Abstract
Molecular imaging of viral infection, using a variety of advanced imaging techniques such as optical and nuclear imaging, can and has been used for direct visualization of the virus as well as assessment of virus-host interactions. Unlike imaging of other pathogens such as bacteria and fungi, challenging aspects of imaging viral infections include the small size of viruses, the complexity of viral infection animal models (eg, species dependence), and the high-level containment needs for many high-consequence pathogens, among others. In this review, using representative viral infections, we discuss how molecular imaging can reveal real-time infection dynamics, improve our understanding of disease pathogenesis, and guide optimization of treatment and prevention strategies. Key findings from human and animal studies are highlighted.
Keywords: Ebola, high-consequence infection, HIV, influenza, molecular imaging, PET scan, reporter virus, RSV, viral infection
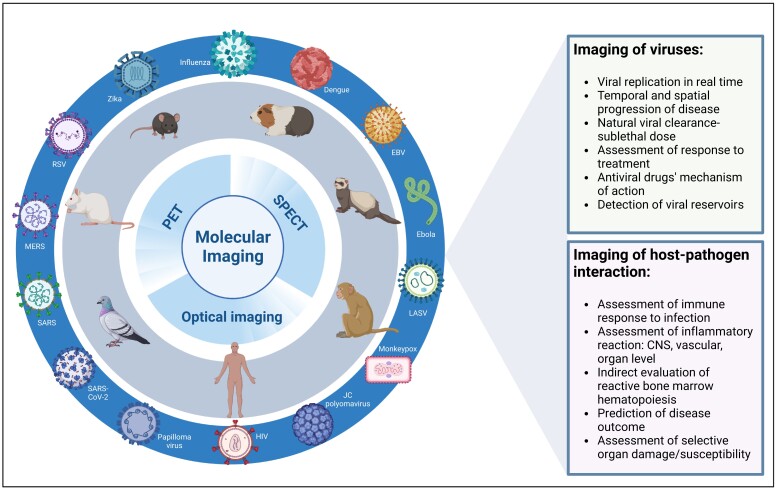
Molecular imaging (MI) of viral infection can provide vital information regarding real-time infection dynamics, including viral replication and spread, as well as progression of disease and viral clearance, both naturally and after treatment. MI provides a noninvasive means to develop treatments, diagnose infections, and increase understanding of pathophysiological responses to viral infections. MI can be performed using bioluminescence, positron emission tomography with computed tomography (PET/CT), magnetic resonance imaging (MRI), and single-photon emission tomography with computed tomography (SPECT/CT). One of the most challenging aspects of imaging viral infections, however, is complexity of the animal models. Animal species have varying susceptibilities to human viral pathogens (eg, hepatitis C virus and human immunodeficiency virus [HIV] infect humans but not rodents). Progression of viral infection can also differ between animals and humans, with animal models potentially requiring different inoculation routes. Logistical issues, safety risks, and costs associated with virus-infected animals tend to increase with biosafety levels (BSL) [1]. Fortunately, existing animal models of viral infections primarily designed to test antimicrobial therapies can often be repurposed for MI, enabling whole-body in vivo real-time insights into pathophysiology of viral disease and immune response (Figure 1).
Figure 1.
Molecular imaging of viral infections: To better understand the mechanism of viral infection and host response, an established or newly developed animal model for the virus in question is paired with an appropriate molecular imaging modality. Considerations related to biosafety levels and availability of the animal models are necessary. A successful combination can provide valuable information related to the pathogen itself as well as the host-pathogen interaction and consequences. Using molecular imaging significantly decreases the number of animals needed compared to standard experimental approaches and each animal becomes its own control, thus decreasing variability across experiments. Abbreviations: EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; LASV, Lassa virus; MERS, Middle East respiratory syndrome; PET, positron-emission tomography; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
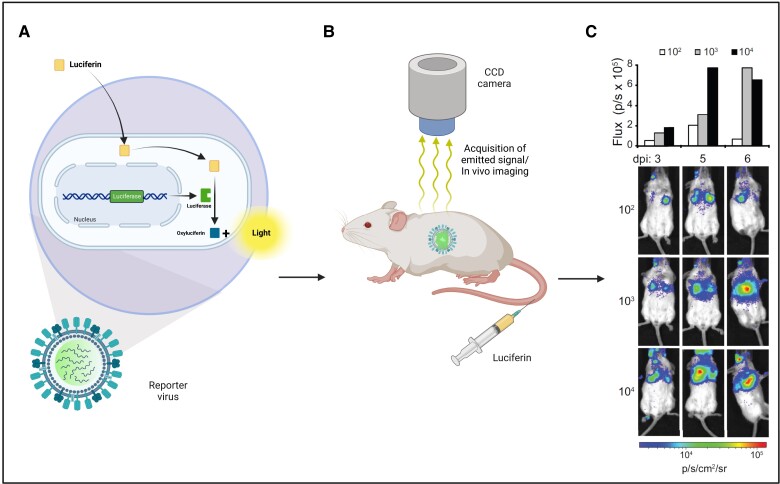
Reporter genes, commonly used in cancer imaging and viral-vector gene therapy [2], can also be used for direct imaging of pathogenic viruses. Multiple challenges exist, however, including the small size of viruses (approximately 20–400 nm), the risk of genome destabilization with gene inserts, the loss of the reporter gene over time, and the reporter gene interfering with virion assembly resulting in attenuated replication. Thus, preservation of virulence and growth characteristics must be confirmed after insertion of the reporter gene into the viral genome before imaging studies are performed. Nonetheless, reporter viruses designed for optical imaging have been used successfully, mainly in rodents [3, 4] (Figure 2). In slightly larger animals like nonhuman primates (NHPs), investigations of reporter genes have been sparse. Examples include use of the sodium iodide symporter for SPECT imaging of Middle East respiratory syndrome coronavirus (MERS-CoV) [6] and a measles virus expressing green fluorescent protein (GFP) to facilitate ex vivo imaging of early target cells [7].
Figure 2.
