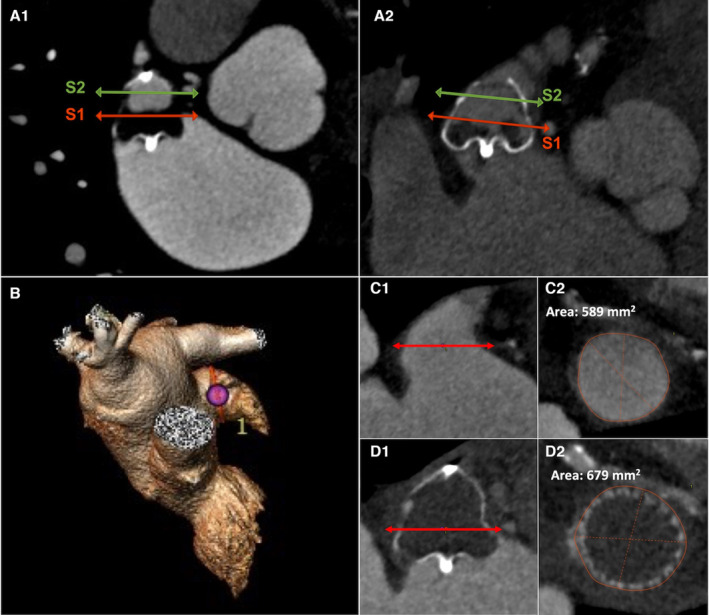
A1 and A2 illustrate the measurements of WMFLX (A1) and WM2.5 (A2) following LAAC using 3‐dimensional CT scan analysis by the 3mensio software. The S1 section (red plane) is located on the device predefined LZ, at the junction of the proximal and mid thirds; the S2 section (green plane) is located at the junction of the device mid and distal thirds. The device length is the distance between initial connector and device tail. B through D describe an example of LZ dimensions measurements before and after a 35‐mm WMFLX device. The LZ was defined as the cross section perpendicular to the axis of the appendage and connecting with the circumflex artery (C1). The LZ area and maximum and minimum diameters were measured to 589 mm2, 28.6 mm, and 26.4 mm, respectively (C2). The postimplantation CT scan (time to procedure, 55 days) displays a correctly positioned device (D1). LZ dimensions increased over time: LZ area was 679 mm2, maximum diameter was 30.8 mm, and minimum diameter was 29.5 mm (D2). The area increase was thus 90 mm2, area expansion was 15.3%, minimum device compression was 12%, and maximum compression was 18.3% at the level of LZ. CT indicates computed tomography; LAAC, left atrial appendage closure; LZ, landing zone; WM2.5, Watchman 2.5; and WMFLX, Watchman FLX.
The implanted device was divided in 3 equal parts/thirds in its long axis for analysis. The S1 plane (junction of the initial and midsection) ideally corresponded to the LZ identified at baseline.
The S2 plane was located at the junction of the mid and distal thirds of the prosthesis. The device length was set as the distance between its proximal connector and its distal extremity. The LZ, S1, and S2 cross sections were analyzed by planimetry, leading to the measurements of the following parameters: minimal diameter, maximal diameter, and section area. The different sections’ eccentricity was defined as the ratio between maximal and minimal diameter. The LZ area variations are expressed as crude variation or percentage: (1) Delta LZ (in mm)=Post LAAC S1 area–Baseline LZ area; and (2) LZ expansion (in %): (Post LAAC S1 area–Baseline LZ area)/Baseline LZ area.
The compression was evaluated in S1 and S2 planes as follows 12 : (1) Maximum compression: 100×(1–[Minimum diameter/Manufacturer device diameter]) (2) Minimum compression: 100×(1–[Maximum diameter/Manufacturer device diameter]).
The presence of LAA patency and the presence of potential leak (Intradevice leak or peridevice leak [PDL]) was assessed according to previously published methods. 18 , 19
Statistical Analysis
All statistical analyses were performed using SPSS 28.0 for MAC (IBM, Chicago, IL). Quantitative variables were described as median (interquartile range) or mean +/−standard error of the mean. Categorical variables were described in terms of counts and percentages. The differences between the variables were compared by the Fisher test for qualitative variables and by the Welch's t‐test (independent group comparison) or Student's paired t‐test (1 group) for quantitative variables. The relationship between baseline and follow‐up LZ area was tested for each device by linear regression analysis. The difference between groups was tested by 1‐way ANCOVA. A P value <0.05 was considered statistically significant.
