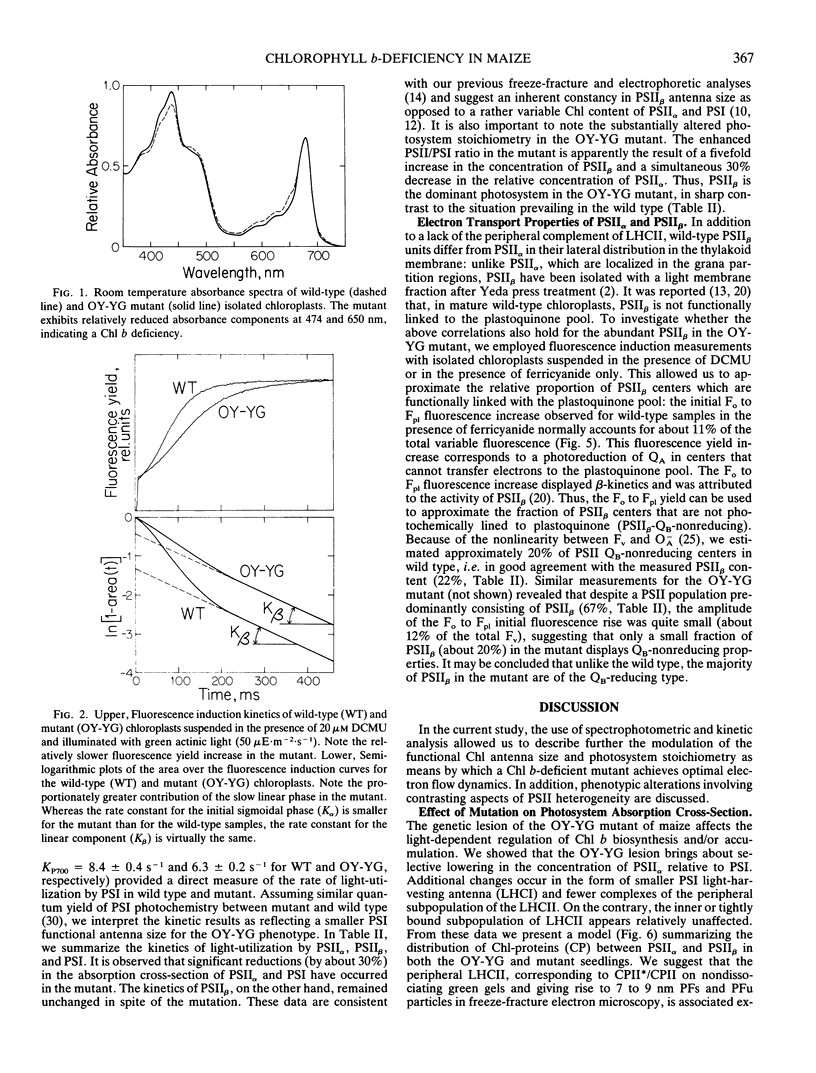
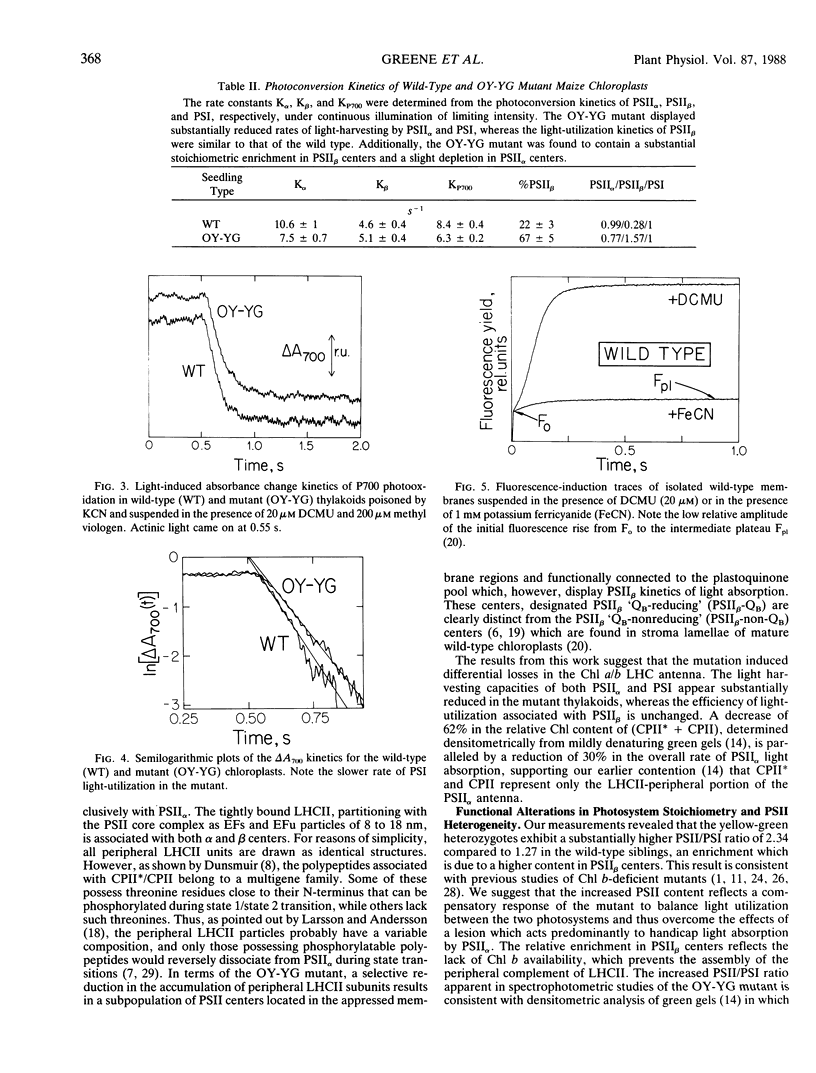
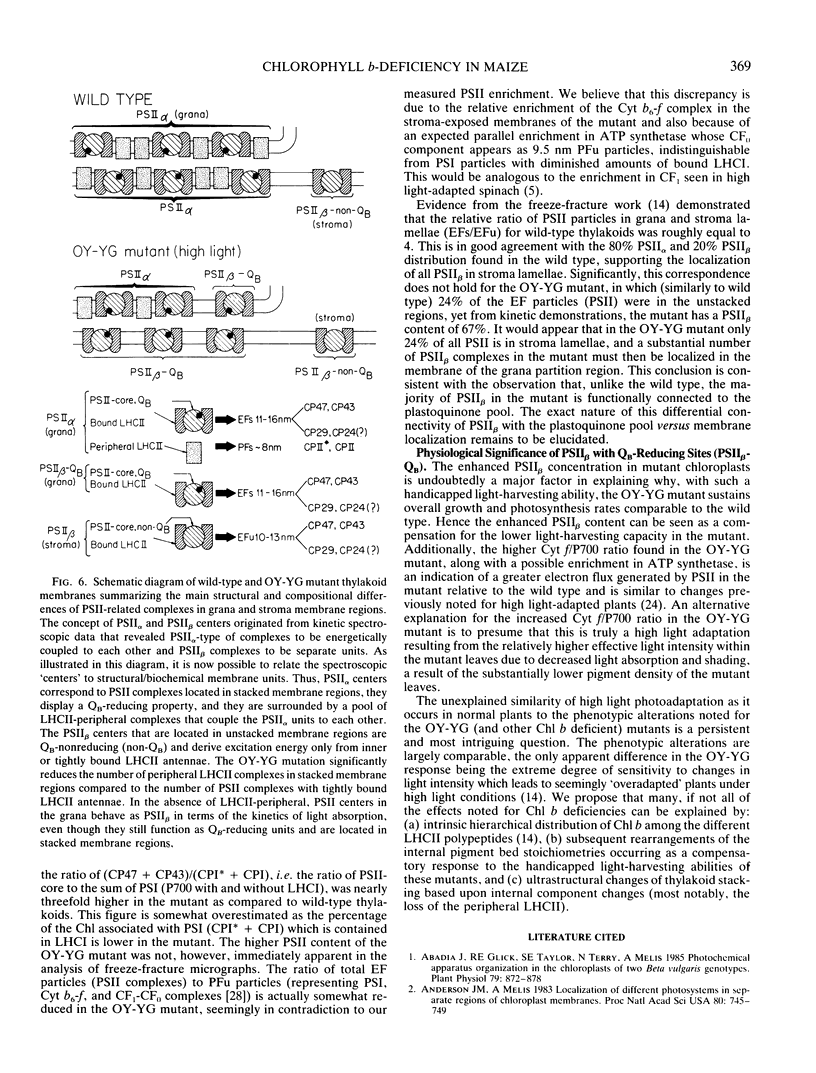
Abstract
Characterization of the functional organization of the photochemical apparatus in the light sensitive chlorophyll b-deficient oil yellow-yellow green (OY-YG) mutant of maize (Zea mays) is presented. Spectrophotometric and kinetic analysis revealed substantially lower amounts of the light harvesting complex of photosystem II (LHCII-peripheral) in high light-grown OY-YG thylakoids. However, accumulation of a tightly bound LHCII appears unaffected by the lesion. Changes in photosystem (PS) stoichiometry include lower amounts of PSII with characteristic fast kinetics (PSIIα) and a substantial accumulation of PSII centers with characteristic slow kinetics (PSIIβ) in the thylakoid membrane of the OY-YG mutant. Thus, PSIIβ is the dominant photosystem in the mutant chloroplasts. In contrast to wild type, roughly 80% of the mutant PSIIβ centers are functionally coupled to the plastoquinone pool and are probably localized in the appressed regions of the thylakoid membrane. These centers, designated PSIIβ-QB-reducing (QB being the secondary electron quinone acceptor of PSII), are clearly distinct from the typical PSIIβ-QB-nonreducing centers found in the stroma lamellae of wild-type chloroplasts. It is concluded that the observed changes in the stoichiometry of