A and B, Optical imaging of bioluminescent and fluorescent reporter genes (eg, luciferase, nanoluciferase, green fluorescent protein) enables longitudinal studies of viral infections in a minimum number of animals. C, This allows for visualization, characterization, and quantification of viruses and their dynamics (adapted with permission from Tran et al [5]). Abbreviation: CCD, charge coupled device; dpi, days postinfection.
Labeling of antiviral drugs is another strategy for MI of viral infection. One example is labeled ganciclovir (18F-FHPG) in rats with herpes encephalitis [8]. Unfortunately, in vitro uptake was cell line-selective [9] and in vivo the labeled drug could not cross an intact blood-brain barrier [8]. Labeled antiviral drugs have been used more successfully to elucidate mechanisms of action and side effects. In the case of Tamiflu (oseltamivir), its labeled metabolite, Ro 64-0802, penetrated the blood-brain barrier faster due to active efflux mediated by a transporter, suggesting the metabolite is likely responsible for the neurological side effects [10].
More commonly, however, MI has been used to study mechanisms of injury associated with viral infection [11], including associated host and immune reactions and organ-specific infectious sequalae [12], with the ultimate goal being the development of imaging biomarkers that shed light on pathophysiology, disease progression, and treatment response.
We herein examine approaches for viral infection imaging, using examples of relevant viruses such as retroviruses (HIV and simian immunodeficiency virus [SIV]), respiratory viruses (influenza, respiratory syncytial virus [RSV], and coronavirus disease 2019 [COVID-19]), and high-consequence viral infections (Ebola, Nipah, Lassa, Marburg, MERS-CoV, and mpox viruses).
RETROVIRUSES
Since its identification in 1984, HIV has been transformed from a death sentence to a chronic disease. However, infection still engenders long-term sequelae and requires life-long antiretroviral therapy (ART). There is no scalable cure for HIV and interruption of ART leads to unchecked disease progression.
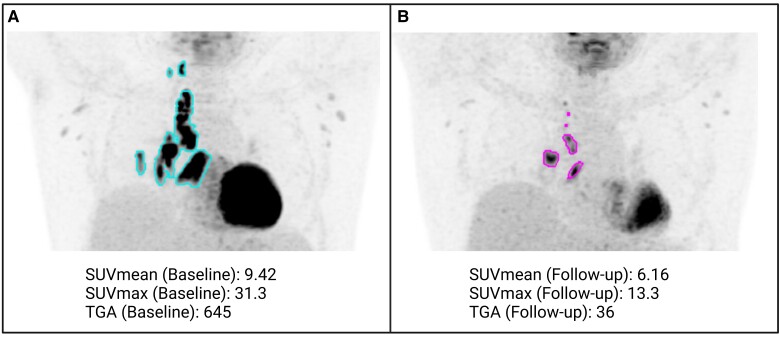
Medical imaging focusing on structural changes (MRI and CT) has long been used to assess the structural consequences of HIV infection during various stages of infection, before and after ART initiation. MI, on the other hand, has been used to investigate the pathophysiology of the infection, such as immune activation in SIV-infected animals [13] and people with HIV (PWH) [12]. Assessing the burden of opportunistic infections by fluorodeoxyglucose (FDG)-PET in ART-naive patients demonstrated that increased baseline, pre-ART metabolic activity (high opportunistic infection burden) was associated with a higher risk of developing immune reconstitution inflammatory syndrome after ART initiation and correlated with inflammatory markers and glucose transporter 1 (Glut-1) expression in CD4+ cells and monocytes [12] (Figure 3). A more recent study in the same population showed lower liver glucose metabolism in PWH compared to controls, which increased longitudinally, approximating control values 2 years after ART initiation [14]. It was concluded that long-term ART may reverse hepatic abnormalities associated with HIV, but that residual liver injury may persist, particularly in PWH who present with low CD4 counts [14].
Figure 3.
Quantifiable opportunistic disease burden in an ART-naive person with HIV before and after treatment with ART. A, 18F-FDG PET imaging allows assessment of hypermetabolism associated with the infectious process (pulmonary tuberculosis) and (B) response to antiretroviral and antimycobacterial therapy. In this case total glycolytic activity of mediastinal and hilar lymph nodes decreased by almost 18-fold, consistent with response to treatment. Abbreviations: ART, antiretroviral therapy; 18F-FDG PET, 18F- fludeoxyglucose positron-emission tomography; SUV, standardized uptake value; TGA, total glycolytic activity.
Imaging of the CD4 T-cell pool with CD4-specific ligands has been used in SIV-infected NHPs, showing stochastic CD4 T-cell pool reconstitution after ART initiation amongst lymph node clusters within the same animal. More interestingly, the authors found that lymph node CD4 pools in ART treated animals with suppressed plasma viral loads were still suboptimally reconstituted compared to healthy controls, unlike splenic CD4 pools which were similar to controls [15]. Another study explored use of radiolabeled tenofovir, 18F-fluoropropan-2-yloxymehtylphosphonic acid, as a marker for viral kinetics but showed more utility for assessment of ART biodistribution as tenofovir was shown to accumulate in the kidneys, consistent with its potential for nephrotoxicity [16].
In light of increased cardiovascular disease risk in PWH [17], the effect of HIV on vascular inflammation and its interplay with cardiovascular risk factors in this population have been evaluated with MI. One study found significantly higher target-to-background ratio of FDG uptake, as a measure of arterial inflammation, in the ascending aorta of PWH compared to uninfected controls, with HIV status being an independent predictor of this increase [18]. The results of this experiment were not always clear cut, which could be partially due to slightly different acquisition and analysis methods between groups. In another study, arterial FDG uptake in PWH was increased compared to controls and was associated with serum sCD163, a marker of monocyte and macrophage activation [17]. Conversely, another study revealed significant overlap of target-to-background ratio values between HIV and controls [19]. In this study, arterial FDG uptake did not correlate with CD4 count, viral load, sex, or infection duration.
An additional use of in vivo measurement of arterial inflammation using MI is assessment of response to therapy. In one randomized, double-blind, placebo-controlled study, the investigators found that although noncalcified plaque volume and high-risk coronary plaque features in PWH decreased after statin therapy, arterial inflammation as measured by FDG PET did not [20]. In a follow-up study by the same group, ART also did not reduce arterial inflammation (as measured by FDG uptake) despite immune function restoration, supporting the need for complementary strategies to reduce arterial inflammation among ART-treated PWH and hopefully decrease cardiovascular events in this vulnerable population [21].
