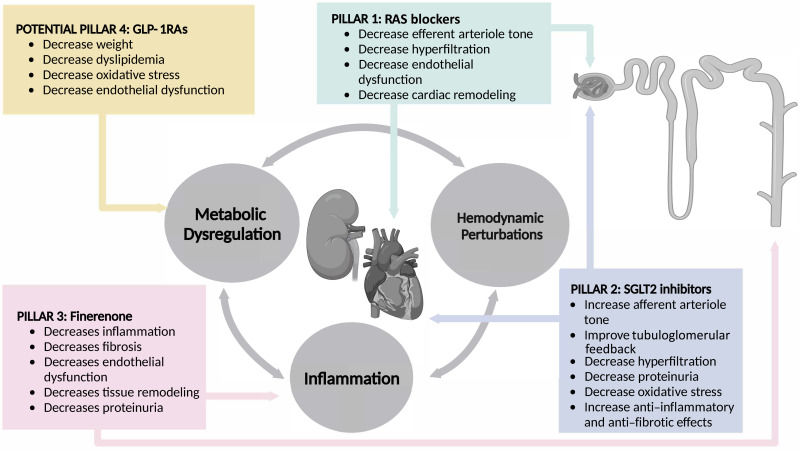
Abstract
Management of diabetic kidney disease (DKD) has evolved in parallel with our growing understanding of the multiple interrelated pathophysiological mechanisms that involve hemodynamic, metabolic, and inflammatory pathways. These pathways and others play a vital role in the initiation and progression of DKD. Since its initial discovery, the blockade of the renin-angiotensin system has remained a cornerstone of DKD management, leaving a large component of residual risk to be dealt with. The advent of sodium–glucose cotransporter 2 inhibitors followed by nonsteroidal mineralocorticoid receptor antagonists and, to some extent, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) has ushered in a resounding paradigm shift that supports a pillared approach in maximizing treatment to reduce outcomes. This pillared approach is like that derived from the approach to heart failure treatment. The approach mandates that all agents that have been shown in clinical trials to reduce cardiovascular outcomes and/or mortality to a greater extent than a single drug class alone should be used in combination. In this way, each drug class focuses on a specific aspect of the disease's pathophysiology. Thus, in heart failure, β-blockers, sacubitril/valsartan, a mineralocorticoid receptor antagonist, and a diuretic are used together. In this article, we review the evolution of the pillar concept of therapy as it applies to DKD and discuss how it should be used based on the outcome evidence. We also discuss the exciting possibility that GLP-1 RAs may be an additional pillar in the quest to further slow kidney disease progression in diabetes.
Graphical Abstract
Introduction
Diabetic kidney disease (DKD) is a serious microvascular complication that affects approximately 40% of individuals with diabetes (1). Presently the leading cause of end-stage kidney disease (ESKD) worldwide, DKD affects 700 million people, and it disproportionately affects those who are socially disadvantaged (2). The global percentage of prevalent ESKD patients with diabetes increased from 19.0% in 2000 to 29.7% in 2015, while the percentage of incident ESKD patients due to diabetes increased from 22.1% to 31.3% (3). In 2017, half of all patients in the U.S. with ESKD had diabetes reported as the primary etiologic cause (4). The current global prevalence of diabetes is 564 million and is projected to grow to 600 million by 2035 (5), reaching 783 million by 2045 (6).
Further, the number of renal replacement treatment (RRT) recipients has steadily climbed from 2.819 million in 2010 to a projected estimate of 4.35 million by 2030 (7). Albeit lifesaving, RRT expansion is not economically tenable, given the unhealthy aging trends of growing populations, and is often inaccessible to many low- and middle-income countries.
