Abstract
Background
Overweight and obesity are associated with adverse functional outcomes in people with peripheral artery disease (PAD). The effects of weight loss in people with overweight/obesity and PAD are unknown.
Methods
The PROVE (Promote Weight Loss in Obese PAD Patients to Prevent Mobility Loss) Trial is a multicentered randomized clinical trial with the primary aim of testing whether a behavioral intervention designed to help participants with PAD lose weight and walk for exercise improves 6‐minute walk distance at 12‐month follow‐up, compared with walking exercise alone. A total of 212 participants with PAD and body mass index ≥25 kg/m2 will be randomized. Interventions are delivered using a Group Mediated Cognitive Behavioral intervention model, a smartphone application, and individual telephone coaching. The primary outcome is 12‐month change in 6‐minute walk distance. Secondary outcomes include total minutes of walking exercise/wk at 12‐month follow‐up and 12‐month change in accelerometer‐measured physical activity, the Walking Impairment Questionnaire distance score, and the Patient‐Reported Outcomes Measurement Information System mobility questionnaire. Tertiary outcomes include 12‐month changes in perceived exertional effort at the end of the 6‐minute walk, diet quality, and the Short Physical Performance Battery. Exploratory outcomes include changes in gastrocnemius muscle biopsy measures of mitochondrial cytochrome C oxidase activity, mitochondrial biogenesis, capillary density, and inflammatory markers.
Conclusions
The PROVE randomized clinical trial will evaluate the effects of exercise with an intervention of coaching and a smartphone application designed to achieve weight loss, compared with exercise alone, on walking performance in people with PAD and overweight/obesity. Results will inform optimal treatment for the growing number of patients with PAD who have overweight/obesity.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04228978.
Keywords: eating behavior, home‐based exercise, mitochondria, obesity, peripheral artery disease, physical function, randomized controlled trial
Subject Categories: Obesity, Exercise, Peripheral Vascular Disease
Nonstandard Abbreviations and Acronyms
- EX
exercise intervention
- GOALS
Group‐Oriented Arterial Leg Study
- ICC
interclass correlation
- LITE
Low Intensity Exercise Intervention in PAD
- PROVE
Promote Weight Loss in Obese PAD Patients to Prevent Mobility Loss
- WL+EX
weight loss plus exercise intervention
Lower extremity peripheral artery disease (PAD) affects >230 million adults worldwide, 1 , 2 and causes significant walking impairment. 2 Walking exercise is first‐line therapy to improve walking performance in PAD. 3 Individuals with PAD and overweight or obesity have greater functional impairment and faster functional decline than people with PAD and normal weight. 4 , 5 , 6 Rates of obesity are increasing nationally 7 and ≈65% of people with PAD have overweight or obesity. 8 , 9 In animals, obesity was associated with impaired limb perfusion 10 and in humans, obesity was associated with reduced skeletal muscle mitochondrial biogenesis and activity. 11 However, the effects of intentional weight loss in people with PAD who have overweight or obesity are unknown. A combined weight loss and exercise intervention may improve walking performance if weight loss improves adherence to exercise activity by making walking exercise easier, increasing calf perfusion, and improving calf mitochondrial activity and inflammation. However, weight loss may not be beneficial if weight loss exacerbates PAD‐related sarcopenia or if the requirements of the weight loss program interfere with adherence to walking exercise. 12
The PROVE (Promote Weight Loss in Obese PAD Patients to Prevent Mobility Loss) Trial was designed to compare an intervention intended to achieve weight loss combined with walking exercise to a walking exercise intervention alone. The weight loss intervention and the walking exercise intervention incorporated behavioral change methods. The primary outcome is change in 6‐minute walk distance at 12‐month follow‐up. This article describes the design and methods for the PROVE Trial.
Methods
The PROVE Trial was reviewed and approved by a single Institutional Review Board through Northwestern University. All participants provide written informed consent. This clinical trial is single‐blind, multicentered, and conducted at Northwestern University (Chicago, IL), the University of Minnesota (Minneapolis, MN), and Tulane University (New Orleans, LA), with the Data Coordinating Center at Wake Forest University School of Medicine (Winston‐Salem, NC). After trial completion, anonymized data and materials will be made publicly available at BioLINCC.
Participant Identification
Patients with established PAD or vascular laboratory testing consistent with PAD are contacted via mailed letters, messaging through patient portals, and referrals from vascular specialists. Brochures, flyers, or posters are displayed in relevant clinics. Postcards advertising the study are mailed to people aged 50 years and older in the communities where the trial is conducted. Advertisements on buses and trains in Chicago, Minneapolis, and New Orleans are used to advertise the clinical trial. Field center investigators also identify potential participants from lists of people with PAD who participated in previous studies and expressed interest in future research. Initial eligibility screening occurs by telephone (Table 1).
Table 1.
Initial Telephone Screening Questions
| Section 1: Demographics |
|
|
|
|
|
| Section 2: PAD |
|
|
|
|
|
| Section 3: Interest |
|
| Section 4: Inclusion/exclusion |
|
|
|
| Section 5: Physical activity |
|
| If yes, on average, how many days per week do you walk for exercise? |
| If yes, on average, how many minutes per day do you walk for exercise? |
| Section 6: Equipment |
|
| If no, are you willing to learn how to use a smartphone to record your diet if one is provided to you during the study? |
| If yes, please provide the type of device and operating system or version. |
| Section 7: Alcohol use |
|
| If yes, how many drinks do you have a week? |
| Section 8: Self‐report inclusion/exclusion criteria |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| If yes, how many pounds? |
| Section 9: Additional information |
|
|
| If yes, has the foot pain gotten worse in the past month? |
|
|
|
Inclusion and Exclusion Criteria
Inclusion and exclusion criteria are intended to maximize both eligibility and generalizability of the randomized sample (Table 2). Eligible participants are people with PAD who have either overweight or obesity. Participants with overweight (ie, body mass index [BMI] 25–29.9 kg/m2) were included for the following reasons. First, prior work showed that among people with PAD, annual declines in 6‐minute walk distance increased across BMI categories of normal BMI, overweight, and obesity. 4 Second, prior work showed a trend across BMI categories of normal BMI, overweight, and obesity with regard to the degree of fatty infiltration of calf skeletal muscle in people with PAD. 6 Third, including participants with either overweight or obesity increases generalizability of the results. Fourth, including participants who are overweight was expected to assist with recruitment. Patients who report treatment for overweight or obesity in the past 6 months are excluded (Table 2). However, patients taking drugs associated with mild‐to‐modest weight loss such as metformin or glucagon‐like peptide receptor agonists to treat diabetes who report no recent treatment for weight loss are not excluded. Data on medication use at baseline and follow‐up is recorded by investigators.