Another current MI objective is identification and characterization of HIV reservoirs. During early infection, copies of the HIV genome integrate into host cell DNA as proviruses, which can remain latent and evade immune detection or can continue their lifecycle to produce virions. These proviruses constitute the HIV reservoir [22] and may or may not produce HIV RNA. Proviruses are present in diverse anatomic sites but are concentrated in lymphoid tissues. If ART is interrupted, proviral reservoirs can reactivate to initiate cycles of viral replication leading to viremia. Reservoirs not only support HIV repopulation when ART is stopped, but also induce chronic inflammation and its sequelae via production of viral proteins during ART [23]. To assess viral reservoirs, Santangelo and colleagues used PET imaging in SIV-infected macaques [24]. Their ligand was based on the full antibody 7D3, which targets the CCR5 coreceptor binding site of gp120. 64Cu-7D3 uptake was greatest in the gastrointestinal and respiratory tracts, lymphoid tissues, and reproductive organs of viremic macaques. In ART-suppressed macaques, on the other hand, while signals were reduced, they were still detectable in colon, some lymph nodes, small bowel, nasal turbinates, genital tract, and lung, suggesting residual viral presence. Their findings were supported by SIV detection by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and immunohistochemistry [24].
No animal model fully reflects human disease, thus results may not directly translate into the human condition. A different study of MRI/PET with 64Cu-3BNC117, which targets the CD4 binding site of gp120, was unable to detect HIV-1 env expression in vivo in PWH [25]. This could potentially be due to a different site of binding compared to 7D3. Another issue is the discordance between the isotope half-life (12.7 hours) and 3BNC117's half-life (9 days in viremic PWH and 17 days in uninfected controls). Using a different isotope, Beckford-Vera and colleagues recently translated the same approach to PET imaging of HIV in humans using the monoclonal antibody VRC01, which also binds a conserved region of the CD4 binding site on HIV gp120 [26]. 89Zr-VRC01 PET uptake was observed in lymph nodes, gut, bone marrow, and nasal turbinates of viremic and suppressed PWH compared to uninfected controls. Interestingly, the high uptake of the labeled antibody in the inguinal lymph nodes of PWH (both viremic and ART suppressed) correlated with tissue HIV protein expression, suggesting the feasibility of using immunoPET to noninvasively characterize residual HIV infection [26].
Imaging using radiolabeled antibodies is generally limited by the large size and half-life of the antibody. In the future, consideration should be given to either antibody fragment or nonantibody ligands such as drug derivatives, aptamers, and other small molecules. An ideal imaging technique would be able to resolve 1 virion; however, that is still unachievable with available techniques. Development and validation of imaging strategies in ART-naive PWH or during analytic treatment interruption will facilitate optimization of imaging applications to improve management of HIV [27].
RESPIRATORY INFECTIONS
Viral respiratory pathogens, including RSV, influenza, and coronaviruses, are a major cause of worldwide morbidity and mortality. Recent pandemics have increased their visibility and the need for more sensitive in situ and in vivo evaluation of treatment and disease dynamics.
RSV is a common infection of both adults and children, with seasonal epidemics causing bronchiolitis and pneumonia, and particularly high morbidity and mortality in children and immunocompromised people [28]. A study using mice infected intranasally with RSV A2 strain expressing luciferase showed the bioluminescent signal correlated with lung viral titers as assessed by traditional plaque assay and qRT-PCR. This animal model was then used to evaluate protective immunity conferred by vaccination and effectiveness of monoclonal antibody treatment. Interestingly, RSV bioluminescence in these animals performed better than plaque assay in detecting viral titers and treatment effect [29]. In another study, a recombinant RSV expressing luciferase was used, enabling the observation of viral replication in the nose and the lungs of the animals at 2 and 4 days postinfection (dpi) [30]. This latter study was intended as a precursor for further subcellular localization of RSV in immune and lung epithelial cells using rHRSV-mCherry and high-resolution 2-photon imaging (Figure 2). The reporter virus labeled with mCherry was subsequently visualized in different cell subtypes identified by morphology, location, and relationship with other cells within the lung tissues [30]. A review of this whole-body imaging-driven multiscale investigative approach to study viral infection was published by Uchil et al, where the authors elaborate on in vivo imaging as a guide for subsequent focused ex vivo imaging with increasing scales of resolution (eg, intravital microscopy and light sheet fluorescence microscopy) [31].
Influenza viruses are constantly mutating and have given rise to several pandemics, including the 1918 Spanish flu and most recently the 2009 H1N1 pandemic. The past, current, and potential future global impact of influenza underscores the importance of integrating MI methods into preclinical and clinical studies of the pathophysiology and disease progress of the viral infection. Ferrets are currently the most suitable influenza infection animal model due to their susceptibility to human influenza viruses [32]. Induction of lower respiratory tract infection with a 2009 H1N1 clinical isolate in the ferret model enabled real-time visualization of inflammation progression by 18F-FDG-PET/CT [33]. The utility of 18F-FDG-PET/CT for real-time visualization of inflammation allows for similar longitudinal assessments in other viral infections.
In smaller viruses like the influenza virus, smaller reporter genes can be used to minimize the risk of disrupting viral genes and growth characteristics, which can occur with traditional reporter genes like luciferase. In one study a small but extremely bright luciferase variant, nanoluciferase, was used to label the single-stranded RNA virus influenza. This resulted in replication-competent reporter virus, which enabled longitudinal observation of viral spread and disease progression with escalating viral inocula as well as viral clearance when using sublethal doses [5] (Figure 2). This work was further expanded by creating derivatives of the NanoLuc reporter virus system using enhanced GFP and monomeric red fluorescent protein to quantify viral load and evaluate the kinetics of the antiviral oseltamivir. Using a multimodal bioluminescent virus with FDG-PET/CT imaging, female BALB/c mice receiving oseltamivir also showed reduced inflammation [34].