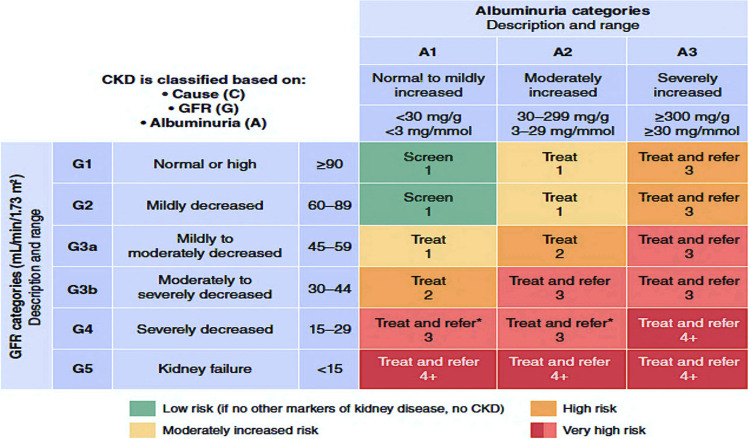
Characterized by progressive kidney fibrosis resulting in loss of function, chronic kidney disease (CKD) from diabetes or other causes is operationally defined by the Kidney Disease: Improving Global Outcomes (KDIGO) group as abnormalities of kidney structure or function, present for >3 months, with implications for health, and it requires one of two criteria documented or inferred for >3 months: either estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 or markers of kidney damage, including albuminuria. The level of albuminuria is defined as >30 mg/g or persistent albuminuria (>300 mg/24 h) spanning repeated measures of 3 or more months irrespective of eGFR (8). Notably, a small subgroup of people progress to ESKD without significant albuminuria (9). While the loss of kidney function can be devastating, there is a remarkable underappreciation of the significant contribution this progressive condition has on cardiovascular disease (CVD) risk, particularly when residual kidney function reaches eGFR <45 mL/min (10–12). The foremost goal of curtailing DKD progression is, therefore, to reduce CVD risk and then to avoid dialysis (11). Notably, death from cardiovascular (CV) causes emerges as the single most competing risk in CKD patients before reaching stage 4 CKD (eGFR <30 mL/min/1.73 m2), with 5.1% of people in stage 3 CKD and only 0.3% of people in stage 4 (13) (Fig. 1). Compared with levels for the general population, DKD confers a threefold higher risk of all-cause mortality and a 16-year loss in life expectancy. Reduced eGFR and albuminuria independently predict increased CV morbidity and mortality, and the presence of both exerts multiplicative effects on CV mortality risk (11,14,15). Recognition of the marked CV risk CKD portends is reflected in changes to clinical practice guidelines (8,16) that now include CKD among the highest-risk groups in screening and treatment recommendations (17).
Figure 1.
Heat map representing CKD staging by GFR and albuminuria and risk of further CKD deterioration. Adapted from Levey et al. (135).
Nothing beyond glycemic and blood pressure control was available to halt CKD progression until a trial of captopril in 1993 in people with type 1 diabetes (18). Results from this trial revealed a key role of the renin-angiotensin system (RAS) blockade for slowing DKD by approximately 5–7 mL/min/year. The following 8 years of research on angiotensin receptor blockers (ARBs) further solidified RAS blockade in all patients with diabetes (18–20). However, substantial residual risk for DKD progression persisted, as the rate of decline with RAS blockers was estimated to be between 4 and 6 mL/min/year and the normal annual decline is approximately 0.7–0.9 mL/min/year. Thus, additional therapies were needed. In 2014, the serendipitous discovery of sodium–glucose cotransporter 2 (SGLT2) inhibitors provided hope that DKD progression could be further slowed. This has clearly been shown in multiple outcomes trials (21). Around the same time as the discovery of SGLT2 inhibitors, trials were started on a novel class of agents, the nonsteroidal mineralocorticoid receptor antagonists (NS-MRAs), specifically finerenone. This compound was distinctly different from spironolactone and also slowed DKD, independent of SGLT2 inhibitor use (22). We now have two evidence-based medications, which, when combined with RAS inhibition, are proven to slow DKD progression to approximately 2.5–3 mL/min/year, provided blood pressure and glucose levels are at guideline goals.
The purpose of this review is to provide an update on what we now have as pillars of therapy to slow DKD. We will also discuss the glucagon-like peptide 1 receptor agonists (GLP-1 RAs), as they show promise and are currently being evaluated for modifying the downward trajectory of DKD.