Results
Baseline Characteristics
A flowchart of the study is provided in Figure S2. A total of 104 patients underwent both pre‐ and postprocedural CT scan and were included in the analysis (48 WM2.5 and 56 WMFLX). The baseline clinical and CT scan characteristics are provided in Tables 1 and 2. There was no significant difference in the clinical profile of the patients, except for a higher proportion of coronary artery disease and myocardial infarction in patients with the WM2.5. The LAA dimensions were comparable between groups. However, there was a significantly higher proportion of chicken‐wing and windsock shape in patients with the WMFLX and WM2.5, respectively. In addition, the device was more frequently oversized in the WMFLX group (Table 2).
Table 1.
Population Baseline Clinical Characteristics
| All patients (n=104) | WM2.5 (n=48) | WMFLX (n=56) | P value | |
|---|---|---|---|---|
| Age, y | 76.0 (72.0–83.0) | 75.5 (71.2–83.0) | 76.5 (72.3–83.5) | 0.90 |
| Male sex, n (%) | 72 (69) | 36 (75) | 36 (64) | 0.24 |
| Cardiovascular risk factors and history | ||||
| Hypertension, n (%) | 86 (83) | 40 (83) | 46 (82) | 0.87 |
| Diabetes, n (%) | 29 (28) | 10 (21) | 19 (34) | 0.14 |
| Coronary artery disease, n (%) | 41 (39) | 26 (54) | 15 (27) | 0.004 |
| Myocardial infarction, n (%) | 17 (16) | 12 (25) | 5 (9) | 0.03 |
| Previous stroke, n (%) | 32 (31) | 17 (35) | 15 (27) | 0.34 |
| Atrial fibrillation type | ||||
| Paroxysmal AF, n (%) | 59 (56) | 28 (58) | 31 (55) | 0.76 |
| Permanent or persistent AF, n (%) | 45 (44) | 20 (42) | 25 (45) | 0.76 |
| LVEF (%) | 60 (51–65) | 60 (53–65) | 60 (47–65) | 0.19 |
| CHA2DS2‐VASc score | 4 (3–5) | 4 (3–5) | 4 (3–5) | 0.77 |
| HAS‐BLED score | 3 (2–4) | 3 (2–4) | 3 (2–3) | 0.12 |
P value is for comparison between patients with WMFLX and patients with WM2.5. Continuous variables are expressed as median (interquartile range). AF indicates atrial fibrillation; LVEF, left ventricular ejection fraction; WM2.5, Watchman 2.5; and WMFLX, Watchman FLX.
Table 2.
Pre‐ and Postintervention CT Scan Characteristics
| All patients (n=104) | WM 2.5 (n=48) | WMFLX (n=56) | P value | |
|---|---|---|---|---|
| Preimplantation CT scan | ||||
| LAA length, mm | 38.9 (34.0–42.9) | 38.0 (32.2–42.9) | 39.3 (36.0–42.8) | 0.12 |
| Landing zone maximum diameter, mm | 22.2 (18.9–25.2) | 22.2 (18.9–25.2) | 22.0 (18.7–25.3) | 0.90 |
| Landing zone minimum diameter, mm | 16.8 (14.4–19.4) | 17.3 (14.6–19.1) | 16.7 (14.3–19.9) | 0.73 |
| Landing zone eccentricity | 1.28 (1.18–1.43) | 1.30 (1.21–1.44) | 1.24 (1.17–1.40) | 0.45 |
| Landing zone area, mm2 | 290 (222–366) | 320 (221–374) | 279 (22é‐355) | 0.98 |
| Chicken–wing morphology, n (%) | 25 (24) | 6 (13) | 19 (34) | 0.01 |
| Windsock morphology, n (%) | 58 (56) | 33 (67) | 25 (45) | 0.01 |
| Cauliflower morphology, n (%) | 11 (11) | 4 (8) | 7 (13) | 0.49 |
| Cactus morphology, n (%) | 10 (10) | 5 (10) | 5 (9) | P>0.99 |
| Device diameter, mm | 27 (24–31) | 27 (24–27) | 27 (27–31) | 0.02 |
| Oversizing degree, mm | 4.8 (2.7–7.2) | 3.6 (1.8–5.4) | 6.2 (3.9–8.9) | <0.001 |
| Oversized prosthesis, n (%) | 37 (36) | 8 (17) | 29 (52) | <0.001 |
| CT scan/LAAC procedure delay, d | 49 (43–64) | 48 (43–69) | 50 (44–64) | 0.18 |