Table 2.
Inclusion and Exclusion Criteria
| Inclusion criteria | Rationale |
|---|---|
|
Accepted criteria for PAD in clinical practice guidelines. 3 |
|
Overweight and obesity are defined as a BMI of 25–29 kg/m2 and >30 kg/m2. Observational evidence demonstrated a linear trend in association of normal BMI, overweight, and obesity with the magnitude of annual decline in 6‐minute walk distance among people with PAD. 4 |
|
PAD is rare in children |
| Exclusion criteria | Rationale |
|
These criteria characterize severe or end stage PAD. Most individuals with PAD do not have severe/end stage PAD. |
|
The WL+EX intervention is designed to improve walking impairment caused by PAD. |
|
These individuals may not adhere to study requirements. |
|
These procedures may influence functional performance, independent of study interventions. |
|
These conditions may interfere with the ability to fully participate in and complete the study. |
|
May interfere with ability to fully adhere to the study intervention or accurately complete questionnaires. |
|
These individuals have morbid obesity that requires more intensive treatment than our intervention provides. |
|
The WL+EX intervention is not intended for individuals with an eating disorder. Patients with recent weight loss may have weight change independently of the study interventions. |
|
These individuals will not be able to fully engage in the intervention. |
|
These individuals may not be able to lose weight. |
|
These programs may influence functional performance, independent of study interventions. |
ABI indicates ankle brachial index; BMI, body mass index; PAD, peripheral artery disease; TBI, toe brachial index; and WL+EX, weight loss plus exercise.
Symptomatic PAD will be defined as leg symptoms associated with exertion that resolved within 10 minutes of rest. The presence of symptomatic PAD will be determined based on the claudication questionnaire, the 6‐minute walk, or principal investigator interview/discussion with the potential participant.
The run‐in period can be extended to assist participants with learning the app, but participants must demonstrate ability to enter at least 800 kcal per day for at least 5 days of the run‐in period.
Potential participants may still qualify if they have had treatment for an early‐stage cancer in the past 2 years and the prognosis is excellent.
After completing a stem cell or gene therapy intervention, participants will become eligible after the final study follow‐up visit of the stem cell or gene therapy study so long as at least 6 months have passed since the final intervention administration. After completing a supplement or drug therapy (other than stem cell or gene therapy), participants will be eligible after the final study follow‐up visit provided at least 3 months have passed since the final intervention of the trial.
The ankle brachial index (ABI) is performed after 5 minutes of supine rest. The systolic blood pressure is measured twice in the right brachial, dorsalis pedis, posterior tibial, and left dorsalis pedis, posterior tibial, and brachial arteries using a hand‐held Doppler probe. The ABI is calculated for each leg by dividing the mean of the dorsalis pedis and posterior tibial pressures by the mean of the 4 brachial pressures. Participants with a borderline ABI (ie, >0.90 and ≤1.00) may undergo a heel‐rise ABI. The heel‐rise test consists of the participant performing 50 heel rises at a rate of 1 per second and then immediately repeating the ABI. 13 Participants with ≥20% drop in the ABI in either leg are eligible. Additional methods of confirming the presence of PAD through angiographic evidence or vascular laboratory testing are summarized in Table 2.
Information regarding medical history and demographic characteristics are obtained by questionnaires administered by research staff.
Run‐In Period
Before randomization, potential participants are asked to complete a run‐in period for up to 14 days. During the run‐in, participants receive the study application (app) on a smartphone and are asked to enter all food consumed into the app. A smartphone with data plan is provided to participants who do not have their own. Successful completion of the run‐in is defined as entering >800 kcal for at least 5 days. Individuals who do not meet this criterion are not eligible. This helps ensure that randomized participants are willing and able to use the study app regularly.
Randomization
Participants unwilling to adhere to the intervention and follow‐up testing, regardless of group assignment, are not randomized. To ensure equipoise, participants are informed of the purpose of the trial and advised that if they are randomized to the exercise group, they should not make changes to their diet, unless advised otherwise by their physician. Potential participants unwilling to continue approximately their same (current) diet if randomized to exercise alone are excluded. Data on participants excluded due to inability to complete run‐in or unwillingness to adhere to the assigned diet and exercise intervention are recorded. For randomization, investigators use permuted block randomization with random block sizes, stratified by site.
Interventions
The study interventions combine connective mobile technology with a Group Mediated Cognitive Behavioral intervention and remote coaching. The Group Mediated Cognitive Behavioral intervention uses psychological empowerment, social support, and supportive accountability to increase self‐efficacy and subjective well‐being to facilitate positive behavioral change in older adults. The intervention for each study group is divided into 3 phases: intensive (months 1–4), transition (months 5–6), and maintenance (months 7–12) (Table 3). During the intensive phase, participants meet weekly in a video conference (Zoom) with other participants in their group and the study coach. Group meetings occur twice per month during the transition phase and once per month during the maintenance phase. All participants receive a manual, a custom smartphone app that is specific to their group assignment, and an ActiGraph accelerometer. Participants randomized to exercise alone receive an app that only tracks exercise but does not track calorie or fat consumption or weight loss. In both groups, participants receive individual coaching calls during the weeks without a group session (Table 4).
Table 3.