Pathophysiologic Mechanisms and Potential Therapeutic Targets
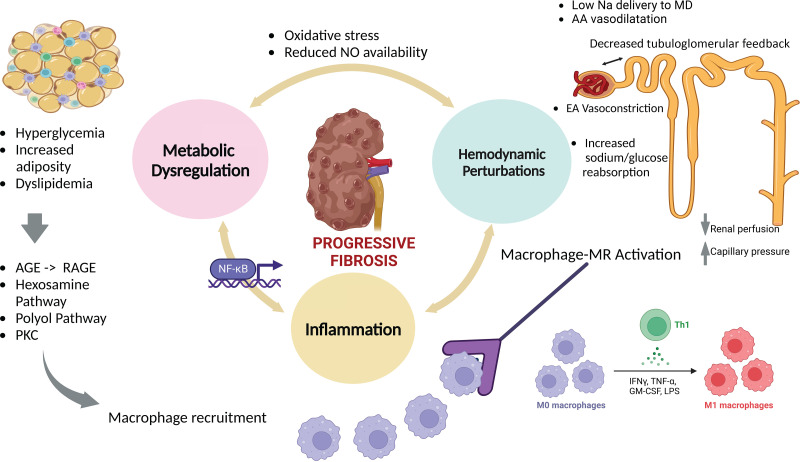
A detailed review of the mechanisms implicated in DKD progression is beyond the scope of this article; however, the reader is referred to some comprehensive articles on this topic (23,24). The mechanisms underlying DKD can be broadly conceptualized as stemming from an interplay of three key processes, each with variable contributions depending on the genetic makeup of an individual, which accounts for heterogeneity in the hemodynamic, metabolic, and inflammatory components (Fig. 2).
Figure 2.
Metabolic, hemodynamic, and inflammatory pathways implicated in the underlying pathophysiology of DKD, underscoring the need for multitargeted therapies to halt disease progression. MR is a pervasive ligand-activated transcription factor that exerts injury beyond the kidney to endothelial cells, adipocytes, smooth muscle cells, and immune cells (55,136). Once released in local tissue, inflammatory cytokines exert pleiotropic effects, setting in motion inflammatory and profibrotic processes that affect adjacent compartments and contribute to increased adverse cross talk between glomeruli, which contributes further to increased scarring (137). AA, afferent arteriole; AGE, advanced glycation end products; EA, efferent arteriole; GM-CSF, granulocyte macrophage colony-stimulating factor; IFNγ, γ-interferon; LPS, lipopolysaccharide; MD, macula densa; NO, nitric oxide; NF-κB, nuclear factor κ light-chain enhancer of activated β-cell; RAGE, receptor-bound advanced glycation end products.
Hemodynamic effects are central to the maintenance of nephron homeostasis and center around the renin-angiotensin-aldosterone system. The enzyme renin is key to the activation of the RAS. Produced by the juxtaglomerular cells of the nephron, renin is found in the area adjacent to the afferent arterioles. Angiotensin II, which is generated by activation of the RAS, binds avidly to two specific receptors, designated AT1 and AT2, that exert pleiotropic effects (25,26). AT1 activation mediates increased efferent arteriolar resistance of the nephron, which increases intraglomerular pressure to maintain the renal filtration rate (27). AT2 receptor activation, in contrast, modulates renal vasodilating prostaglandin release, thereby exerting protective counterregulatory action on blood pressure regulation, which opposes AT1 receptor action (28). High angiotensin II levels exert several nonhemodynamic effects that contribute to renal injury, including increased adrenal aldosterone secretion, induction of fibrogenic chemokines (monocyte chemoattractant protein-1 [MCP-1] and transforming growth factor-β [TGF-β]), and macrophage activation, which creates an inflammatory milieu (29–31) (Fig. 2).
Increases in intraglomerular pressure induced by RAS activation are among the early and well-characterized findings noted in up to 75% and 40% of individuals with type 1 and type 2 diabetes, respectively (32). Angiotensin II and endothelin contribute to the earliest changes in glomeruli exposed to hyperglycemia, which results in a mesangial expansion (33–36). These changes, along with inflammation, contribute to glomerulosclerosis over time (37).
