SUMMARY
OBJECTIVE:
The aim of this study was to investigate the levels of leptin, growth hormone, insulin-like growth factor-1, and insulin-like growth factor binding protein-3 and their relations with clinical parameters in patients with primary fibromyalgia and healthy controls.
METHODS:
Our study was performed on 30 female patients with primary fibromyalgia and 30 healthy controls. The levels of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 were measured by a two-site immunoradiometric assay. The serum level of leptin was measured by the ELISA kit.
RESULTS:
The serum level of leptin was significantly higher, but the serum levels of insulin-like growth factor-1 were significantly lower in patients with fibromyalgia syndrome than healthy controls (p<0.001). The leptin level was positively correlated with the Visual Analog Scale, Fibromyalgia Impact Questionnaire score, Beck Depression Inventory score, tender point count, age, and duration of disease (p<0.001), but it was negatively correlated with insulin-like growth factor-1 (p<0.001). The insulin-like growth factor-1 level was negatively correlated with age, Visual Analog Scale, Fibromyalgia Impact Questionnaire and Beck Depression Inventory scores, duration of disease, and tender point count (p<0.001).
CONCLUSION:
Our results indicate that high levels of serum leptin and low levels of serum insulin-like growth factor-1 may play a role in the physiopathogenesis of fibromyalgia and may be related to some symptoms.
KEYWORDS: Fibromyalgia, Leptin, Growth hormone, IGF-1, IGFBP-3
INTRODUCTION
Primary fibromyalgia syndrome (FMS) is considered a multifactorial disorder characterized by widespread musculoskeletal pain, diffuse tenderness, psychological distress, fatigue, and sleep disturbances. Although several mechanisms are suggested, the etiology and pathophysiology of fibromyalgia have not yet been understood completely. Sleep disorders, changes in muscle oxygenation, and psychological, biochemical, hormonal, and immunological factors are suggested to be effective in the etiopathogenesis of FMS 1–3 . It is demonstrated that FMS symptoms are originated from the interaction between the autonomic nervous system, hypothalamus-pituitary-adrenal axis, and immune system. It was indicated that insulin-like growth factor (IGF-1) and growth hormone (GH) levels are low and this is a negative factor for skeletal muscle homeostasis. In experimental studies, it was shown that GH administration to fibromyalgia patients with low IGF-1 levels provides an improvement in FMS symptoms. IGF-1 has an anabolic effect and is the major mediator for GH needed in muscle homeostasis. In most studies, the level of serum IGF-1 is generally measured instead of GH because its half-life is very short. IGF-1 level is an indicator of GH secretion. Symptoms such as lack of energy seen in deficiency of GH in adults, poor general health, reduced exercise capacity, muscle weakness, cold intolerance, and impaired cognitive functions are similar to the symptoms described in FMS patients 4–8 . It is stated that the levels of leptin and growth factors (IGF-1 and insulin-like growth factor binding protein-3 (IGFBP-3)) vary in patients with fibromyalgia and this is a negative factor for homeostasis of the hypothalamic-pituitary-adrenal axis and skeletal muscle 5 . Leptin is in close interaction with HPA, thyroid, and GH axes. Both the sympathetic nervous system and the HPA axis regulate the secretion of leptin and are stress-activated. Leptin secretion is inhibited by the sympathetic nervous system while activated by the HPA system 8,9 . In recent years, the possible role of leptin in the etiology and pathogenesis of fibromyalgia has been suggested in some studies, but the results are conflicting 6–14 . Finding out the causes of symptoms can play a key role in the treatment of fibromyalgia. This study was planned to evaluate the relationship between FMS and leptin, a hormone that has a wide range of physiological effects, and GH, IGF-1, and IGFBP-3, which have important roles in different processes in organisms with clinical parameters.