Another approach to assess disease pathophysiology in influenza virus infections is immuno-PET. In one study, 89Zr anti-CD8α nanobody was used in mice intranasally infected with H1N1, revealing increased signal intensity in the lungs 6 dpi and in the mediastinal lymph nodes and thymus 4 dpi. CD8+ cells, a critical component of host defense, were tracked through different lung regions, eventually accumulating in specific regions over the disease course, in correlation with morbidity and weight loss [35]. Such dynamic monitoring can be applied preclinically and potentially translated to study responses to vaccines and therapeutics.
Appropriate preclinical models of influenza are needed to understand disease course and to advance noninvasive approaches that support adequate diagnosis and therapies. Influenza A subtype H5N1 is a less common, but typically fatal, virus that has been used in preclinical modeling. NHPs intratracheally exposed to H5N1 can successfully clear the virus and rarely develop severe disease. However, disease severity was increased by using aerosolized H5N1 and the NHPs developed a fulminant pneumonia. The clinical manifestations resembled human acute respiratory distress syndrome (ARDS), with infection of macrophages, virus-mediated damage to alveoli, and compromised epithelial barrier function. After optimization of the model, FDG-PET/CT was used to measure the degree of inflammation in the lungs compared to baseline [36]. This supports use of FDG-PET/CT to monitor disease progression and regression, thus providing a platform to test countermeasures and improve clinical care.
MI studies evaluating lung inflammation and disease in human influenza infection are rare in the literature. A 2010 case report of a 52-year-old man infected with H1N1 and diagnosed with ARDS showed abnormally high uptake on 18F-FDG PET/CT of the lung. Uptake was seen in areas of both loss and preserved aeration, showing that viral infection triggers an inflammatory response, including recruitment of activated neutrophils, that can affect lung tissue regardless of ventilation [37]. Although such results are not surprising, the findings support the use of MI to noninvasively identify inflammatory and other immune responses throughout the infectious process, paving the way towards using those measures as surrogate end points for treatment response.
When the COVID-19 pandemic started, animal models were unavailable and characterization of human disease was just beginning. Initially, incidental detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia on FDG PET/CT was reported in several countries [38, 39]. A systematic review of reports including 52 asymptomatic patients with a mean age of 60 years for whom PET/CT scans were performed for other clinical indications using 18F-FDG, 18F-choline, or 68Ga-prostate-specific membrane antigen showed lung changes including bilateral hypermetabolic ground-glass opacities, consolidation, and interlobular thickening [40].
Research surged as part of the unprecedented rapid public health response to the pandemic. Mice were the first animal models considered, but were resistant to SARS-CoV-2, largely due to deficient interactions between the SARS-CoV-2 spike protein and the angiotensin converting enzyme 2 (ACE2) receptor [41]. Transgenic mice expressing human ACE2 and susceptible to the infection were eventually developed and used; however, these mice can develop a severe encephalitis which is not typically observed in humans [42]. Ferrets were noted to be susceptible to SARS-CoV-2 but did not develop severe disease [43]. Syrian hamsters seemed to be the most representative small animal model because they develop a respiratory disease and clinical manifestations such as weight loss, although the hamsters do begin to clear disease by day 8–10 postexposure [44]. NHPs are being used as the large animal model [45], but have limited utility due to lack of a severe disease phenotype seen in humans.
To characterize COVID-19 pathogenesis more specifically, MI with ligands that target particular cell types and functions has been used in animal models and to a lesser extent in infected patients. Syrian hamster studies with 124I-iodo-DPA-713, a translocator protein (TSPO)-specific ligand, showed accumulation and colocalization of the ligand within pneumonic lesions. The uptake was higher in male versus female hamsters, suggesting sex-based differences in immune reaction and susceptibility to the infection [46]. In SARS-CoV-2–infected NHPs, uptake of another TSPO ligand, 18F-DPA-714, was seen corresponding to CT pulmonary ground-glass opacities and coincided with dendritic cell activation in the blood [47]. Tracking SARS-CoV-2 using a radioligand based on the anti–SARS-CoV-2 spike antibody CR3022 revealed lung pathology in NHPs, which correlated with CT abnormalities. Increased signal was also seen in the infected male genital track and validated by immunofluorescence imaging [48].
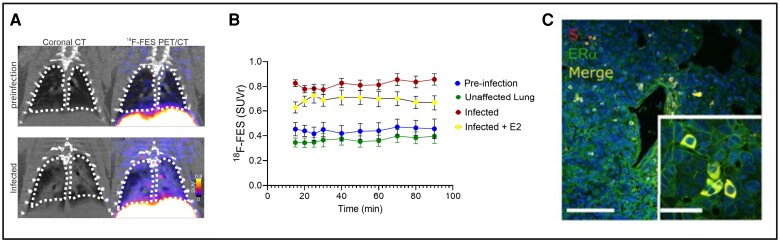
The SARS-CoV-2 spike protein has been shown to interact with multiple human receptors in addition to ACE2. The targets of these interactions present opportunities for radiolabeled ligands to longitudinally assess COVID-19 infection. PET/CT imaging was used to examine one such interaction between the SARS-CoV-2 spike protein and endogenous estrogen receptor α (ERα). ERα molecules are present in alveolar macrophages and are important in the immune response to infection [49–51]. 18F radiolabeled estradiol (18F-FES), targeting ERα, was administered to SARS-CoV-2–infected Syrian golden hamsters. PET/CT imaging revealed colocalization of ERα and lung pathology, and overall increase in ERα levels in the lung of SARS-CoV-2–infected hamsters compared to control animals [52] (Figure 4). These data suggest an interaction between spike protein and ERα, in which the spike protein may cause the activation of ERα. The correlation of spike protein and ERα allow for the 18F-FES radioligand to be used as a biomarker for longitudinal assessment of COVID-19.
Figure 4.
A, 18F-FES PET/CT coronal lung images from SARS-CoV-2–infected hamsters at preinfection (day −1) and infection (day 7). B, Time activity curves show tissue/blood SUV ratios greater during infection versus preinfection. C, Hamster lung immunohistochemistry showing colocalization of S and ERα immunoreactivity (adapted with permission from Solis et al [52]). Abbreviations: E2, estradiol; 18F-FES, 18F-estradiol; Erα, estrogen receptor α; PET/CT, positron-emission tomography/computed tomography; S, spike; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SUVr, standardized uptake value ratio of tissue to blood.