| Postimplantation CT scan | ||||
| Device section 1/landing zone | ||||
| Landing zone maximum diameter, mm | 25.1 (22.2–27.0) | 23.7 (20.8–26.2) | 25.7 (22.9–27.8) | 0.03 |
| Landing zone minimum diameter, mm | 23.1 (20.5–24.8) | 22.2 (19.4–23.8) | 24.0 (21.7–26.2) | 0.008 |
| S1 plane eccentricity | 1.06 (1.04–1.09) | 1.08 (1.05–1.11) | 1.05 (1.03–1.08) | 0.11 |
| S1/landing zone area, mm2 | 446 (363–523) | 401 (314–482) | 486 (395–548) | 0.01 |
| Device section 2 | ||||
| Maximum diameter, mm | 20.4 (17.8–23.8) | 18.2 (16.1–20.4) | 22.5 (20.0–25.8) | <0.001 |
| Minimum diameter, mm | 18.2 (15.4–21.8) | 15.5 (13.7–17.7) | 21.3 (18.7–23.6) | <0.001 |
| S2 plane eccentricity | 1.08 (1.05–1.17) | 1.14 (1.08–1.24) | 1.06 (1.05–1.09) | <0.001 |
| S2 area, mm2 | 291 (225–405) | 233 (171–273) | 372 (297–454) | <0.001 |
| Device length, mm | 20.7 (17.9–23.7) | 20.7 (18.3–22.3) | 21.1 (17.8–24.0) | 0.42 |
| LAA patency, n (%) | 58 (56) | 28 (58) | 30 (52) | 0.63 |
| Intradevice leak, n (%) | 29 (30) | 18 (38) | 11 (19) | 0.05 |
| Peridevice leak, n (%) | 39 (37) | 21 (44) | 18 (31) | 0.22 |
Continuous variables are expressed as median (interquartile range). AF indicates atrial fibrillation; CT, computed tomography; LAA, left atrial appendage; LAAC, left atrial appendage closure; WM2.5, Watchman 2.5; and WMFLX, Watchman FLXc.
Postimplantation LZ Dimensions
The postprocedural LAA dimensions are provided in Table 2. The LZ area and maximal and minimal diameter significantly increased after implantation (Figure 2A through 2C). Absolute and relative increase of LZ area were 114% (40%–184%) mm2 and 37% (8%–61%), respectively. A total of 88% of patients had an enhanced LZ area in the whole cohort. We observed a reduction in LAA eccentricity following implantation (Figure 2D): The ratio between LZ maximal and minimal diameter significantly decreased for all patients (R=1.29 [1.18–1.43] versus 1.06 [1.05–1.09]; P<0.001). The LZ dimension enlargement was significantly greater in patients who underwent oversized device implantation (n=37) compared with the others (n=67), as assessed by the absolute (157 [98–220] versus 85 [18–148] mm2; P<0.001) and relative (50% [32%–79%] versus 26% [4%–50%]; P<0.001) increases in LZ area (Figure 3A through 3E). We did not observe any statistically significant association between LAA shape and postimplantation LZ dimension increase or eccentricity (Table S1). In addition, the patients with permanent/persistent AF displayed larger LAA dimensions at baseline, but there was no evidence of significant larger remodeling according to the LZ area relative increase (Table S2). Finally, we also observed that the variations in LZ area and dimensions were more pronounced in the WMFLX group (Figures S3 and S4).
Figure 2. LAA LZ dimensions and eccentricity measured by CT scan before and after percutaneous closure procedure.
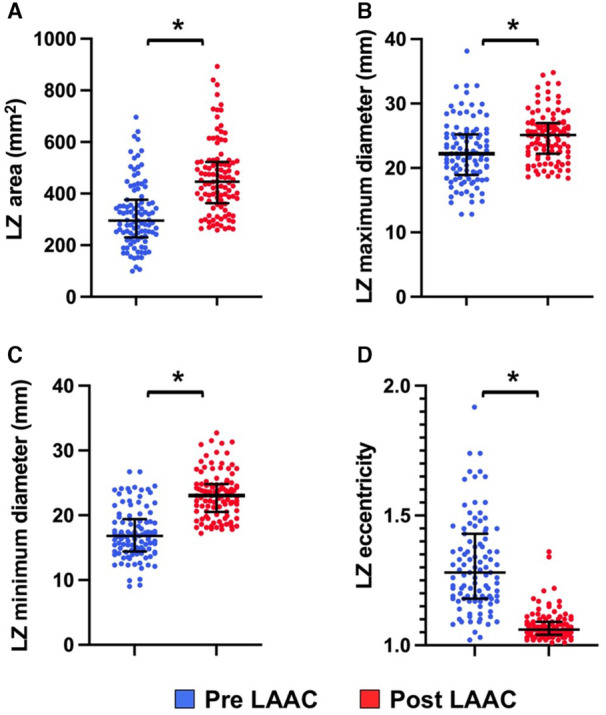
A, LZ area (mm2) values before and after LAAC; B, LZ maximum diameter values before and after LAAC; C, LZ minimum diameter values before and after LAAC; D, LZ eccentricity values before and after LAAC. CT indicates computed tomography; LAA, left atrial appendage; LAAC, left atrial appendage closure; and LZ, landing zone. *P<0.01.