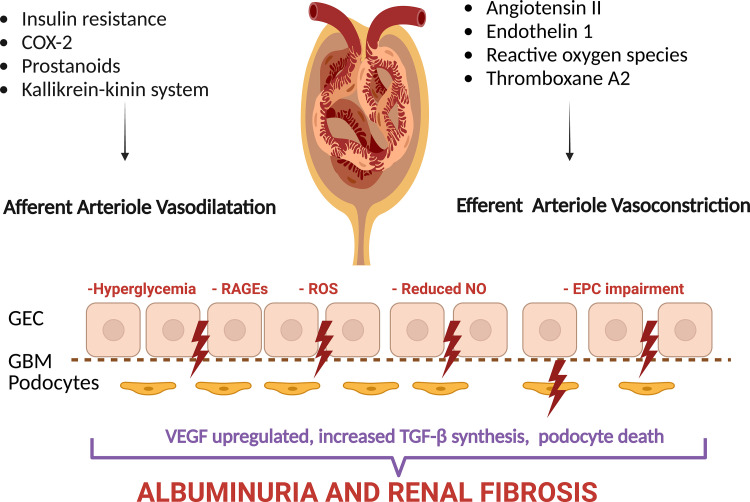
Podocytes are critical for maintaining the permselectivity of the glomerular filtration barrier (38). Podocyte injury leads to foot process effacement and podocyte loss, the unifying mechanism underlying albuminuria in diabetes (Fig. 3). RAS blockade improves permselectivity, whereas dihydropyridine calcium blockers like nifedipine, when used alone, worsen permselectivity (39).
Figure 3.
Local mechanisms underlying glomerular hypertension. Endothelin 1, reactive oxygen species, and thromboxane A2 increase efferent vessel tone, whereas insulin resistance upregulates cyclooxygenase 2 (COX-2), prostanoids, and the kallikrein-kinin system, resulting in afferent arteriole dilatation (138). RAS activation damages glomerular endothelial cells (GEC), which increases fenestrations and induces apoptosis. Hyperglycemia leads to advanced glycation end products (AGEs), which bind to their receptors (RAGEs), which decreases nitric oxide (NO) availability and stimulates transforming growth factor-β (TGF-β), a profibrotic factor. Diabetes further accelerates endothelial progenitor cell (EPC) aging, which reduces their reparative function. Vascular endothelial growth factor (VEGF) synthesis by podocytes is dysregulated. Podocyte injury leads to foot process effacement and podocyte loss, the unifying mechanism underlying albuminuria in diabetes (33–37).
Changes in tubular function can trigger glomerular hemodynamic changes via impaired tubuloglomerular feedback (40). In diabetes, supraphysiologic levels of glucose delivered to the proximal tubule upregulate SGLT1 and SGLT2 to maximize the reabsorption of glucose and sodium (41,42). Reduced sodium delivery to the distal nephron results in negative tubuloglomerular feedback (Fig. 2). Studies of individuals with type 1 diabetes demonstrate that to increase glomerular perfusion, local angiotensin production is upregulated, which triggers afferent arteriole dilatation and efferent arteriole constriction (41,43). This explains, in part, the renoprotective role of SGLT2 inhibitors under conditions of normal kidney function. By blocking glucose reabsorption at the proximal tubule and diverting it into the urine, tubuloglomerular balance is restored, with the net effect of lowering intraglomerular pressure and reducing hyperfiltration (44,45). However, this is not feasible when the eGFR is below 45 mL/min/1.73 m2, when autoregulation is not functional (46). Hence, the mechanism of renoprotection in advanced kidney disease is unclear. Note that these mechanisms have not been studied in type 1 diabetes.
Hyperglycemia, insulin resistance, and dyslipidemia commonly coexist, which sets in motion several dysregulated metabolic pathways inextricably related to oxidative stress and inflammatory processes, ultimately creating a vicious cycle where one process potentiates another (47,48). Most notable are the polyol and protein kinase C (PKC) pathways, which augment oxidative stress and deplete endothelial nitric oxide synthase, respectively, leading to higher endothelin-1 and vascular endothelial growth factor levels.