Clinical evaluation has also employed 18F labeled integrin αvβ6-binding peptide, which targets an epithelium-specific cell surface receptor known to modulate inflammatory responses during lung injury and fibrotic diseases. Integrin αvβ6-binding peptide was found to be increased in the lungs of a 71-year-old man who had been COVID-19 positive 2 months prior, consistent with elevated integrin levels [53].
In another approach, ACE2-specific PET imaging was used to assess the anatomical concentration and distribution of ACE2 expression. The probe 68Ga-HZ20, obtained by radiolabeling the DX600 peptide (a competitive ACE2 inhibitor), was initially tested in pigeons to track ACE2 biodistribution, with kidneys and lungs showing the highest uptake 20 minutes after injection [54]. A related study using the 68Ga-HZ20 PET radiotracer in 20 healthy volunteers and 1 individual 7 months after COVID-19 showed high uptake in ACE2-expressing organs including the kidney and gallbladder with little uptake in the lungs [55]. The infected participant, on the other hand, demonstrated higher uptake in the gallbladder and testis relative to the healthy volunteers, potentially reflecting ACE2 upregulation in response to SARS-CoV-2 infection [55].
MI continues to make important contributions to understanding COVID-19. In January 2022, the Society of Nuclear Medicine and Molecular Imaging warned that imaging reports from cancer patients infected with the COVID-19 Omicron variant showed unusual imaging patterns on FDG-PET/CT [56]. Omicron primarily affected the upper aerodigestive tract and cervical lymph nodes, whereas previous SARS-CoV-2 strains primarily showed lung involvement. MI may thus be particularly useful for understanding disease and guiding clinical management in high-risk populations.
HIGH-CONSEQUENCE INFECTIOUS DISEASES
High-consequence infectious disease (HCID) outbreaks can spread rapidly in the community, cause acute infections with high case-fatality rates, and have no routinely available vaccines. HCIDs includes hemorrhagic fever viruses (eg, Ebola [EBOV], Marburg, Lassa, and Nipah) as well as airborne-spreading infections such as SARS, MERS, and SARS-CoV-2, among others. Multiple outbreaks in endemic regions with eventual spread to nonendemic areas have occurred in recent decades. Understanding the pathophysiology and assessing the efficacy of prevention and management strategies is thus imperative for containing HCIDs and minimizing their local and global impact.
HCIDs are difficult to study, mainly because they generally occur in less developed countries with limited resources, and because they require BSL-3 or BSL-4 containment measures in nonendemic regions. Studying HCIDs diseases through animal models often becomes necessary due to relative rarity and ethical concerns about exposing humans to such pathogens. One such example is MERS, which has a 35% human case fatality rate [57], but produces only transient respiratory illness without discernible CT lung lesions, change in body weight, or change in temperature in NHPs [58, 59]. Nonetheless, FDG-PET analysis by Chefer and colleagues [59] did show increased mediastinal and axillary lymph node uptake, indicating FDG-PET may be useful for quantification of small changes in the host immune system response even in suboptimal animal models. In a mouse model of dengue virus, which manifests thrombocytopenia, inflammation, and vascular damage, FDG-PET could distinguish mice that received treatment from untreated mice and could differentiate lethal and nonlethal disease [60]. Using macaque models of mpox infected with a nonuniformly lethal dose, nonsurvivors showed increased bone marrow and lymph node uptake compared to survivors, indicating that FDG PET can be used to assess temporal immune responses to a viral infection and may be useful in predicting disease outcomes [61].
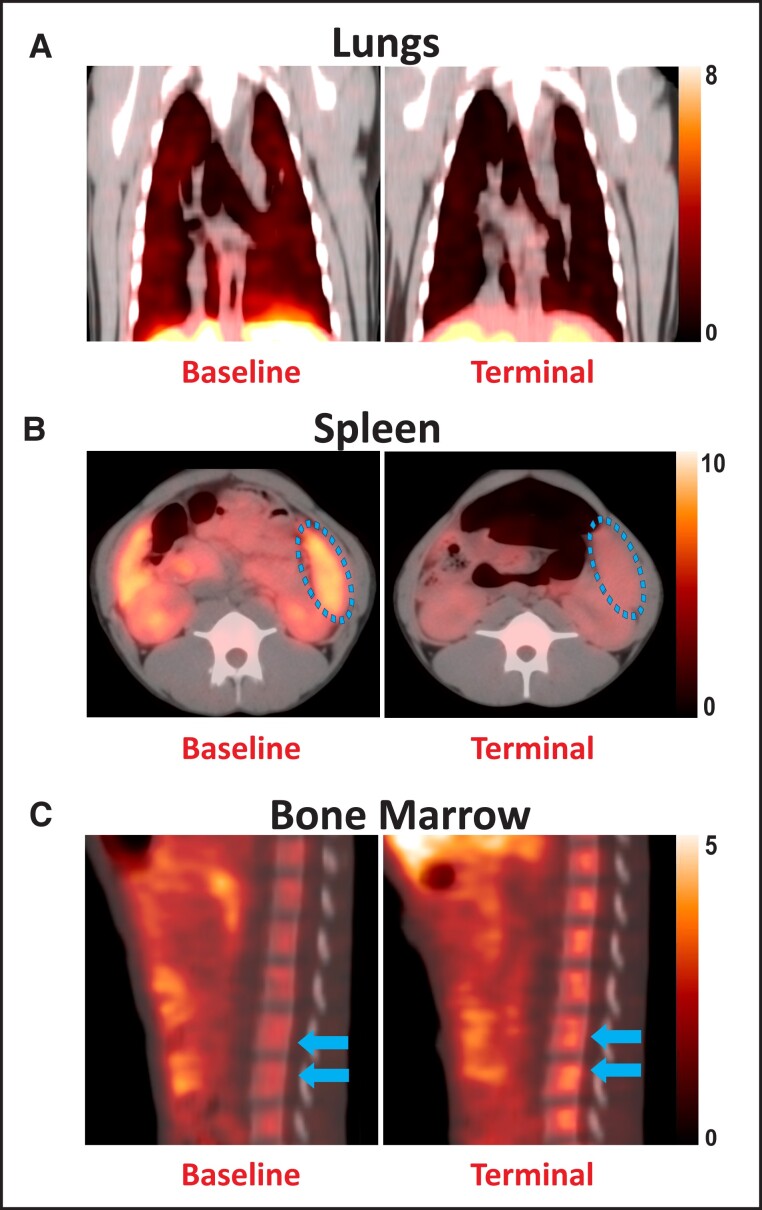
Other PET ligands have also been used, albeit sparsely, to characterize disease pathophysiology of HCIDs. For instance, the TSPO-binding ligand DPA-714 was used to delineate organ-level inflammation and immune-cell loss in EBOV-infected NHPs. The infected animals showed the pathological hallmarks of human EBOV disease such as lymphopenia, coagulopathy, cytokine storm, and multiorgan failure. Longitudinal TSPO PET imaging in these animals showed progressive decline of binding in the spleen and lungs, which coincided with monocytic and lymphocytic cellular depletion and increased binding in bone marrow, suggesting hematopoietic activation to counter widespread immune cell death in the infected animals [11] (Figure 5).