Figure 3. Variations of LZ dimensions before and after LAAC (A through E) and device compression (F) according to the existence of an initial prosthesis oversizing.
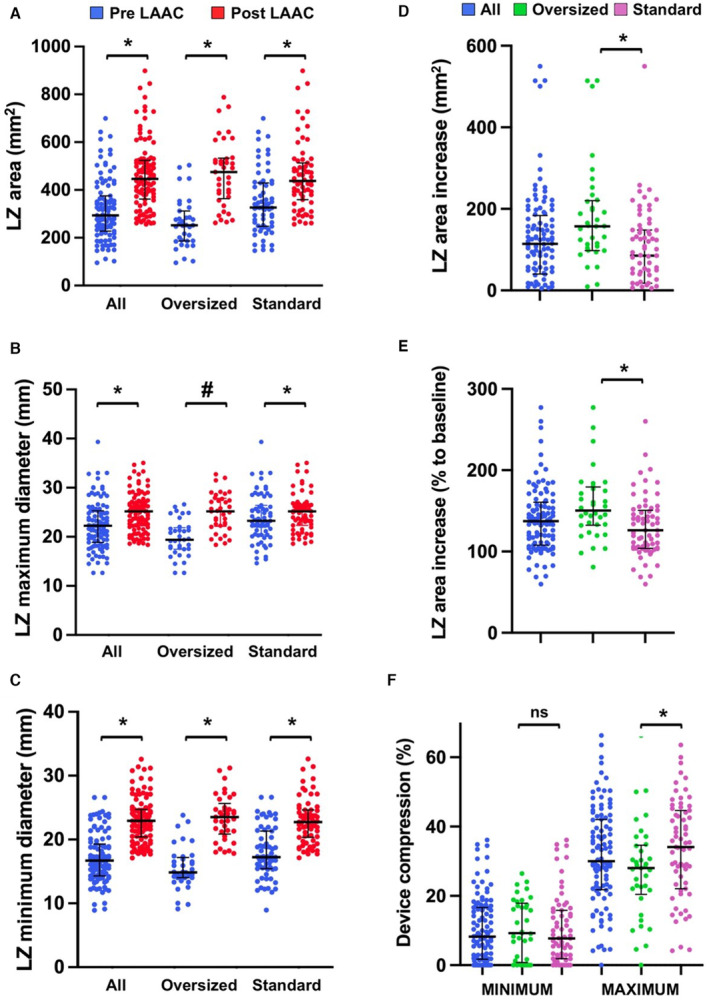
LAAC indicates left atrial appendage closure; LZ, landing zone; and ns, non significant. *P<0.01; #P<0.05.
Postimplantation Dimensions Within the Device
We observed differences in final device dimensions between proximal S1 and distal S2 sections (Table 2). The S1 area was significantly larger than the S2 area in all patients (446 [363–523] versus 291 [225–405]; P<0.001) and in both WM2.5 (401 [314–482] versus 233 [171–273]; P<0.001) and WMFLX (486 [395–548] versus 372 [297–454]; P<0.001) groups. However S1 area‐S2 area was larger in patients with the WM2.5 compared with patients with the WMFLX (187 [138–228] versus 100 [69–121] mm2; P<0.001). In addition, we also observed that S2 eccentricity was more pronounced in patients with the WM2.5 compared with patients with the WMFLX (1.14 [1.08–1.24] versus 1.06 [1.05–1.09]; P<0.001) and that S2 was significantly more eccentric than S1 in the WM2.5 group (1.14 [1.08–1.24] versus 1.08 [1.05–1.11]; P<0.001) but not in the WMFLX group (1.06 [1.05–1.09] versus 1.05 [1.03–1.08]; P=0.13).