Endothelial instability and nuclear factor-κB (NF-κB)–mediated cytokines (tumor necrosis factor-α [TNF-α] and interleukin-6 [IL-6]) favor an inflammatory response. The hyperglycemic milieu also encourages the accumulation of advanced glycation end products (AGEs). AGEs are a heterogeneous group of nonenzymatically glycated molecules. Upon engaging with their receptors (RAGEs), which are found throughout the kidney, they trigger cellular function perturbations, including NF-κB upregulation, which induces a cascade of proinflammatory cytokines (TNF-α and IL-6). AGEs reduce the bioavailability of endothelium-derived nitric oxide and increase reactive oxygen species production, which is linked to impaired vasodilatation in diabetes (49) (Fig. 2).
Inflammation and fibrosis are major interrelated contributors to DKD progression. Mounting evidence implicates an intricate interaction between the mineralocorticoid receptor (MR) aldosterone and RAS-related C3 botulinum toxin substrate 1 (Rac1) in driving the inflammatory processes that lead to the final common pathway of fibrosis in DKD. The deleterious effects of MR activation in modulating inflammation and fibrosis were long recognized in animal studies of the heart and provide the therapeutic basis for MR antagonism (50,51). Animal studies showed similar benefits of MR blockade on the kidneys (52) and vasculature (53).
MR activation occurs in the aldosterone-responsive distal nephron, causing sodium reabsorption and potassium excretion. Aldosterone secretion is stimulated by RAS activation in response to decreased circulating plasma volume or significant increases in serum potassium levels. While critical for survival in states of low sodium intake, it becomes pathologic in the setting of persistently high sodium intake (54), as exemplified by Western and many Asian diets.
Inappropriate aldosterone signaling combined with high sodium intake results in hypertension, a direct contributor to glomerular injury and fibrosis. The MR possesses a binding affinity for cortisol and corticosterone similar to that of aldosterone. These cells typically coexpress 11B-dehydrogenase isoenzyme 2 (11B-HSD2), which neutralizes cortisol and thereby mitigates MR overactivation. Outside the distal nephron, MRs are expressed on other cell types, including podocytes, fibroblasts, vascular cells, and macrophages; these cells, however, do not uniformly coexpress the steroid-blunting effects of 11B-HSD2, which permits unbridled MR activation (55). MR is upregulated in hyperglycemia, insulin resistance, dyslipidemia, and obesity. This results in increased gene transcription of profibrotic factors plasminogen activator inhibitor-1 (PAI-1) and TGF-β1, connective tissue growth factor, and extracellular matrix proteins, all of which contribute to progressive DKD (56) (Fig. 3).
Innate immunity plays a critical role in the pathogenesis of DKD, but a detailed discussion is beyond the scope of this review. Briefly, macrophage infiltration has been identified as one of the hallmarks of DKD, the burden of which is associated with worse disease (57). Hyperglycemia, endothelial cell dysfunction, angiotensin II, AGEs, and oxidized LDL recruit macrophages (58). Macrophage-MR activation polarizes macrophage differentiation toward the M1 phenotype, which promotes inflammation via a cascade of injurious cytokines (59) (Figs. 2 and 4). Of note, however, is that MR inhibition favors macrophage switching toward an M2 anti-inflammatory phenotype with demonstrated beneficial effects in CKD (60). The therapeutic effects of MR antagonism in curtailing DKD were first reported in 2001 using animal models (61). There are no outcome trials with the steroidal MRAs due to tolerability issues. However, they have been shown in people with early DKD to reduce albuminuria and blood pressure significantly. In contrast, the NS-MRAs in the phase 2 trials of the Mineralocorticoid Receptor Antagonist Tolerability Study-Diabetic Nephropathy (ARTS-DN) and ARTS-DN Japan demonstrate up to 38% albuminuria reduction with finerenone compared with placebo in patients with albuminuric DKD (62,63). Other NS-MRAs, namely, finerenone, esaxerenone, and apararenone, also have demonstrated significant albuminuria reduction and a very low adverse effect profile in advanced DKD (64,65), although esaxerenone is only available in Japan (66). None of these agents have ever been tested in outcome trials.