METHODS
A total of 30 fibromyalgia patients, applied to Physical Medicine and Rehabilitation Outpatient Clinics of Dicle University Faculty of Medicine, having complaints of pain and fatigue, lasting for at least 3 months, existing 11 painful of 18 tender points, and aged between 25 and 55 years were included in the study, and 30 healthy females with similar age were included for the control group. These patients were examined by a rheumatologist to confirm or to exclude the FMS diagnosis according to the 1990 classification criteria of the American College of Rheumatology 15 . All individuals were assessed for clinical findings. Beck Depression Inventory (BDI) score for depression, Fibromyalgia Impact Questionnaire score (FIQ) for functional disability, Visual Analog Scale (VAS) for intensity of pain, and tender point count were evaluated. A standard form was fulfilled, evaluating whether there were complaints accompanied or not for both groups. Exclusion criteria were as follows: a recent or history of a defined systemic, metabolic, endocrine, tumoral, infectious, neurological, or cardiovascular disease, pregnancy, any drug or alcohol addict, those who have pain and limitation of movement in their lower extremity joints, and any medical treatment except for analgesics within the last month. The Human Studies Research Committee of the University of Dicle approved all procedures, and written informed consent was obtained from each subject before inclusion in the study.
Complete blood count, routine biochemical tests (i.e., serum glucose, insulin, urea, creatinine, uric acid, calcium, P, ALP, ALT, AST, LDH, CK, and GGT), erythrocyte sedimentation rate, C-reactive protein, PTH, GH, and thyroid function tests were performed. After 12 h of the hunger period, 5 mL of antecubital venous blood samples were taken from all subjects, and their sera were separated with centrifugation under 37°C for 5 min at 4,000 rpm. Separated sera were kept at −70°C until study. Within the working day, all sera were dissolved, and parameters were studied on the same day and at one time in the calibrated test machines.
The levels of IGF-1 and IGFBP-3 were measured by a two-site immunoradiometric assay (Diagnostic Systems Laboratories, Inc., TX, USA). For IGF-1, the sensitivity was 0.8 ng/mL, and intra-assay and inter-assay coefficients of variation were 3.8 and 4.9%, respectively. For IGFBP-3, the sensitivity was 0.8 ng/mL, and the intra-assay and inter-assay coefficients of variation were 5.6 and 7.1%, respectively. The assays were analyzed in duplicate. The level of leptin was measured by the ELISA kit (Cayman Chemicals, USA). The detection limit was 0.5 ng/mL. The intra-assay coefficients of variation were 6.0% (n=8) at 7.6 ng/mL and 2–3% (n=8) at 20.12 ng/mL. Serum insulin was measured by the electro-chemiluminescence immunoassay analyzers (Roche Elecsys-1010 and Modular Analytics E-170 Indianapolis, USA). The minimum detection limit was 0.2 μg/mL, and the intra-assay coefficient of variation was 4.9%. Common biochemical parameters were determined by standard laboratory methods.
Statistical analysis
SPSS 25.0 for the Windows program was used to perform statistical analysis. Definitive tests were applied to the data, and the mean value and standard deviation were found. The Kolmogorov-Smirnov distribution test was used for the examination of normal dispersion. Within two groups state, related to the comparison of quantitative data, independent-sample test and Mann-Whitney U test were used in the comparison of groups. Pearson correlation analysis was used within the cases showing normal distribution in the comparison of two quantitative data. Results were evaluated two-way, with 95% confidence interval and p<0.05 significance level.
RESULTS
The demographic, clinical, and biochemical characteristics of fibromyalgia and control groups are given in Table 1.
Table 1. Demographic and clinical characteristics and biochemical parameters of the fibromyalgia and control groups.
| Fibromyalgia group (n=30) | Control group (n=30) | p | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 37.90 | 9.37 | 38.23 | 8.76 | 0.784 |
| BMI (kg/m2) | 25.81 | 1.98 | 25.74 | 1.93 | 0.824 |
| Duration of disease (years) | 3.06 | 1.51 | – | – | – |
| Tender point count | 14.12 | 2.46 | 1.93 | 0.67 | <0.001 |
| VAS (pain intensity) | 5.85 | 1.26 | 2.57 | 1.09 | <0.001 |
| FIQ score | 58.19 | 9.53 | – | – | – |
| BDI score | 12.31 | 4.02 | 3.55 | 1.90 | <0.001 |
| Glucose (mg/dL) | 92.07 | 10.51 | 91.57 | 9.81 | 0.850 |
| Insulin (μU/mL) | 7.56 | 2.83 | 7.27 | 2.79 | 0.700 |
| GH (ng/mL) | 0.31 | 0.14 | 1.61 | 0.91 | <0.001 |
| Leptin (ng/mL) | 36.34 | 10.04 | 16.18 | 7.40 | <0.001 |
| IGF-1 (ng/mL) | 158.23 | 13.83 | 243.84 | 83.87 | <0.001 |
| IGFBP-3 (ng/mL) | 2561.64 | 597.37 | 2539.00 | 605.24 | 0.885 |
VAS: Visual Analog Scale; FIQ: Fibromyalgia Impact Questionnaire; BDI: Beck Depression Inventory; GH: growth hormone; IGF-1: insulin-like growth factor; IGFBP-3: insulin-like growth factor binding protein-3.