Postimplantation Device Compression and PDL
The compression rate of the prostheses was highly variable (Table 2). The median minimum and maximum compression rates were 8.2% (1.7%–16.6%) and 30.0% (21.8%–42.0%), respectively. The minimum compression rate was identified in the landing zone/S1 area in all cases. The maximum compression rate was lower with patients with an initial oversized device compared with the others (28.0% [20.5%–34.6%] versus 34.1% [22.1%–44.7%]; P<0.01), yet no significant difference was observed in minimal compression (9.3% [1.0%–17.9%] versus 7.7% [1.9%–15.8%]; P=0.5) (Figure 3F).
The minimal device compression rate was <10% in 82 patients (64%) on control CT scan, whereas all patients had a minimum device compression >10% on periprocedural TEE. Altogether, these data suggested an LAA remodeling around the device following intervention and leading to less prosthesis compression. We observed that the PDL rate was higher on follow‐up CT scan compared with the periprocedural PDL incidence observed by TEE (37% versus 5%; P≤0.001).
We did not observe any significant difference in device compressions and LZ dimensions increase between patients with and without PDL, but we identified lower compression in patients with residual LAA patency compared with those without (Data S1, Table S3). However, we did not observe a significant difference in PDL incidence in patients with “oversized” device versus the others (27% versus 43%, respectively; P=0.1) and between the different LAA morphologies (Data S1).
Factors Associated With Significant LZ Overexpansion
Table 3 displays the differences in selected variables of interest among patients with or without LZ area expansion >10%. We observed that the baseline LAA dimensions were smaller and LZ eccentricity was larger in patients with significant overexpansion compared with the others. In addition, oversized Watchman devices were more frequently implanted in patients with subsequent significant overexpansion.
Table 3.
Characteristics of Patients With or Without LZ Area Expansion >10%
| LZ area expansion >10% (n=76) | LZ area expansion <10% (n=28) | P value | |
|---|---|---|---|
| Age, per y | 76 (71–83) | 77 (73–83) | 0.23 |
| Diabetes, n (%) | 23 (30) | 6 (21) | 0.46 |
| Permanent AF, n (%) | 32 (42) | 13 (46) | 0.82 |
| LVEF, % | 60 (50–65) | 60 (52–65) | 0.53 |
| LAA windsock morphology, n (%) | 39 (51) | 19 (68) | 0.18 |
| Baseline LZ eccentricity | 1.27 (1.18–1.38) | 1.18 (1.11–1.21) | 0.01 |
| LAA length, per mm | 38.0 (33.4–41.3) | 41.6 (34–46.5) | 0.02 |
| LZ baseline maximum diameter, mm | 21.3 (18.2–24.7) | 23.5 (20.9–28.1) | 0.01 |
| LZ baseline minimum diameter, mm | 15.9 (14.0–18.9) | 18.1 (16.5–22.5) | 0.001 |
| Device diameter, per mm | 27 (24–31) | 27 (24–30) | 0.61 |
| Oversized device, n (%) | 32 (42) | 5 (18) | 0.02 |
| WMFLX, n (%) | 45 (59) | 11 (39) | 0.08 |
| Delay between LAAC and CT scan, d | 48 (43–62) | 57 (43–85) | 0.24 |
Continuous variables are expressed as median (interquartile range). AF indicates atrial fibrillation; CT, computed tomography; LAA, left atrial appendage; LAAC, left atrial appendage closure; LZ, landing zone; WM2.5, Watchman 2.5; and WMFLX, Watchman FLX.
Discussion
The main results of the present study are (1) the dimensions of LAA increase following its closure, suggesting a positive remodeling phenomenon; (2) the prosthesis compression rate decreases after the procedure; and (3) this phenomenon appears more pronounced in the case of oversized devices.