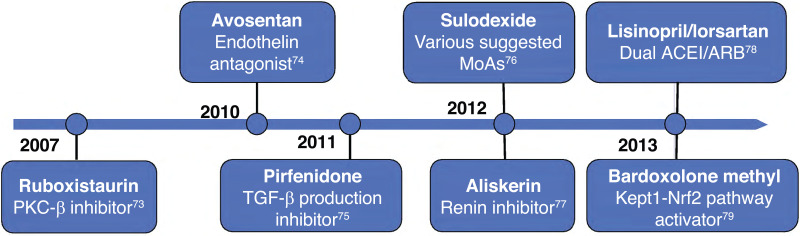
Figure 4.
The timeline of the major therapeutic outcome trials focused on delaying DKD in patients with type 2 diabetes and CKD following the publication of RENAAL and IDNT (73–79). MoAs, mechanisms of action.
Mechanism Application to Outcome Data and Guidelines: Pillars of Therapy
DKD management has evolved over the last 50 years. However, following the 2001 trials that showed a clear benefit of ARBs in slowing DKD progression, nothing was proven to further curtail advancing DKD until the advent of SGLT2 inhibitors. Many trials using novel targeted therapies were attempted between 2002 and 2010, all of which failed to show any benefit in changing the trajectory of DKD (Fig. 5). Since the approval of the first SGLT2 inhibitor, dapagliflozin, in January 2014, the field has added other drugs to this class. Additionally, approval of the NS-MRA finerenone, in July 2021, has further advanced the field from one to three therapies in the tool kit. Questions remain concerning when and how to use these drugs.
Figure 5.
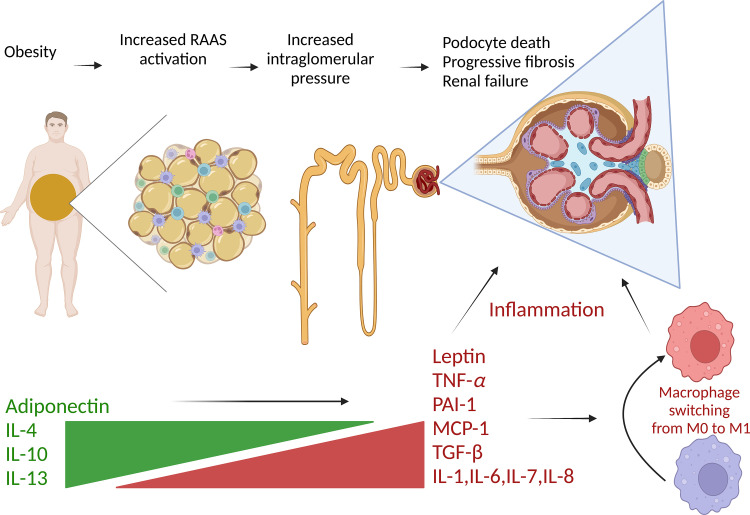
Obesity reduces the production of adiponectin in favor of leptin. Gene transcription of inflammatory mediators such as IL-1, IL-6, IL-7, IL-8, and TNF-α is increased, which creates a proinflammatory state and oxidative stress. Profibrotic factors plasminogen activator inhibitor-1 (PAI-1) and TGF-β1, connective tissue growth factor, and extracellular matrix proteins are increased, all contributing to progressive diabetic nephropathy. IL, interleukin; TNF-α, tumor necrosis factor-α; MCP-1, monocyte chemoattractant protein-1.
Albuminuria is an established continuous variable, where levels exceeding 30 mg/day predict adverse CV outcomes and those exceeding 300 mg/day are typically diagnostic of underlying kidney disease and are associated with accelerated decline in DKD (67,68). Many trials have established that a reduction in albuminuria is associated with slowed DKD progression and reduced CV event rates (69–72).