The levels of serum leptin, tender point score, VAS, FIQ score, and BDI score were significantly increased in women with FMS than in healthy women (p<0.01). Serum IGF-1 and GH levels were significantly lower in women with FMS than in healthy women (p<0.01). Fasting blood glucose, insulin, and IGFBP-3 were not found significantly different between healthy subjects and women with FMS (p>0.05).
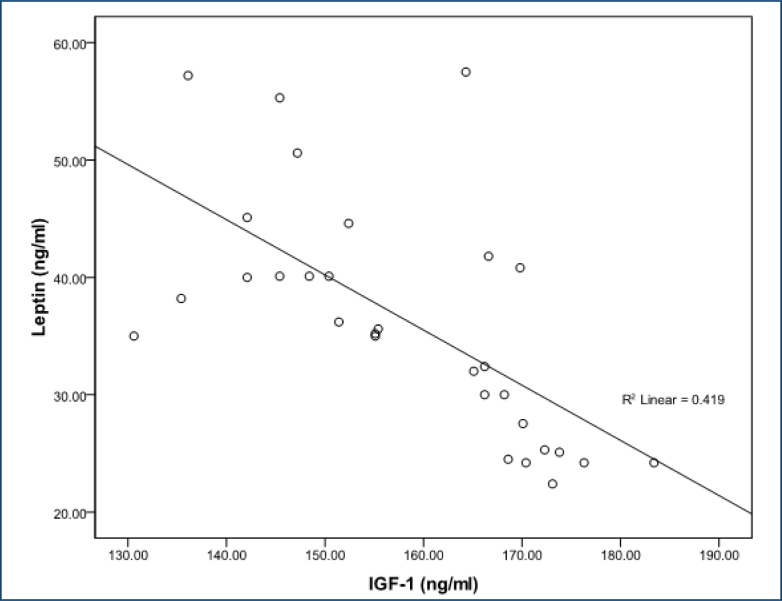
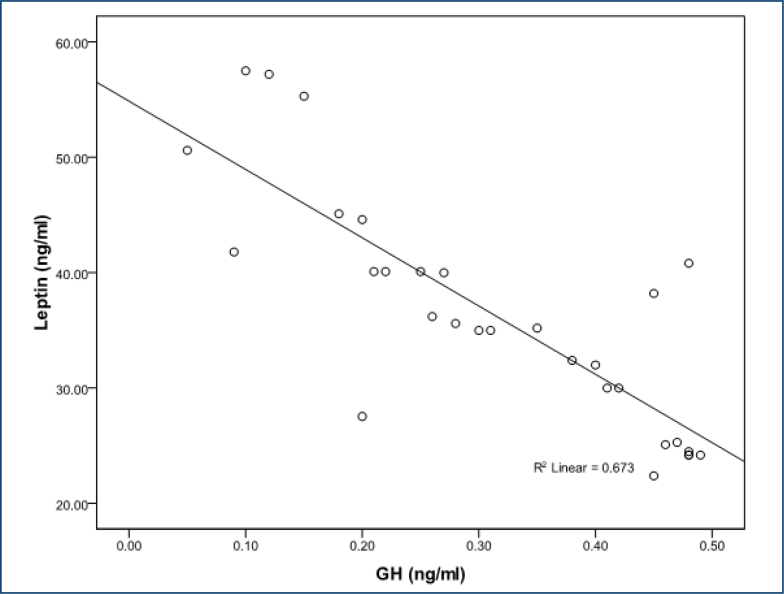
In women with FMS, serum leptin level was positively correlated with VAS score (r=0.643; p<0.001), FIQ score (r=0.681; p<0.001), tender point score (r=0.674; p<0.001), age (r=0.760; p<0.001), BDI score (r=0.783;p<0.001), and duration of disease (r=0.755; p<0.001), and leptin level was negatively correlated with IGF-1 (r=-0.648; p<0.001) and GH (r=-0.820 p<0.001) (Figures 1 and 2). The IGF-1 level was negatively correlated with age (r=-0.665; p<0.001), VAS score (r=-0.718; p<0.001), FIQ score (r=-0.545; p<0.001), tender point count (r=-0.460; p<0.001), BDI score (r=-0.543; p<0.001), and duration of disease (r=-0.798; p<0.001) (Figure 2). We found no association between glucose, insulin, and IGFBP-3 or other parameters in either subjects with fibromyalgia.