LAAC procedures aim to exclude the LAA cavity from the circulation by occluding its ostium. The stability of the single‐lobe occluders (such as Watchman) is obtained by combining 2 factors: lateral hooks anchoring the prosthesis to LAA walls and the device radial strength that is created by its compression within the appendage. 20 However, the dynamic interplay between the implanted device expansion and the surrounding tissue compliance is not fully understood. Hence, the data regarding the short‐ and long‐term LAA dimensions variations are scarce. 21 In the present series, we observed a significant increase in the LAA LZ dimensions over a 6‐ to 8‐week period, suggesting that the prosthesis expansion, resulting from its initial compression within the chamber and the nitinol inherent physical properties, was greater than the resistance opposed by the appendage wall. Whether this local “positive remodeling” (as a reference to the positive Glagov's remodeling phenomenon observed in atherosclerotic coronary arteries that leads to the increase of their dimensions over time 22 ) is related to local tissue stretching or involves structural modifications in the atrial wall remains unknown. The deferred increase in LZ dimensions that we observed with the endovascular Watchman devices might explain the appearance of late‐acquired peridevice leak that are observed after up to 35% of LAAC cases in this series and in the literature. 12 , 23 , 24 However, the clinical relevance of these residual leaks remains to be clarified. 19 , 24 Interestingly, different results were observed in patients with percutaneous LAA ligation, in which the LAA size is reduced and the myocardium is atrophied. 21 These discrepancies could be explained by the different mechanisms used by the 2 devices to obtain the cavity exclusion. Hence, the use of LARIAT involves LAA neck strangulation (which occludes the epicardial blood vessels supplying blood flow to the LAA myocardium) without leaving a remnant foreign body within the appendage.
Our data show that the remodeling was not homogeneous among the patients and within the device. The phenomenon was influenced by several factors and appeared to be more pronounced in the proximal part of the device. This remodeling could also afford for the eccentricity reduction in elliptical LAA and might also explain the reduction in Watchman compression on follow‐up CT scan. Hence, the majority of our patients displayed a minimal compression below the requested 10% on control CT scan. These observations are in line with previous preliminary reports 11 , 25 and involve both generations of Watchman devices. The remodeling was more pronounced and the compression lower in the proximal part of both devices, which is probably explained by LAA anatomic features (that frequently exhibit larger neck compared with the tail). Interestingly, the device minimal compression was lower in the case of patent LAA on control CT scan, suggesting that a certain degree of residual compression should be obtained to get an optimal occlusive result.
We observed that the implantation of an oversized device (according to the manufacturer's instructions) was associated with a larger increase in LZ dimensions. Single‐lobe appendage occluders display variable radial strength according to their degree of compression, with the highest outward force values observed for the most compressed prosthesis. 20 The implantation of an oversized device is related to several factors, including the operator's experience and skill, as well as anatomic factors. 13 Recently published data from the NCDR LAAO (National Cardiovascular Data Registry Left Atrial Appendage Registry) show that this strategy was frequently used in patients with WM2.5 implantation and tended to increase over time. Oversizing was not associated with increased rate of periprocedural adverse events. 13 Interestingly, we observed in the present series that the percentage of oversized devices was lower in the WM2.5 group compared with the other, which could be explained by the differences in manufacturer recommendations for sizing between prostheses (Figure S1). These discrepancies are related to the greater radial and chronic outward forces displayed by the WMFLX and the higher optimal target compression with the WMFLX (10%–30%) compared with the WM2.5 (8%–20%). This more liberal use of oversizing in WMFLX implantation probably supports the larger remodeling we observed in this subgroup of patients. Whether the modifications in the latter prosthesis design (closed‐end design, reduced height and higher number of open frame cells that enhance the “stored energy” restitution phenomenon) could play a role in the remodeling remains unknown 11 but might also explain the differences in the distal sections of WMFLX and WM2.5 dimensions we observed.
Study Limitations
This study is a retrospective analysis with a limited number of patients. There was no randomization between the WM2.5 and WMFLX devices. We included only patients who underwent pre‐ and post‐LAAC CT scan analysis, which might have created a patient selection. The rhythm status of the patient with paroxysmal AF was not collected at the time of the pre and postintervention CT scans, which might have affected the results. The implantation depth of the device was not analyzed in a standardized fashion. The study included only WM devices, and the translation of the results to other occluders has to be determined. Moreover, this analysis focused on imaging end points, and there was no correlation between findings and long‐term clinical follow‐up. Finally, the follow‐up duration was relatively short, and the persistence of the positive remodeling phenomenon over time remains to be determined.
In conclusion, this study shows that LAA dimensions increased at the site of WM prosthesis implantation, suggesting a local positive appendage remodeling after percutaneous LAAC. This phenomenon was observed with the different generations of WM devices and appears to be more pronounced in the case of oversized prostheses. The potential clinical impact of this observation needs to be determined.
Sources of Funding
None.