Intervention Summary and Frequency of Contact
| Intensive (mo 1–4) | Transition (mo 5–6) | Maintenance (mo 7–12) | |
|---|---|---|---|
| GMCB sessions | Weekly | Every other wk | 1 per mo |
| Coach telephone calls | None | Every other wk | 3 per mo |
| Coach remote monitoring | Weekly | Weekly | Weekly |
| Walking exercise recorded using ActiGraph | 5 d per wk | 5 d/wk | 5 d/wk |
| Weight loss goal (WL+EX only) | ≥0.50 pounds/wk | ≥0.50 pounds/wk | |
| PROVE study app to record dietary intake (WL+EX only) | Daily | Daily | Daily |
| Weight recorded using digital scale (WL+EX) | Daily | Daily | Daily |
GCMB indicates group‐mediated cognitive behavioral; EX, exercise; WL+EX, weight loss plus exercise; and PROVE, Promote Weight Loss in Obese PAD Patients to Prevent Mobility Loss.
Table 4.
Items Used to Monitor Fidelity to the Study Intervention
| WL+EX | EX | |
|---|---|---|
| Coaching skills | ||
| Expresses empathy and asks permission | X | X |
| Uses active and reflective listening to generate the most appropriate responses and questions | X | X |
| Supports self‐efficacy | X | X |
| Elicits change talk | X | X |
| Uses nonjudgmental curiosity and uses open‐ended questions | X | X |
| Treatment fidelity | ||
| Discusses adherence to weighing and self‐monitoring | X | … |
| Discusses walking exercise goals | X | X |
| Discusses calorie goals | X | … |
| Discusses the fat gram goal | X | … |
| Discusses my action plan | X | X |
EX indicates exercise; and WL+EX, weight loss plus exercise.
Exercise Intervention
The exercise (EX) component in each group is identical and modeled after the GOALS (Group‐Oriented Arterial Leg Study) and LITE (Low Intensity Exercise Intervention in PAD) home‐based exercise interventions. 14 , 15 GOALS used a group‐mediated cognitive behavioral framework to help participants with PAD adhere to a home‐based walking exercise program and improved 6‐minute walk distance, compared with the control group. 16 Participants are asked to walk for exercise 5 days/wk, beginning with 10 to 15 minutes of walking exercise/session and working up to 40 to 50 minutes/session. Exercise is tracked in the study app. Exercise goals are individualized and intended to increase walking duration by 5 minutes per session each week to facilitate progress toward the goal of 40 to 50 minutes of walking exercise per session.
Participants are instructed to walk for exercise at a pace that elicits moderate to severe ischemic leg symptoms. 15 Walking duration and intensity are monitored using an ActiGraph accelerometer. Before the start of the intervention, participants are instructed to wear the ActiGraph while walking for exercise for 5 minutes at a pace eliciting moderate‐to‐severe discomfort defined as attaining ischemic leg discomfort of 4 to 5 on a 1 to 5 scale, where 5 is the most severe ischemic leg symptoms. The ActiGraph‐measured intensity (activity counts) during this walking activity is the desired intensity during walking exercise activity. The ActiGraph‐measured walking exercise intensity corresponding to ischemic leg discomfort of 4 to 5 on the 1 to 5 scale is remeasured at scheduled intervals, every 3 months, for each participant and after hospitalizations or major illness.
Participants wear the ActiGraph during walking exercise and data are uploaded to CentrePoint. Walking progress is displayed on the study app to the participant (Figure 1) and on the study website to the coach. The coach and participant use the data to monitor exercise intensity, frequency, and minutes of exercise per session.
Figure 1. Screenshot of PROVE Trial app showing progress toward walking exercise goal for a participant in the exercise (EX) group.
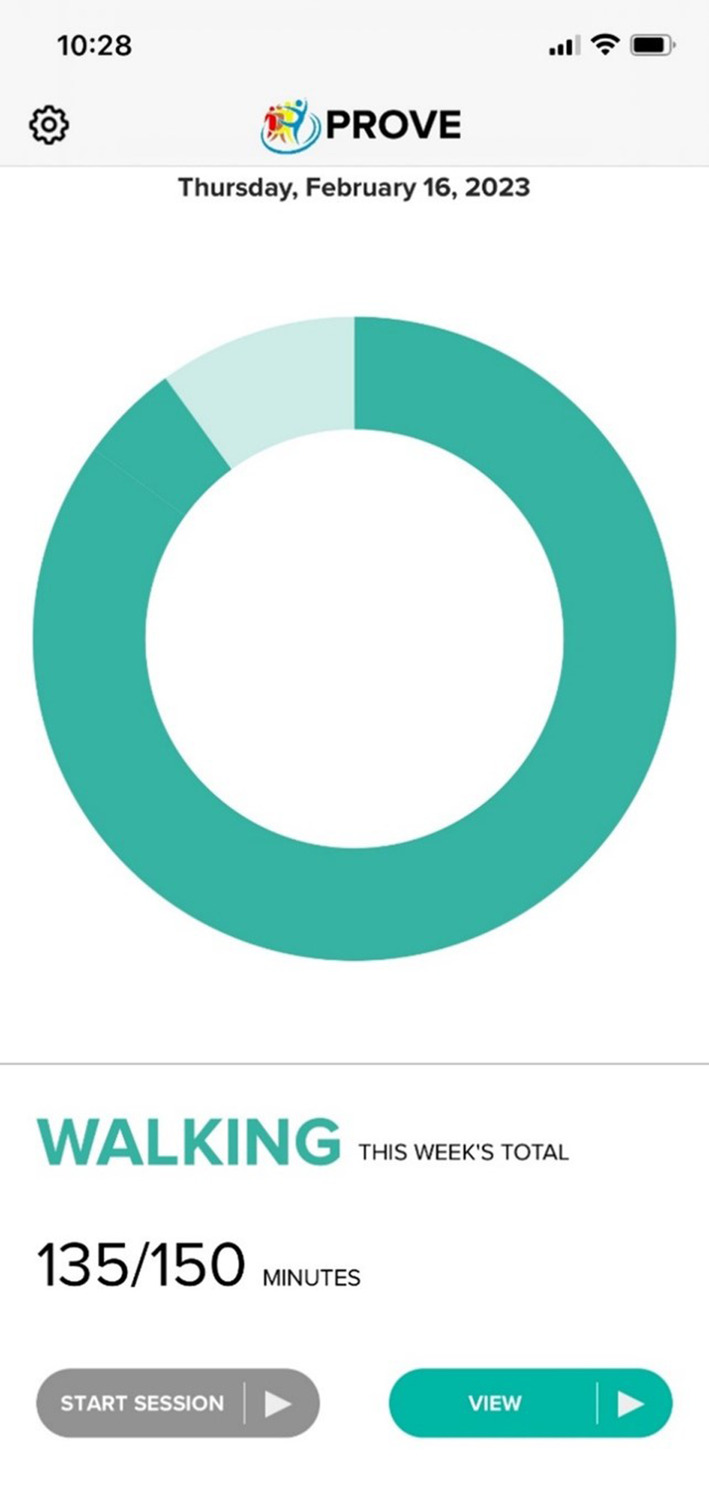
PROVE indicates Promote Weight Loss in Obese PAD Patients to Prevent Mobility Loss.