Figure 1. The correlations between leptin and the levels of insulin-like growth factor-1.
Figure 2. The correlations between leptin and the levels of growth hormone.
DISCUSSION
The etiology and pathogenesis of fibromyalgia have not been fully explained 1–3 . In our study, we measured serum leptin, IGF-1, and IGFBP-3 levels in patients with FMS and evaluated their relationship with clinical parameters. The results of the studies which show that changes in serum IGF-1 levels play a role in FMS physiopathogenesis are inconsistent 5,6,9,12,15–20 .
In their study on 47 patients with fibromyalgia and 28 healthy women, Tander et al. 5 found that GH and IGF-1 and IGFBP-3 levels were not significantly different from controls, but serum ghrelin levels were lower. In a study, McCall et al. 19 found the level of IGF-1 similar to those of the healthy control group; hence, with an increase in age and obesity, IGF-1 levels decrease. Also, Bjersing et al. 21 found that serum IGF-1 and IGFB3 levels remained same during 15 weeks of aerobic exercise in FMS patients. However, Bennett et al. 16 found that circulating IGF-1 and GH were low in patients compared with the control group. Atalay et al. 15 also found that low serum IGF-1 levels were associated with the number of tender points, muscle spasm, and stiffness in female patients with FMS.
Lack of GH secretion may also cause muscle microtrauma and/or distortion in the recovery of microtrauma in normal processes in patients at the same time. Many FMS patients have low serum GH levels, with a hypothesized etiology of dysregulated GH/IGF-I axis and in the sympathetic stress axis as well 17,18 . In another study, they were reported that the pituitary function was normal in fibromyalgia and the changes in the hypothalamus-GH-IGF-1 axis were largely due to hypothalamic origin and that GH cure should be recommended accordingly in future studies 20 .
Serum IGF-1 and GH levels in women with FMS were lower than in healthy women in our study compatible with the literature 15,16 . Consistent with the literature, IGFBP-3 levels in FMS were not different from the control group 5,21 .
Both hypothalamo-pituitary-IGF-1 axis and leptin levels may be altered in FMS due to increased somatostatin tone 5,19 . Ataoglu et al. 10 found the serum leptin level to be significantly higher in patients compared with the healthy group, but they could not find a significant relationship between leptin level and clinical and inflammatory parameters. On the other hand, another study found levels of serum leptin significantly higher in fibromyalgia patients 13 . In this study, it was recommended that the interaction of leptin and the HPA could play a role in FMS etiopathogenesis and further studies on leptin had to be done. Koca et al. 7 found that leptin (high), GH (low), and IGF-1 (low) levels in the FMS group were independent of disease severity but were statistically different and correlated with BMI. They suggested that these findings may be related to hypothalamo-pituitary axis dysfunction, BMI, and energy metabolism 22 .
In our study, we found out that serum leptin levels are high in FMS patients than those of healthy controls, while IGF-1 and GH are low; leptin levels were negatively correlated with IGF-1 and GH significantly. Increased leptin levels may play a role in the severity of fibromyalgia. Our results showed compatibility with the studies showing that there was an important relationship between high levels of leptin, low levels of IGF-1, and primary FMS. A decrease in IGF-1 and GH levels seems to be associated with pain scores. The duration of illness in the FMS group was similar to the literature. These findings about FMS associated with pain and depression are consistent with previous studies 23 .