Disclosures
Dr Räber received research grants to the institution from Abbott, Biotronik, BostonScientific, Heartflow, Sanofi, and Regeneron and consultation fees from Abbott, Amgen, AstraZeneca, Canon, Medtronic, NovoNordisk, Occlutech, and Sanofi. Dr Amabile received institutional research grants from Abbott; consulting fees from Abbott, Boston Scientific, and Shockwave Medical; and proctoring fees from Abbott and Boston Scientific. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S4
Figures S1–S4.
This manuscript was sent to Kevin F. Kwaku, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030037
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 2. Doshi SK, Kar S, Sadhu A, Horton R, Osorio J, Ellis C, Stone J, Shah M, Dukkipati SR, Adler S, et al. Two‐year outcomes with a next‐generation left atrial appendage device: final results of the PINNACLE FLX trial. J Am Heart Assoc. 2023;12:e026295. doi: 10.1161/JAHA.122.026295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non‐inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X [DOI] [PubMed] [Google Scholar]
- 4. Reddy VY, Doshi SK, Kar S, Gibson DN, Price MJ, Huber K, Horton RP, Buchbinder M, Neuzil P, Gordon NT, et al. 5‐year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964–2975. doi: 10.1016/j.jacc.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 5. Boersma LVA, Schmidt B, Betts TR, Sievert H, Tamburino C, Teiger E, Pokushalov E, Kische S, Schmitz T, Stein KM, et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri‐procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37:2465–2474. doi: 10.1093/eurheartj/ehv730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collado FMS, Buchwald CML, Anderson CK, Madan N, Suradi HS, Huang HD, Jneid H, Kavinsky CJ. Left atrial appendage occlusion for stroke prevention in nonvalvular atrial fibrillation. J Am Heart Assoc. 2021;10:e022274. doi: 10.1161/JAHA.121.022274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Phan QT, Shin SY, Cho IS, Lee WS, Won H, Sharmin S, Lee DY, Kim TH, Kim CJ, Kim SW. Impact of left atrial appendage closure on cardiac functional and structural remodeling: a difference‐in‐difference analysis of propensity score matched samples. Cardiol J. 2019;26:519–528. doi: 10.5603/CJ.a2018.0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li YG, Gong CQ, Zhao MZ, Sun J, Wang QS, Zhang PP, Feng XF, Yu Y, Yu YC, Liang BE. Determinants of postoperative left atrial structural reverse remodeling in patients undergoing combined catheter ablation of atrial fibrillation and left atrial appendage closure procedure. J Cardiovasc Electrophysiol. 2019;30:1868–1876. doi: 10.1111/jce.14094 [DOI] [PubMed] [Google Scholar]
- 9. Luani B, Groscheck T, Genz C, Tanev I, Rauwolf T, Herold J, Medunjanin S, Schmeisser A, Braun‐Dullaeus RC. Left atrial enlargement and clinical considerations in patients with or without a residual interatrial shunt after closure of the left atrial appendage with the WATCHMAN™‐device. BMC Cardiovasc Disord. 2017;17:294. doi: 10.1186/s12872-017-0728-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jalal Z, Iriart X, Dinet ML, Corneloup O, Pillois X, Cochet H, Thambo JB. Evaluation of left atrial remodelling following percutaneous left atrial appendage closure. J Geriatr Cardiol. 2017;14:496–500. doi: 10.11909/j.issn.1671-5411.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chow DH, Bieliauskas G, Sawaya FJ, Millan‐Iturbe O, Kofoed KF, Sondergaard L, De Backer O. A comparative study of different imaging modalities for successful percutaneous left atrial appendage closure. Open Heart. 2017;4:e000627. doi: 10.1136/openhrt-2017-000627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qamar SR, Jalal S, Nicolaou S, Tsang M, Gilhofer T, Saw J. Comparison of cardiac computed tomography angiography and transoesophageal echocardiography for device surveillance after left atrial appendage closure. EuroIntervention. 2019;15:663–670. doi: 10.4244/EIJ-D-18-01107 [DOI] [PubMed] [Google Scholar]