During each group Zoom session, the coach presents a topic of the week, such as “overcoming negative thoughts,” “goal setting and self‐monitoring,” or “walking exercise in cold weather.” The coach leads group discussions that facilitate participants sharing their experiences in the walking exercise program, including successes and challenges. At the end of each session, each participant selects their individual walking exercise goals for the next week.
Weight Loss Plus Exercise Intervention
The exercise component of the weight loss plus exercise (WL+EX) intervention is the same as that described for the EX intervention above.
Participants randomized to WL+EX receive a digital scale and a version of the app that enables participants to track their daily dietary intake (Figure 2A) and weight, in addition to walking exercise. The app provides immediate feedback to participants regarding their adherence to self‐selected goals related to daily intake of kilocalories, total grams of fat and protein, body weight, and walking exercise (Figure 2B). The PROVE Trial app has been successfully used previously to achieve weight loss in adults with overweight and obesity who did not have PAD. 17 , 18 , 19
Figure 2. Screenshots of progress toward walking exercise and dietary intake goals in the PROVE Trial app for a participant in the weight loss plus exercise (WL+EX) group.
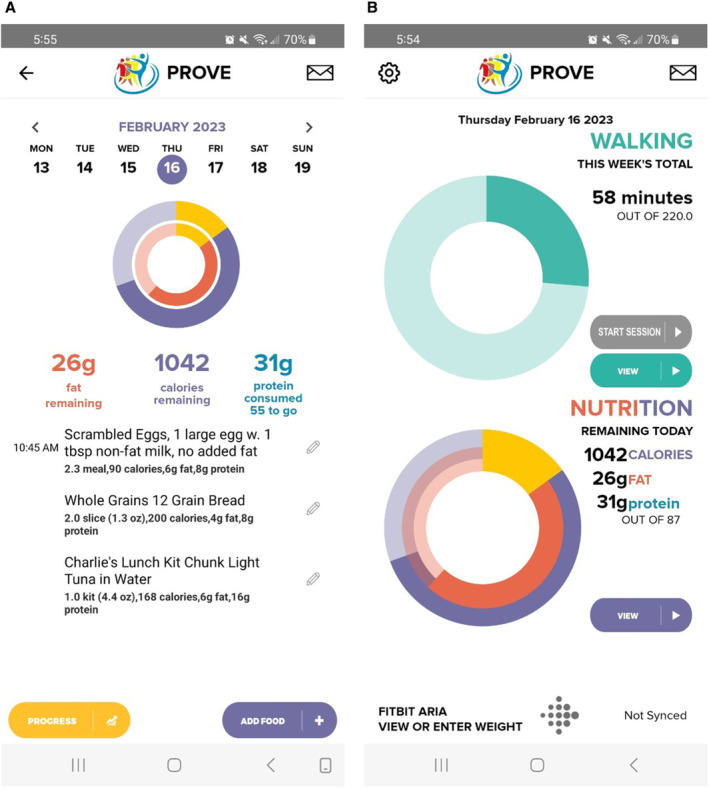
A, Shows examples of food entered in the app and progress toward dietary goals. B, Shows progress toward exercise goals. PROVE indicates Promote Weight Loss in Obese PAD Patients to Prevent Mobility Loss.
The weight loss component of the WL+EX intervention is designed to reduce calorie intake to achieve ≈0.3 kg/wk of weight loss for months 1 to 6 and maintain weight loss during months 7 to 12, with the goal of attaining weight loss of 7% to 10% at 12‐month follow‐up, compared with baseline. An individualized reduced‐kilocalorie goal is determined for each participant according to baseline weight. Participants are encouraged to consume ≈15% to 20% of kilocalories from protein and <7% of kilocalories from saturated fat. These 2 dietary components are monitored using the PROVE Trial app and coach dashboard. Participants are coached on increasing dietary protein‐rich foods if self‐selected intake is inadequate.
Monitoring Intervention Adherence
Throughout the intervention, coaches remotely monitor each participant's progress at least once/wk using the coaching dashboard that displays walking exercise relative to goals for all participants. For those assigned to WL+EX, the dashboard also displays weight, nutrient, and calorie consumption relative to goals (Figure 2A). This information is used to provide individualized feedback during individual coaching calls.