Figure 5.
18F-DPA-714 binding in Ebola virus infection. Representative positron-emission tomography/computed tomography (PET/CT) images show 18F-DPA-714 binding in the (A) lungs (coronal plane), (B) spleen (axial plane), and (C) bone marrow (sagittal plane) of macaques at pre-infection baseline (left) and the terminal stages (right) after infection with the Ebola virus.
Reporter genes expressing luciferases or fluorescent proteins have also been used to quantitatively study disease pathogenesis in Nipah, dengue, EBOV, mpox, and Lassa viruses for purposes of monitoring disease progression, tissue tropism, and cellular entry dynamics in animal models. For example, Fang and colleagues developed a reporter dengue virus to assess the kinetics of neurovirulence of dengue-4 (DENV-4) Ban18, which has a point mutation causing hypervirulence, in live mice [62]. Similar reporter virus constructs were produced for 2 clades of mpox viruses (Congo and United States), which were used to compare viral spread and tissue tropism in mouse models [63].
Findings from MI studies of HCIDs not only shed light on the pathophysiology of the infections but can also inform prevention and management approaches by applying preclinical findings to humans, considering the high mortality rates and narrow windows for effective treatment associated with those infections.
CONCLUSIONS
In this review, we have discussed examples of MI applications in key retroviruses, respiratory infections, and HCID. Such applications can be expanded to cover many other viral infections where MI can contribute significantly to understanding the pathogenesis and spread kinetics of those infections as well as optimizing treatment and prevention. This potential is bolstered by technologic advances in the imaging field such as the wider availability of high-resolution imaging of small animals (small animal PET, SPECT, CT, MRI), ability to use small reporter genes that would not affect virulence and growth characteristics of the virus in question (eg, nanoluciferase [5]), as well as advances in metabolic PET and immuno-PET imaging coupled with an ever-expanding assortment of specific probes that could be repurposed from their original use in other fields (eg, cancer imaging) to be used in viral infections.
MI of viral infections has flourished in recent years and many hurdles have been overcome, but many challenges remain. Notably, collaborations between the key players in the field, namely virologists and infectious diseases specialists on the one hand and imaging experts on the other, are still suboptimal. This could be improved by increasing awareness of the potential benefits of such collaborations through review articles such as this one, virtual seminars, and even imaging-dedicated sessions in national and international virology and infectious disease meetings. On the other hand, increasing the awareness and interest of imaging experts in the potential of repurposing existing animal models of viral infections to be used in imaging can be increased if the necessary expertise and virology knowledge is provided by collaborating specialists in the virology field.
The burgeoning field of MI of viral infections has not yet achieved its full potential. A glimpse of this potential was seen with COVID-19, and we hope to see MI utilized with future viral epidemics as part of a rapid research response. We also anticipate the use of MI to better understand how licensed preventive and therapeutic antiviral products impact disease. As an example, MI techniques could help us understand how the recently licensed RSV vaccines [64, 65] affect immune responses, disease transmission, and susceptibility to related infections. Unique attributes of MI make it a particularly attractive strategy for noninvasively assessing anatomic sanctuary sites where a virus may not be affected by the same factors that are acting in the periphery.
MI of viral infections has the potential of positively impacting diverse aspects of research and clinical care related to viral infections. Future MI investigations will benefit from development of new imaging ligands and techniques, optimization of animal models, and advancing technologies.
Contributor Information
Chuen-Yen Lau, HIV Dynamics and Replication Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA.
Neysha Martinez-Orengo, Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, Maryland, USA.
Anna Lyndaker, Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, Maryland, USA.
Kelly Flavahan, Center for Infection and Inflammation Imaging Research, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Reed F Johnson, SARS-CoV-2 Virology Core, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Swati Shah, Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, Maryland, USA.
Dima A Hammoud, Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, Maryland, USA.
Notes
Disclaimer. The contents of this publication do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Financial support. This work was supported by the Intramural Research Program at the Center for Infectious Disease Imaging, Department of Radiology and Imaging Sciences, Clinical Center, National Institutes of Health; and the Intramural Research Program at the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Supplement sponsorship . This article appears as part of the supplement “Seeing Is Believing: The Potential of Molecular Imaging Approaches for Infectious Diseases,” sponsored by the Center for Infectious Disease Imaging, Clinical Center, NIH (Intramural Research Program), and the Center for Infection and Inflammation Imaging Research, Johns Hopkins University School of Medicine, with individual contributions from Long Island Jewish Medical Center; University of California, San Francisco; and University of Maryland School of Pharmacy.