electron-transport complexes reflect the existence of a regulatory mechanism for the adjustment of photosystem stoichiometry in chloroplasts designed to correct any imbalance in light absorption by the two photosystems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abadia J., Glick R. E., Taylor S. E., Terry N., Melis A. Photochemical Apparatus Organization in the Chloroplasts of Two Beta vulgaris Genotypes. Plant Physiol. 1985 Nov;79(3):872–878. doi: 10.1104/pp.79.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. M., Melis A. Localization of different photosystems in separate regions of chloroplast membranes. Proc Natl Acad Sci U S A. 1983 Feb;80(3):745–749. doi: 10.1073/pnas.80.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmuir P. The petunia chlorophyll a/b binding protein genes: a comparison of Cab genes from different gene families. Nucleic Acids Res. 1985 Apr 11;13(7):2503–2518. doi: 10.1093/nar/13.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardi M. L., Melis A. Localization of photosynthetic electron transport components in mesophyll and bundle sheath chloroplasts of Zea mays. Arch Biochem Biophys. 1983 Jul 1;224(1):19–28. doi: 10.1016/0003-9861(83)90186-8. [DOI] [PubMed] [Google Scholar]
- Melis A., Brown J. S. Stoichiometry of system I and system II reaction centers and of plastoquinone in different photosynthetic membranes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4712–4716. doi: 10.1073/pnas.77.8.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A., Homann P. H. A selective effect of Mg2+ on the photochemistry at one type of reaction center in photosystem II of chloroplasts. Arch Biochem Biophys. 1978 Oct;190(2):523–530. doi: 10.1016/0003-9861(78)90306-5. [DOI] [PubMed] [Google Scholar]
- Melis A., Schreiber U. The kinetic relationship between the C-550 absorbance change, the reduction of Q(delta A320) and the variable fluorescence yield change in chloroplasts at room temperature. Biochim Biophys Acta. 1979 Jul 10;547(1):47–57. doi: 10.1016/0005-2728(79)90094-x. [DOI] [PubMed] [Google Scholar]
- Melis A., Thielen A. P. The relative absorption cross-sections of photosystem I and photosystem II in chloroplasts from three types of Nicotiana tabacum. Biochim Biophys Acta. 1980 Feb 8;589(2):275–286. doi: 10.1016/0005-2728(80)90044-4. [DOI] [PubMed] [Google Scholar]
- Pulles M. P., Van Gorkom H. J., Verschoor G. A. Primary reactions of photosystem II at low pH. 2. Light-induced changes of absorbance and electron spin resonance in spinach chloroplasts. Biochim Biophys Acta. 1976 Jul 9;440(1):98–106. doi: 10.1016/0005-2728(76)90116-x. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A., Arntzen C. J. Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol. 1983 Nov;97(5 Pt 1):1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]