For all participants, the coach contacts participants outside of their normally scheduled calls for the following: (1) the participant is not recording any exercise minutes for >4 days; (2) exercise minutes in 1 week are >50% less than the participant's walking exercise goal; (3) the participant has difficulty adhering to walking exercise activity at the desired intensity (ie, not walking at a pace that elicits moderate‐to‐severe ischemic leg symptoms) at least 4 to 5 days per week. Individuals in either group who meet 1 or more of these criteria may receive additional unscheduled individual calls from the coach, as appropriate. For WL+EX the coach additionally contacts the participant between weekly scheduled interactions if dashboard data show 1 or more of the following: (1) absence of self‐monitoring for >4 days, (2) overly rapid weight loss (>1.36 kg [≈3 pounds]/wk for 3 consecutive weeks); (3) a 2‐week period without at least 0.23 kg [0.50 pounds] weight loss/wk (ie, 0.46 kg [1.0 pound] per 2 weeks) (this monitoring begins in week 3 of the intervention); or (4) nutritional concerns (ie, inadequate protein intake). If a participant randomized to WL+EX does not lose weight for a 3‐week period and they are adherent to dietary goals, the calorie goal is reduced.
Monitoring Intervention Fidelity
Group meetings and individual coaching calls are audio recorded and a 10% subsample of sessions is selected quarterly by the Data Coordinating Center for review. Investigators evaluate and rate assigned sessions using a fidelity checklist (Table 5). Coaches with a mean score of <70% undergo additional training and recertification.
Table 5.
Outcomes of the PROVE Trial. For Each Outcome, We Hypothesize That Weight Loss Plus Exercise (WL+EX) Will Have Greater Improvement, Compared With Exercise (EX)
| Primary outcome |
|
| Secondary outcomes |
|
| Tertiary outcomes |
|
| Exploratory outcomes |
|
EX indicates exercise; IL, interleukin; PGC, peroxisome proliferator‐activated receptor‐gamma coactivator; PROMIS, Patient‐Reported Outcomes Measurement Information System; PROVE, Promote Weight Loss in Obese PAD Patients to Prevent Mobility Loss; TNF, tumor necrosis factor; WIQ, Walking Impairment Questionnaire; and WL+EX, weight loss plus exercise.
Outcomes
Outcomes are shown in Table 5. Outcome assessors are blinded to group assignment. Outcome assessors at each site are trained in data collection by the principal investigator's (MMM) staff and certified by the principal investigator or her designee, using a detailed checklist developed for the study protocol. Study coordinators undergo recertification approximately every 6 months. When deficiencies are identified, interviewers are retrained and reassessed.
6‐Minute Walk
In the 6‐minute walk, participants walk back and forth along a 100‐ft hallway for 6 minutes after standardized instructions are read from a script. The script instructs participants to complete as many laps as possible during the 6 minutes. The primary outcome is the total distance covered during the 6 minutes. The BORG scale of perceived exertion is administered at the end of the walk to evaluate perceived effort at baseline and follow‐up.
Questionnaires
Health‐related quality of life is assessed with the Patient‐Reported Outcomes Measurement Information System mobility questionnaire 20 and the Walking Impairment Questionnaire. 21 Leg symptoms are assessed with the San Diego Claudication Questionnaire. 22 Frequency of exercise activity at baseline and follow‐up is collected via administered questionnaires.
Physical Activity
Physical activity during daily life is measured over 10 days at baseline and follow‐up, using the ActiGraph accelerometer. Up to 7 days of wear time will be used in analyses. Participants are instructed to put on the ActiGraph when they wake up and take it off when they go to sleep, removing it only for bathing.
Dietary Assessment
The standard semi‐quantitative food frequency questionnaire, the Diet History Questionnaire III, 23 is used to estimate energy, macronutrient, and micronutrient intake at baseline and 12‐month follow‐up.
Short Physical Performance Battery
The short physical performance battery is a composite score (range 0–12, 12 = best) that includes assessment of 4‐meter walking velocity at usual pace, standing balance, and time required to stand 5 times from a seated position. 24 , 25 Each of the 3 short physical performance battery components is scored on a 0 to 4 scale. The 4‐m walking velocity is performed by asking the participant to walk ≈16 feet in a corridor at their usual pace, as if they are “walking down the street to go to the store.” The time required to walk the first 4 m is recorded. The measure is performed twice, and the faster time is used in analyses. Standing balance is measured by asking participants to hold 3 separate standing positions for 10 s each. The 3 standing positions are side‐by‐side stand, semi‐tandem stand, and the tandem stand. Time required to stand from a seated position 5 times as fast as possible is also measured.
Height, Weight, and Waist Circumference
Height and weight and waist circumference are measured at baseline using standard procedures. Weight and waist circumference are repeated at 6‐ and 12‐month follow‐up.
Muscle Biopsies
For an exploratory‐specific aim, 50 participants randomized at Northwestern undergo muscle biopsies. Gastrocnemius muscle biopsy measures consist of mitochondrial activity (cytochrome c oxidase activity), mitochondrial biogenesis (peroxisome proliferator‐activated receptor‐gamma coactivator enzyme activity), mitochondrial biogenesis (PGC‐1α), capillary density (capillaries per muscle fiber), calf muscle inflammation (interleukin‐6, tumor necrosis factor‐α, and interleukin‐1β), and abundance of senescent cells, compared with EX. These measures will help delineate biologic pathways that may mediate improved 6‐minute walk distance in response to WL+EX, compared with EX. Gastrocnemius muscle biopsies are obtained from an incision in the medial head of the gastrocnemius muscle in the leg with lower ABI, at ≈67% of the distance between the medial malleolus and the medial aspect of the proximal tibia. 26 Biopsies are performed at baseline and 12 month follow‐up, with the 12‐month biopsy incision performed adjacent to the site of the baseline biopsy, denoted by the incision scar.