References
- 1. Schreiber-Stainthorp W, Solomon J, Lee JH, et al. Longitudinal in vivo imaging of acute neuropathology in a monkey model of Ebola virus infection. Nat Commun 2021; 12:2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alauddin MM, Gelovani JG. Radiolabeled nucleoside analogues for PET imaging of HSV1-tk gene expression. Curr Top Med Chem 2010; 10:1617–32. [DOI] [PubMed] [Google Scholar]
- 3. Luker KE, Luker GD. Bioluminescence imaging of reporter mice for studies of infection and inflammation. Antiviral Res 2010; 86:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luker GD, Luker KE, Sharma V, et al. In vitro and in vivo characterization of a dual-function green fluorescent protein–HSV1-thymidine kinase reporter gene driven by the human elongation factor 1 alpha promoter. Mol Imaging 2002; 1:65–73. [DOI] [PubMed] [Google Scholar]
- 5. Tran V, Moser LA, Poole DS, Mehle A. Highly sensitive real-time in vivo imaging of an influenza reporter virus reveals dynamics of replication and spread. J Virol 2013; 87:13321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chefer S, Seidel J, Cockrell AS, et al. The human sodium iodide symporter as a reporter gene for studying Middle East respiratory syndrome coronavirus pathogenesis. mSphere 2018; 3:e00540-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lemon K, de Vries RD, Mesman AW, et al. Early target cells of measles virus after aerosol infection of non-human primates. PLoS Pathog 2011; 7:e1001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buursma AR, de Vries EF, Garssen J, et al. [18f]FHPG positron emission tomography for detection of herpes simplex virus (HSV) in experimental HSV encephalitis. J Virol 2005; 79:7721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Vries EF, van Waarde A, Harmsen MC, Mulder NH, Vaalburg W, Hospers GA. [(11)C]FMAU and [(18)F]FHPG as PET tracers for herpes simplex virus thymidine kinase enzyme activity and human cytomegalovirus infections. Nucl Med Biol 2000; 27:113–9. [DOI] [PubMed] [Google Scholar]
- 10. Hatori A, Arai T, Yanamoto K, et al. Biodistribution and metabolism of the anti-influenza drug [11C]oseltamivir and its active metabolite [11C]Ro 64-0802 in mice. Nucl Med Biol 2009; 36:47–55. [DOI] [PubMed] [Google Scholar]
- 11. Shah S, Sinharay S, Patel R, et al. PET Imaging of TSPO expression in immune cells can assess organ-level pathophysiology in high-consequence viral infections. Proc Natl Acad Sci U S A 2022; 119:e2110846119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hammoud DA, Boulougoura A, Papadakis GZ, et al. Increased metabolic activity on 18F-fluorodeoxyglucose positron emission tomography-computed tomography in human immunodeficiency virus-associated immune reconstitution inflammatory syndrome. Clin Infect Dis 2019; 68:229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sinharay S, Srinivasula S, Schreiber-Stainthorp W, et al. Monitoring immune activation with whole-body fluorodeoxyglucose-positron-emission tomography in simian immunodeficiency virus-infected Rhesus macaques. Immunohorizons 2021; 5:557–67. [DOI] [PubMed] [Google Scholar]
- 14. Patel R, Manion MM, Laidlaw E, et al. Improvement of liver metabolic activity in people with advanced HIV after antiretroviral therapy initiation. AIDS 2022; 36:1655–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Mascio M, Srinivasula S, Kim I, et al. Total body CD4+ T cell dynamics in treated and untreated SIV infection revealed by in vivo imaging. JCI Insight 2018; 3:e97880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di Mascio M, Srinivasula S, Bhattacharjee A, et al. Antiretroviral tissue kinetics: in vivo imaging using positron emission tomography. Antimicrob Agents Chemother 2009; 53:4086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taglieri N, Bonfiglioli R, Bon I, et al. Pattern of arterial inflammation and inflammatory markers in people living with HIV compared with uninfected people. J Nucl Cardiol 2021; 29:1566–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lawal IO, Ankrah AO, Popoola GO, Lengana T, Sathekge MM. Arterial inflammation in young patients with human immunodeficiency virus infection: a cross-sectional study using F-18 FDG PET/CT. J Nucl Cardiol 2019; 26:1258–65. [DOI] [PubMed] [Google Scholar]
- 20. Lo J, Lu MT, Ihenachor EJ, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2015; 2:e52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zanni MV, Toribio M, Robbins GK, et al. Effects of antiretroviral therapy on immune function and arterial inflammation in treatment-naive patients with human immunodeficiency virus infection. JAMA Cardiol 2016; 1:474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sengupta S, Siliciano RF. Targeting the latent reservoir for HIV-1. Immunity 2018; 48:872–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lau CY, Adan MA, Maldarelli F. Why the HIV reservoir never runs dry: clonal expansion and the characteristics of HIV-infected cells challenge strategies to cure and control HIV infection. Viruses 2021; 13:2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Santangelo PJ, Rogers KA, Zurla C, et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat Methods 2015; 12:427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McMahon JH, Zerbato JM, Lau JSY, et al. A clinical trial of non-invasive imaging with an anti-HIV antibody labelled with copper-64 in people living with HIV and uninfected controls. EBioMedicine 2021; 65:103252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beckford-Vera DR, Flavell RR, Seo Y, et al. First-in-human immunoPET imaging of HIV-1 infection using (89)Zr-labeled VRC01 broadly neutralizing antibody. Nat Commun 2022; 13:1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lau CY, Adan MA, Earhart J, et al. Imaging and biopsy of HIV-infected individuals undergoing analytic treatment interruption. Front Med (Lausanne) 2022; 9:979756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022; 399:2047–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fuentes S, Arenas D, Moore MM, Golding H, Khurana S. Development of bioluminescence imaging of respiratory syncytial virus (RSV) in virus-infected live mice and its use for evaluation of therapeutics and vaccines. Vaccine 2017; 35:694–702. [DOI] [PubMed] [Google Scholar]