In light of our study and literature, we can say that many of the clinical features of FMS may be related to neuroendocrine or metabolic events, that the levels of GH and IGF-1 in FMS are lower than in healthy controls, and that there is a deficiency in GH secretion. The greatest advantage of our study is that it is the first study analyzing the relationship between leptin levels based on clinical data such as BDI, VAS, and FIQ scores in addition to HPA axis hormones such as GH and IGF-1 in FMS patients. On the contrary, the subjective evaluation of clinical findings and the absence of an assessment tool such as an algometer are the shortcomings of our study. Besides, our study will be a good guide for studies to find the relationship between clinical parameters such as VAS, BDI, and FIQ scores, and leptin-IGF-1-GH axle.
Footnotes
Funding: none.
REFERENCES
- 1.Wolfe F, Butler SH, Fitzcharles M, Häuser W, Katz RL, Mease PJ, et al. Revised chronic widespread pain criteria: development from and integration with fibromyalgia criteria. Scand J Pain. 2019;20(1):77–86. doi: 10.1515/sjpain-2019-0054. [DOI] [PubMed] [Google Scholar]
- 2.Ruschak I, Montesó-Curto P, Rosselló L, Aguilar Martín C, Sánchez-Montesó L, Toussaint L. Fibromyalgia syndrome pain in men and women: a scoping review. Healthcare (Basel) 2023;11(2):223–223. doi: 10.3390/healthcare11020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deveci H. Relationship between fibromyalgia clinical and laboratory parameters with obesity. Pamukkale Med J. 2020;13:207–214. [Google Scholar]
- 4.Estrada-Marcén NC, Casterad-Seral J, Montero-Marin J, Serrano-Ostáriz E. Can an aerobic exercise programme ımprove the response of the growth hormone in fibromyalgia patients? A randomised controlled trial. Int J Environ Res Public Health. 2023;20(3):2261–2261. doi: 10.3390/ijerph20032261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tander B, Atmaca A, Aliyazicioglu Y, Canturk F. Serum ghrelin levels but not GH, IGF-1 and IGFBP-3 levels are altered in patients with fibromyalgia syndrome. Joint Bone Spine. 2007;74(5):477–481. doi: 10.1016/j.jbspin.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Hulens M, Dankaerts W, Rasschaert R, Bruyninckx F, Mulder P, Bervoets C. The link between empty sella syndrome, fibromyalgia, and chronic fatigue syndrome: the role of ıncreased cerebrospinal fluid pressure. J Pain Res. 2023;16:205–219. doi: 10.2147/JPR.S394321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koca TT, Berk E, Seyithanoğlu M, Koçyiğit BF, Demirel A. Relationship of leptin, growth hormone, and insulin-like growth factor levels with body mass index and disease severity in patients with fibromyalgia syndrome. Acta Neurol Belg. 2020;120(3):595–599. doi: 10.1007/s13760-018-01063-6. [DOI] [PubMed] [Google Scholar]
- 8.Katz RS. Leptin, a Hypothalamic signaling hormone, is elevated in fibromyalgia patients [abstract] [cited on Jan 28, 2023];Arthritis Rheumatol. 2017 69(suppl10) https://acrabstracts.org/abstract/leptin-a-hypothalamic-signaling-hormone-is-elevated-in-fibromyalgia-patients . [Google Scholar]
- 9.Musker M, McArthur A, Munn Z, Wong ML. Circulating leptin levels in patients with myalgic encephalomyelitis, chronic fatigue syndrome or fibromyalgia: a systematic review protocol. JBI Evid Synth. 2021;19(3):695–701. doi: 10.11124/JBIES-20-00125. [DOI] [PubMed] [Google Scholar]