Statistical Considerations
Comparisons will be made by using contrasts at 12 months in a mixed‐model repeated measures analysis of covariance model with an unstructured covariance matrix to account for the fact that multiple measurements within a participant over time are not independent. We focus on the contrast at 12 months because our interest is on that time point. However, including all time points (ie, 6 and 12 months) will allow us to borrow strength across the time points when there may be missing data at 12 months. Predictor variables as fixed effects will include treatment (EX versus WL+EX), time (6 versus 12 months), and the baseline (prerandomization) value of the end point being analyzed. Field center and recruitment wave within center will be included as random effects. All participants will be asked to complete follow‐up testing, regardless of adherence to their intervention. Tests of hypotheses and reported P values will be 2‐sided using the intention‐to‐treat principle at the 5% level. We have 1 primary outcome, 5 secondary outcomes, and 7 exploratory outcomes. We do not plan an explicit adjustment for multiplicity for multiple outcomes. However, we will make explicit the number of outcomes explored. Subgroups of interest defined a priori include sex, race or ethnicity (Black versus non‐Black), baseline BMI (median split), baseline 6‐minutes walk performance (median split), baseline smoking status (current versus never and past), age (median split), presence/absence of diabetes, ABI <0.6 versus ≥0.6, presence/absence of classical claudication symptoms, and presence/absence of exertional leg symptoms.
To account for correlation of baseline and follow‐up, we used data from GOALS 16 to estimate residual SDs after adjustment for the baseline level of the response and correlations. For the primary outcome of the 6‐minute walk distance, the root mean squared error was 63.0 m. For the calculation of power, we used a 2‐sample t test and assumed a sample size of 212 participants, a 12‐month retention of at least 85% (a net sample size of at least 180), and 2‐sided tests at the 5% level. To account for the group‐based intervention delivery, we calculated the design effect, which is the ratio of variances comparing a simple randomized design to a cluster randomized design as 1+ICC[n−1], where n is the size of each group and ICC is the interclass correlation coefficient. The design effect is also the ratio of effective sample sizes for designs where the subjects are randomized independently compared with a group‐randomized design. 27 In the GOALS study, we delivered the intervention in a group setting and we used data from that study to estimate the ICC within a group for 6‐minute walk at 12 months as 0.0297. We assumed a similar ICC for the PROVE Trial and an average cluster size of 7 (the most we expect) to get a design effect of 1+0.0297 [7−1] =1.178. Therefore, we have assumed an effective sample size of 212×0.85/1.178=152.9 to account for loss to follow‐up and clustering. Power for the primary outcome is 0.681 for a difference of at least 25 m in the 6‐minute walk test and goes up to 0.83 for 30 m, 0.925 for 35 m, and 0.973 for 40 m. Note that these power estimates are slightly conservative as we will borrow strength from the 6‐month visit in our mixed model. Meaningful change has been defined for change in 6‐minute walk performance: a 20‐ to 30‐m change in the 6‐minute walk represents a small meaningful change, and 50 m represents a large meaningful change. 28 , 29 , 30 The GOALS Trial achieved a 53.5‐m difference in 6‐minute walk between the intervention and control groups. 16 We believe that WL+EX will achieve at least 60% of the benefit of the home exercise alone, or 32.1 m. Our own (unpublished) pilot study demonstrated an improvement of 64.1 m (95% CI, 10.6–118) over 7 weeks. Therefore, we believe that WL+EX will improve 6‐minute walk distance by at least 30 m over EX.
Discussion
Among people with PAD who have overweight or obesity, the PROVE Trial will examine whether a diet and weight loss intervention combined with walking exercise results in significantly greater improvement in 6‐minute walk distance at 12‐month follow‐up, compared with walking exercise alone. There are at least 3 potential mechanisms by which weight loss could improve walking outcomes in people with overweight or obesity and PAD. First, in individuals with PAD with overweight/obesity, weight loss may improve walking performance by reducing the energy or perceived effort required during the 6‐minute walk. Second, weight loss in people with PAD with overweight/obesity may improve adherence to regular walking exercise, thereby facilitating greater response to a walking exercise intervention. Third, based on preclinical evidence, weight loss may improve the pathophysiologic changes that characterize PAD. Pathophysiologic changes may include improved lower extremity perfusion, 31 improved skeletal muscle mitochondrial bioenergetics, and/or reduced inflammation, which may have direct adverse effects on muscle, 32 , 33 , 34 , 35 thereby improving walking performance. For example, in a rat model of PAD, obese rats, when compared with rats that were not obese, had hind limb arterial vasoconstriction and reduced vasodilation in response to nitric oxide. 31 , 33
However, there are several potential reasons that weight loss may not improve walking performance in people with PAD with overweight/obesity. First, in older people, including people with PAD, weight loss has been associated with loss of skeletal muscle. 36 , 37 , 38 In a longitudinal observational study of 389 people with PAD and mean BMI of 28.1 (SD, 5.1) at baseline, people who engaged in intentional weight loss had significantly greater decline in calf muscle area, measured by computed tomography scan, at 3.23 years (SD, 1.37) follow‐up, compared with those who did not engage in intentional weight loss. 36 People with PAD already have reduced calf muscle area compared with those without PAD. 6 , 39 Weight loss that exacerbates sarcopenia could be detrimental for people with PAD. Second, although recent literature 40 indicates that attending to multiple health behaviors simultaneously can be beneficial and create a synergistic effect on positive health behavior, it is unclear whether these synergistic effects will also be observed in individuals with PAD. Third, while weight loss initially relies on dietary changes, physical activity is often necessary to realize weight loss maintenance, which may be challenging in this often physically inactive population. 41 Fourth, the added burden of discomfort, pain, and heaviness that individuals with PAD with overweight or obesity may experience may make behavior change to attain weight loss more difficult compared with people without PAD who have overweight or obesity. For participants randomized to WL+EX, extreme difficulty making the behavior changes in diet or lack of perceived success could discourage progress or sustained engagement in the intervention.
It is also possible that the technological component of the study interventions may be too burdensome for some participants. However, the app has been used successfully to support physical activity and weight loss in other studies, and weight loss has been achieved in prior clinical trials of people who are overweight and obese and who have metabolic syndrome. 17 , 18 , 19 , 40 , 42 , 43 , 44 If the WL+EX group sufficiently improves physical functioning above that of EX alone, investigators will have established an additional intervention that could improve the lives of some individuals with PAD without undue burden of additional in‐person health care visits.