- 13. Sandhu A, Varosy PD, Du C, Aleong RG, Tumolo AZ, West JJ, Tzou WS, Curtis JP, Freeman JV, Friedman DJ, et al. Device‐sizing and associated complications with left atrial appendage occlusion: findings from the NCDR LAAO registry. Circ Cardiovasc Interv. 2022;15:e012183. doi: 10.1161/CIRCINTERVENTIONS.122.012183 [DOI] [PubMed] [Google Scholar]
- 14. Glikson M, Wolff R, Hindricks G, Mandrola J, Camm AJ, Lip GYH, Fauchier L, Betts TR, Lewalter T, Saw J, et al. EHRA/EAPCI expert consensus statement on catheter‐based left atrial appendage occlusion–an update. EP Europace. 2019;22:184. doi: 10.1093/europace/euz258 [DOI] [Google Scholar]
- 15. Cruz‐Gonzalez I, Korsholm K, Trejo‐Velasco B, Thambo JB, Mazzone P, Rioufol G, Grygier M, Möbius‐Winkler S, Betts T, Meincke F, et al. Procedural and short‐term results with the new Watchman FLX left atrial appendage occlusion device. JACC Cardiovasc Interv. 2020;13:2732–2741. doi: 10.1016/j.jcin.2020.06.056 [DOI] [PubMed] [Google Scholar]
- 16. Faletra FF, Saric M, Saw J, Lempereur M, Hanke T, Vannan MA. Imaging for patients selection and guidance of LAA and ASD percutaneous and surgical closure. JACC Cardiovasc Imaging. 2021;14:3–21. doi: 10.1016/j.jcmg.2019.06.032 [DOI] [PubMed] [Google Scholar]
- 17. Donal E, Lip GYH, Galderisi M, Goette A, Shah D, Marwan M, Lederlin M, Mondillo S, Edvardsen T, Sitges M, et al. EACVI/EHRA expert consensus document on the role of multi‐modality imaging for the evaluation of patients with atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2016;17:355–383. doi: 10.1093/ehjci/jev354 [DOI] [PubMed] [Google Scholar]
- 18. Galea R, Mahmoudi K, Gräni C, Elhadad S, Huber AT, Heg D, Siontis GCM, Brugger N, Sebag F, Windecker S, et al. Watchman FLX versus Watchman 2.5 in a dual‐center left atrial appendage closure cohort: the WATCH‐DUAL study. Europace. 2022;24:1441–1450. doi: 10.1093/europace/euac021 [DOI] [PubMed] [Google Scholar]
- 19. Galea R, De Marco F, Meneveau N, Aminian A, Anselme F, Gräni C, Huber AT, Teiger E, Iriart X, Babongo Bosombo F, et al. Amulet or Watchman device for percutaneous left atrial appendage closure: primary results of the SWISS‐APERO randomized clinical trial. Circulation. 2022;145:724–738. doi: 10.1161/CIRCULATIONAHA.121.057859 [DOI] [PubMed] [Google Scholar]
- 20. Menne MF, Schrickel JW, Nickenig G, Al‐Kassou B, Nelles D, Schmitz‐Rode T, Steinseifer U, De Backer O, Sedaghat A. Mechanical properties of currently available left atrial appendage occlusion devices: a bench‐testing analysis. Artif Organs. 2019;43:656–665. doi: 10.1111/aor.13414 [DOI] [PubMed] [Google Scholar]
- 21. Kreidieh B, Rojas F, Schurmann P, Dave AS, Kashani A, Rodríguez‐Mañero M, Valderrábano M. Left atrial appendage remodeling after Lariat left atrial appendage ligation. Circ Arrhythm Electrophysiol. 2015;8:1351–1358. doi: 10.1161/CIRCEP.115.003188 [DOI] [PubMed] [Google Scholar]
- 22. Korshunov VA, Schwartz SM, Berk BC. Vascular remodeling. Arterioscler Thromb Vasc Biol. 2007;27:1722–1728. doi: 10.1161/ATVBAHA.106.129254 [DOI] [PubMed] [Google Scholar]
- 23. Glassy MS, Wung W, Westcott S, Smith TWR, Fan D, Rogers JH, Singh GD. Watchman occlusion in long‐standing persistent atrial fibrillation: larger left atrial appendages with greater residual leak. JACC Cardiovasc Interv. 2019;12:1018–1026. doi: 10.1016/j.jcin.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 24. Afzal MR, Gabriels JK, Jackson GG, Chen L, Buck B, Campbell S, Sabin DF, Goldner B, Ismail H, Liu CF, et al. Temporal changes and clinical implications of delayed peridevice leak following left atrial appendage closure. Clinical Electrophysiol. 2022;8:15–25. [DOI] [PubMed] [Google Scholar]
- 25. Korsholm K, Samaras A, Andersen A, Jensen JM, Nielsen‐Kudsk JE. The Watchman FLX device: first European experience and feasibility of intracardiac echocardiography to guide implantation. JACC Clin Electrophysiol. 2020;6:1633–1642. doi: 10.1016/j.jacep.2020.06.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S4
Figures S1–S4.