- 10.Ataoglu S, Ankarali H, Samanci R, Ozsahin M, Admis O. The relationship between serum leptin level and disease activity and inflammatory markers in fibromyalgia patients. North Clin Istanb. 2018;5(2):102–108. doi: 10.14744/nci.2017.31644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ablin JN, Aronov N, Shimon I, Kanety H, Pariente C, Aloush V, et al. Evaluation of leptin levels among fibromyalgia patients before and after three months of treatment, in comparison with healthy controls. Pain Res Manag. 2012;17(2):89–92. doi: 10.1155/2012/350654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Homann D, Carvalho HM, Stefanello JM, Góes SM, Lopes AL, Oliveira AR, et al. Hyperleptinemia independent of body adiposity in women with fibromyalgia. Rheumatol Int. 2014;34(11):1593–1598. doi: 10.1007/s00296-014-2988-0. [DOI] [PubMed] [Google Scholar]
- 13.Paiva ES, Andretta A, Batista ED, Lobo MMMT, Miranda RC, Nisihara R, et al. Serum levels of leptin and adiponectin and clinical parameters in women with fibromyalgia and overweight/obesity. Arch Endocrinol Metab. 2017;61(3):249–256. doi: 10.1590/2359-3997000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghavidel-Parsa B, Bidari A, Hajiabbasi A, Shenavar I, Ghalehbaghi B, Sanaei O. Fibromyalgia diagnostic model derived from combination of American College of Rheumatology 1990 and 2011 criteria. Korean J Pain. 2019;32(2):120–128. doi: 10.3344/kjp.2019.32.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gümüş Atalay SG. The association of IGF-1 with clinical symptoms in female patients with fibromyalgia syndrome. Ankara Med J. 2018;18(3):410–418. [Google Scholar]
- 16.Bennett RM, Cook DM, Clark SR, Burckhardt CS, Campbell SM. Hypothalamic-pituitary-insulin-like growth factor-I axis dysfunction in patients with fibromyalgia. J Rheumatol. 1997;24(7):1384–1389. [PubMed] [Google Scholar]
- 17.Yuen KC, Bennett RM, Hryciw CA, Cook MB, Rhoads SA, Cook DM. Is further evaluation for growth hormone (GH) deficiency necessary in fibromyalgia patients with low serum insulin-like growth factor (IGF)-I levels? Growth Horm IGF Res. 2007;17(1):82–88. doi: 10.1016/j.ghir.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Hulens M, Dankaerts W, Rasschaert R, Bruyninckx F, Mulder P, Bervoets C. The link between empty sella syndrome, fibromyalgia, and chronic fatigue syndrome: the role of increased cerebrospinal fluid pressure. J Pain Res. 2023;16:205–219. doi: 10.2147/JPR.S394321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCall-Hosenfeld JS, Goldenberg DL, Hurwitz S, Adler GK. Growth hormone and insulin-like growth factor-1 concentrations in women with fibromyalgia. J Rheumatol. 2003;30(4):809–814. [PubMed] [Google Scholar]
- 20.Nadal-Nicolás Y, Rubio-Arias JÁ, Martínez-Olcina M, Reche-García C, Hernández-García M, Martínez-Rodríguez A. Effects of manual therapy on fatigue, pain, and psychological aspects in women with fibromyalgia. Int J Environ Res Public Health. 2020;17(12):4611–4611. doi: 10.3390/ijerph17124611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjersing JL, Dehlin M, Erlandsson M, Bokarewa MI, Mannerkorpi K. Changes in pain and insulin-like growth factor 1 in fibromyalgia during exercise: the involvement of cerebrospinal inflammatory factors and neuropeptides. Arthritis Res Ther. 2012;14(4):R162–R162. doi: 10.1186/ar3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batista JG, Soares JM, Maganhin CC, Simões RS, Tomaz G, Baracat EC. Assessing the benefits of rosiglitazone in women with polycystic ovary syndrome through its effects on insulin-like growth factor 1, insulin-like growth factor-binding protein-3 and insulin resistance: a pilot study. Clinics (Sao Paulo) 2012;67(3):283–287. doi: 10.6061/clinics/2012(03)14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao C, Zhong H, Chen L, Wang L, Yao H, Huang X, et al. Clinical and psychological assessment of patients with rheumatoid arthritis and fibromyalgia: a real-world study. Clin Rheumatol. 2022;41(4):1235–1240. doi: 10.1007/s10067-021-06026-6. [DOI] [PubMed] [Google Scholar]