Conclusions
The PROVE Trial is a multicentered randomized clinical trial that will evaluate the effect of weight loss combined with home‐based walking exercise, compared with home‐based walking exercise alone on walking performance in individuals with PAD. If the proposed hypotheses are supported, the PROVE Trial will have important implications for the large and growing number of people with PAD who have overweight or obesity.
Source of Funding
This study is funded by National Heart Lung and Blood Institute, UH3HL141729 (PI McDermott) and U24HL141732 (PI Ambrosius) and supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Disclosures
Dr. McDermott reported receiving research funding from Regeneron and Helixmith and other research support from ArtAssist, Chromadex, ReserveAge, Mars Company, and personal feeds from Cambrain biopharma. Dr. Spring reported receiving research funding from Actigraph scientific advisory board.
This manuscript was sent to Amgad Mentias, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Disclosures, see page 11.
References
- 1. Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, Rudan I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–e1030. doi: 10.1016/s2214-109X(19)30255-4 [DOI] [PubMed] [Google Scholar]
- 2. Polonsky TS, McDermott MM. Lower extremity peripheral artery disease without chronic limb‐threatening ischemia: a review. JAMA. 2021;325:2188–2198. doi: 10.1001/jama.2021.2126 [DOI] [PubMed] [Google Scholar]
- 3. Gerhard‐Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FGR, Hamburg NM, Kinlay S, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2017;135:e726–e779. doi: 10.1161/CIR.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McDermott MM, Criqui MH, Ferrucci L, Guralnik JM, Tian L, Liu K, Greenland P, Tan J, Schneider JR, Clark E, et al. Obesity, weight change, and functional decline in peripheral arterial disease. J Vasc Surg. 2006;43:1198–1204. doi: 10.1016/j.jvs.2006.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Golledge J, Leicht A, Crowther RG, Clancy P, Spinks WL, Quigley F. Association of obesity and metabolic syndrome with the severity and outcome of intermittent claudication. J Vasc Surg. 2007;45:40–46. doi: 10.1016/j.jvs.2006.09.006 [DOI] [PubMed] [Google Scholar]
- 6. Raval Z, Liu K, Tian L, Ferrucci L, Guralnik JM, Liao Y, Criqui MH, McDermott MM. Higher body mass index is associated with more adverse changes in calf muscle characteristics in peripheral arterial disease. J Vasc Surg. 2012;55:1015–1024. doi: 10.1016/j.jvs.2011.10.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. GBD 2015 Obesity Collaborators , Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi‐Lakeh M, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McDermott MM, Leeuwenburgh C, Guralnik JM, Tian L, Sufit R, Zhao L, Criqui MH, Kibbe MR, Stein JH, Lloyd‐Jones D, et al. Effect of resveratrol on walking performance in older people with peripheral artery disease: the RESTORE randomized clinical trial. JAMA Cardiol. 2017;2:902–907. doi: 10.1001/jamacardio.2017.0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naqvi AZ, Davis RB, Mukamal KJ. Nutrient intake and peripheral artery disease in adults: key considerations in cross‐sectional studies. Clin Nutr. 2014;33:443–447. doi: 10.1016/j.clnu.2013.06.011 [DOI] [PubMed] [Google Scholar]
- 10. Wieczór R, Wieczór AM, Gadomska G, Stankowska K, Fabisiak J, Suppan K, Pulkowski G, Budzyński J, Rość D. Overweight and obesity versus concentrations of VEGF‐A, sVEGFR‐1, and sVEGFR‐2 in plasma of patients with lower limb chronic ischemia. J Zhejiang Univ Sci B. 2016;17:842–849. doi: 10.1631/jzus.B1600009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wells GD, Noseworthy MD, Hamilton J, Tarnopolski M, Tein I. Skeletal muscle metabolic dysfunction in obesity and metabolic syndrome. Can J Neurol Sci. 2008;35:31–40. doi: 10.1017/s0317167100007538 [DOI] [PubMed] [Google Scholar]
- 12. Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65 years and older: a review of the controversy. Exp Gerontol. 2013;48:1054–1061. doi: 10.1016/j.exger.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amirhamzeh MM, Chant HJ, Rees JL, Hands LJ, Powell RJ, Campbell WB. A comparative study of treadmill tests and heel raising exercise for peripheral arterial disease. Eur J Vasc Endovasc Surg. 1997;13:301–305. doi: 10.1016/s1078-5884(97)80102-5 [DOI] [PubMed] [Google Scholar]
- 14. McDermott MM, Domanchuk K, Liu K, Guralnik JM, Tian L, Criqui MH, Ferrucci L, Kibbe M, Jones DL, Pearce WH, et al. The Group Oriented Arterial Leg Study (GOALS) to improve walking performance in patients with peripheral arterial disease. Contemp Clin Trials. 2012;33:1311–1320. doi: 10.1016/j.cct.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McDermott MM, Spring B, Tian L, Treat‐Jacobson D, Ferrucci L, Lloyd‐Jones D, Zhao L, Polonsky T, Kibbe MR, Bazzano L, et al. Effect of low‐intensity vs high‐intensity home‐based walking exercise on walk distance in patients with peripheral artery disease: the LITE randomized clinical trial. JAMA. 2021;325:1266–1276. doi: 10.1001/jama.2021.2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McDermott MM, Liu K, Guralnik JM, Criqui MH, Spring B, Tian L. Home‐based walking exercise interevention in peripheral artery disease. JAMA. 2013;310:57–65. doi: 10.1001/jama.2013.7231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spring B, Duncan JM, Janke EA, Andrea T, McFadden HG, DeMott A, Pictor A, Epstein LH, Siddique J, Pellegrini CA, et al. Integrating technology into standard weight loss treatment: a randomized controlled trial. JAMA Intern Med. 2013;173:105–111. doi: 10.1001/jamainternmed.2013.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spring B, Pellegrini CA, Pfammatter A, Duncan JM, Pictor A, McFadden HG, Siddique J, Hedeker D. Effects of an abbreviated obesity intervention supported by mobile technology: the ENGAGED randomized clinical trial. Obesity. 2017;25:1191–1198. doi: 10.1002/oby.21842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spring B, Pfammatter AF, Marchese SH, Stump T, Pellegrini C, McFadden HG, Hedeker D, Siddique J, Jordan N, Collins LM. A factorial experiment to optimize remotely delivered behavioral treatment for obesity: results of the Opt‐IN study. Obesity (Silver Spring). 2020;28:1652–1662. doi: 10.1002/oby.22915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hays RD, Spritzer KL, Amtmann D, Lai JS, Dewitt EM, Rothrock N, Dewalt DA, Riley WT, Fries JF, Krishnan E. Upper‐extremity and mobility subdomains from the Patient‐Reported Outcomes Measurement Information System (PROMIS) adult physical functioning item bank. Arch Phys Med Rehabil. 2013;94:2291–2296. doi: 10.1016/j.apmr.2013.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Regensteiner JG, Steiner J, Panzer R, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142–152. [Google Scholar]