- 30. Frétaud M, Descamps D, Laubreton D, et al. New look at RSV infection: tissue clearing and 3D imaging of the entire mouse lung at cellular resolution. Viruses 2021; 13:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Uchil PD, Haugh KA, Pi R, Mothes W. In vivo imaging-driven approaches to study virus dissemination and pathogenesis. Annu Rev Virol 2019; 6:501–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Belser JA, Eckert AM, Huynh T, et al. A guide for the use of the ferret model for influenza virus infection. Am J Pathol 2020; 190:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jonsson CB, Camp JV, Wu A, et al. Molecular imaging reveals a progressive pulmonary inflammation in lower airways in ferrets infected with 2009 H1N1 pandemic influenza virus. PLoS One 2012; 7:e40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tran V, Poole DS, Jeffery JJ, et al. Multi-modal imaging with a toolbox of influenza A reporter viruses. Viruses 2015; 7:5319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rothlauf PW, Li Z, Pishesha N, et al. Noninvasive immuno-PET imaging of CD8+ T cell behavior in influenza A virus-infected mice. Front Immunol 2021; 12:777739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wonderlich ER, Swan ZD, Bissel SJ, et al. Widespread virus replication in alveoli drives acute respiratory distress syndrome in aerosolized H5N1 influenza infection of macaques. J Immunol 2017; 198:1616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bellani G, Guerra L, Pesenti A, Messa C. Imaging of lung inflammation during severe influenza A: H1N1. Intensive Care Med 2010; 36:717–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Amin R, Grinblat L, Husain M. Incidental COVID-19 on PET/CT imaging. CMAJ 2020; 192:E631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Doroudinia A, Tavakoli M. A case of coronavirus infection incidentally found on FDG PET/CT scan. Clin Nucl Med 2020; 45:e303–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rafiee F, Keshavarz P, Katal S, et al. Coronavirus disease 2019 (COVID-19) in molecular imaging: a systematic review of incidental detection of SARS-CoV-2 pneumonia on PET studies. Semin Nucl Med 2021; 51:178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saravanan UB, Namachivayam M, Jeewon R, Huang JD, Durairajan SSK. Animal models for SARS-CoV-2 and SARS-CoV-1 pathogenesis, transmission and therapeutic evaluation. World J Virol 2022; 11:40–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumari P, Rothan HA, Natekar JP, et al. Neuroinvasion and encephalitis following intranasal inoculation of SARS-CoV-2 in K18-hACE2 mice. Viruses 2021; 13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shou S, Liu M, Yang Y, et al. Animal models for COVID-19: hamsters, mouse, ferret, mink, tree shrew, and non-human primates. Front Microbiol 2021; 12:626553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Imai M, Iwatsuki-Horimoto K, Hatta M, et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci U S A 2020; 117:16587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bi Z, Hong W, Yang J, Lu S, Peng X. Animal models for SARS-CoV-2 infection and pathology. MedComm (2020) 2021; 2:548–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ruiz-Bedoya CA, Mota F, Ordonez AA, et al. (124)I-iodo-DPA-713 positron emission tomography in a hamster model of SARS-CoV-2 infection. Mol Imaging Biol 2022; 24:135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meijer L, Boszormenyi KP, Bakker J, et al. Novel application of [(18)F]DPA714 for visualizing the pulmonary inflammation process of SARS-CoV-2-infection in rhesus monkeys (Macaca mulatta). Nucl Med Biol 2022; 112–113:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Madden PJ, Thomas Y, Blair RV, et al. An immunoPET probe to SARS-CoV-2 reveals early infection of the male genital tract in rhesus macaques. bioRxiv. 2022. In press. doi: 10.1101/2022.02.25.481974. [DOI] [Google Scholar]
- 49. Vegeto E, Cuzzocrea S, Crisafulli C, et al. Estrogen receptor-alpha as a drug target candidate for preventing lung inflammation. Endocrinology 2010; 151:174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Keselman A, Fang X, White PB, Heller NM. Estrogen signaling contributes to sex differences in macrophage polarization during asthma. J Immunol 2017; 199:1573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fuentes N, Pesantez J, Nicoleau M, Wal RV, Chroneos Z, Silveyra P. Role of estrogen on alveolar macrophage polarization in response to particulate matter exposure. FASEB J 2019; 33(S1):735.2. [Google Scholar]
- 52. Solis O, Beccari AR, Iaconis D, et al. The SARS-CoV-2 spike protein binds and modulates estrogen receptors. Sci Adv 2022; 8:eadd4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Foster CC, Davis RA, Hausner SH, Sutcliffe JL. alphavbeta6-targeted molecular PET/CT imaging of the lungs after SARS-CoV-2 infection. J Nucl Med 2020; 61:1717–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang Z, Liu Z, Yang L, et al. Noninvasive mapping of angiotensin converting enzyme-2 in pigeons using micro positron emission tomography. Life (Basel) 2022; 12:793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhu H, Zhang H, Zhou N, et al. Molecular PET/CT profiling of ACE2 expression in vivo: implications for infection and outcome from SARS-CoV-2. Adv Sci (Weinh) 2021; 8:e2100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. SNMMI statement: possible effect of omicron infection on (18)F-FDG-based imaging. J Nucl Med 2022; 63:11N. [PubMed] [Google Scholar]
- 57. Centers for Diseases Control and Prevention . Middle East respiratory syndrome (MERS) clinical features. https://www.cdc.gov/coronavirus/mers/clinical-features.html. Accessed 29 December 2022.
- 58. de Wit E, Rasmussen AL, Falzarano D, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci U S A 2013; 110:16598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chefer S, Thomasson D, Seidel J, et al. Modeling [(18)F]-FDG lymphoid tissue kinetics to characterize nonhuman primate immune response to Middle East respiratory syndrome-coronavirus aerosol challenge. EJNMMI Res 2015; 5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chacko AM, Watanabe S, Herr KJ, et al. 18F-FDG As an inflammation biomarker for imaging dengue virus infection and treatment response. JCI Insight 2017; 2:e93474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dyall J, Johnson RF, Chefer S, et al. [(18)F]-fluorodeoxyglucose uptake in lymphoid tissue serves as a predictor of disease outcome in the nonhuman primate model of monkeypox virus infection. J Virol 2017; 91:e00897-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fang E, Liu X, Li M, et al. Construction of a dengue NanoLuc reporter virus for in vivo live imaging in mice. Viruses 2022; 14:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Osorio JE, Iams KP, Meteyer CU, Rocke TE. Comparison of monkeypox viruses pathogenesis in mice by in vivo imaging. PLoS One 2009; 4:e6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Papi A, Ison MG, Langley JM, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med 2023; 388:595–608. [DOI] [PubMed] [Google Scholar]
- 65. Walsh EE, Perez Marc G, Zareba AM, et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med 2023; 388:1465–77. [DOI] [PubMed] [Google Scholar]