- 22. Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non‐invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. doi: 10.1177/1358863X9600100112 [DOI] [PubMed] [Google Scholar]
- 23. Thompson FE, Subar AF, Brown CC, Smith AF, Sharbaugh CO, Jobe JB, Mittl B, Gibson JT, Ziegler RG. Cognitive research enhances accuracy of food frequency questionnaire reports: results of an experimental validation study. J Am Diet Assoc. 2002;102:212–225. doi: 10.1016/s0002-8223(02)90050-7 [DOI] [PubMed] [Google Scholar]
- 24. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.M85 [DOI] [PubMed] [Google Scholar]
- 25. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower‐extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/nejm199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115 [DOI] [PubMed] [Google Scholar]
- 27. Donner A, Birkett N, Buck C. Randomization by cluster. Sample size requirements and analysis. Am J Epidemiol. 1981;114:906–914. doi: 10.1093/oxfordjournals.aje.a113261 [DOI] [PubMed] [Google Scholar]
- 28. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 29. Holland AE, Hill CJ, Conron M, Munro P, McDonald CF. Small changes in six‐minute walk distance are important in diffuse parenchymal lung disease. Respir Med. 2009;103:1430–1435. doi: 10.1016/j.rmed.2009.04.024 [DOI] [PubMed] [Google Scholar]
- 30. Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schunemann HJ. Interpretation of treatment changes in 6‐minute walk distance in patients with COPD. Eur Respir J. 2008;32:637–643. doi: 10.1183/09031936.00140507 [DOI] [PubMed] [Google Scholar]
- 31. Frisbee JC, Delp MD. Vascular function in the metabolic syndrome and the effects on skeletal muscle perfusion: lessons from the obese Zucker rat. Essays Biochem. 2006;42:145–161. doi: 10.1042/bse0420145 [DOI] [PubMed] [Google Scholar]
- 32. Marzetti E, Wohlgemuth SE, Anton SD, Bernabei R, Carter CS, Leeuwenburgh C. Cellular mechanisms of cardioprotection by calorie restriction: state of the science and future perspectives. Clin Geriatr Med. 2009;25:715–732, ix. doi: 10.1016/j.cger.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frisbee JC, Stepp DW. Impaired NO‐dependent dilation of skeletal muscle arterioles in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol. 2001;281:H1304–H1311. doi: 10.1152/ajpheart.2001.281.3.H1304 [DOI] [PubMed] [Google Scholar]
- 34. Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW. Sarcopenic obesity and the pathogenesis of exercise intolerance in heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2015;12:205–214. doi: 10.1007/s11897-015-0257-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high‐fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926 [DOI] [PubMed] [Google Scholar]
- 36. Polonsky TS, Tian L, Zhang D, Bazzano LA, Criqui MH, Ferrucci L, Guralnik JM, Kibbe MR, Leeuwenburgh C, Sufit RL, et al. Associations of weight change with changes in calf muscle characteristics and functional decline in peripheral artery disease. J Am Heart Assoc. 2019;8:e010890. doi: 10.1161/JAHA.118.010890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento‐Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beavers KM, Ambrosius WT, Rejeski WJ, Burdette JH, Walkup MP, Sheedy JL, Nesbit BA, Gaukstern JE, Nicklas BJ, Marsh AP. Effect of exercise type during intentional weight loss on body composition in older adults with obesity. Obesity (Silver Spring). 2017;25:1823–1829. doi: 10.1002/oby.21977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McDermott MM, Hoff F, Ferrucci L, Pearce WH, Guralnik JM, Tian L, Liu K, Schneider JR, Sharma L, Tan J, et al. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2007;55:400–406. doi: 10.1111/j.1532-5415.2007.01092.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spring B, Pellegrini C, McFadden HG, Pfammatter AF, Stump TK, Siddique J, King AC, Hedeker D. Multicomponent mHealth intervention for large, sustained change in multiple diet and activity risk behaviors: the Make Better Choices 2 randomized controlled trial. J Med Internet Res. 2018;20:e10528. doi: 10.2196/10528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, et al. The ankle brachial index is associated with leg function and physical activity: the walking and leg circulation study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008 [DOI] [PubMed] [Google Scholar]
- 42. Spring B, Schneider K, McFadden HG, Vaughn J, Kozak AT, Smith M, Moller AC, Epstein LH, Demott A, Hedeker D, et al. Multiple behavior changes in diet and activity: a randomized controlled trial using mobile technology. Arch Intern Med. 2012;172:789–796. doi: 10.1001/archinternmed.2012.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rejeski WJ, Ambrosius WT, Burdette JH, Walkup MP, Marsh AP. Community weight loss to combat obesity and disability in at‐risk older adults. J Gerontol A Biol Sci Med Sci. 2017;72:1547–1553. doi: 10.1093/gerona/glw252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rejeski WJ, Brubaker PH, Goff DC Jr, Bearon LB, McClelland JW, Perri MG, Ambrosius WT. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011;171:880–886. doi: 10.1001/archinternmed.2010.522 [DOI] [PMC free article] [PubMed] [Google Scholar]