Given the advent of many new therapies over the past decade, we put forth the concept of pillars of therapy, originally adopted by heart failure cardiologists. The concept is akin to any building structure where no single beam alone can support its standing. Hence, we now have three established, proven therapies that, when used together, will maximally decelerate DKD progression.
Before discussing DKD outcome studies, it should be clear that these trials included people at high risk for DKD progression, defined by eGFR <60 mL/min/1.73 m2 as well as a urine albumin excretion rate (urine albumin-to-creatinine ratio [UACR]) >300 mg/day. This does not imply that those with high albuminuria, formerly called microalbuminuria, do not derive benefit from treatment—in fact, they do, and the benefits are predominantly CV. However, the slowing of the DKD trajectory in these cohorts was detected in post hoc analyses.
Pillar 1: RAS Blockers
The captopril trial was the first study to conclusively establish the value of ACE inhibition in delaying DKD, in patients with type 1 diabetes, where using an angiotensin I converting enzyme (ACEI) achieved a slower rate of decline in creatinine clearance (11% vs. 17% in placebo) and a striking 50% risk reduction in combined renal end points (dialysis, transplantation, or death) independent of blood pressure, which was managed with agents other than the experimental drug (18). This ushered in two additional landmark trials conducted in patients with type 1 diabetes, Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) (20) and Irbesartan Diabetic Nephropathy Trial (IDNT) (19), before RAS blockers became formally integrated into the standard of care (16). The modest improvement in eGFR decline to 4 mL/min/year in these trials implied a high residual risk of DKD progression; hence, other targeted therapies were developed. These included dual ACEI/ARB blockade (Veterans Affairs Nephropathy in Diabetes Study [VA NEPHRON D]), renin inhibition (Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints [ALTITUDE]), inhibition of PKC-β, endothelin antagonism, and inhibition of tumor growth factor-β production inhibition, among others, the results of which were all disappointing in attenuating DKD progression (73–79) (Fig. 5).
Pillar 2: SGLT2 Inhibitors
The introduction of SGLT2 inhibitors in January 2014 created a resounding paradigm shift and renewed excitement in improving DKD management. Although they were originally designed to lower glucose by promoting urinary glucose excretion, it was ultimately appreciated that SGLT2 inhibitors were, in effect, cardiorenal risk–reducing agents (80).
The renoprotective benefits of SGLT2 inhibitors were initially gleaned from secondary data analyses of trials with time to major adverse CV events as primary outcomes. In a meta-analysis of six double-blinded randomized trials of SGLT2 inhibitors in patients with type 2 diabetes, a consistent reduction in hospitalization for heart failure and progression to ESKD was found (81). Four of the trials, however, included patients with baseline GFR 60–98 mL/min/1.73 m2 or albuminuria that did not exceed 300 mg/day, arguing for a patient population that was at relatively low risk for rapid DKD decline. However, in three landmark trials that followed, where kidney outcomes were the defined primary end points in a high-risk cohort, the benefits of SGLT2 inhibitors when combined with optimized RAS blockade became universally accepted.
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial was the first nephropathy outcome trial to specify its primary outcome as ESKD, doubling of creatinine level, or death from renal or CV causes with an SGLT2 inhibitor. Canagliflozin achieved a 30% lower relative risk of reaching the primary end point and a 32% lower relative risk of ESKD progression. The unequivocal renal benefits prompted early trial termination (82). The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial, which was published a year later than the CREDENCE trial, randomized a mixed cohort of participants, i.e., two-thirds with diabetes and one-third without diabetes, to receive dapagliflozin versus placebo. Similar to findings in the CREDENCE trial, dapagliflozin achieved a 44% reduction in relative risk of sustained ≥50% reduction in eGFR, ESKD, or death from renal or CV causes and a 29% relative risk reduction in death from CV causes, a finding that was consistent across patients irrespective of diabetes status (83).
Most recently, The Study of Heart and Kidney Protection With Empagliflozin (EMPA-KIDNEY) outcome trial adds to the substantial body of evidence underscoring the robust salutary effects of SGLT2 inhibitors on renal outcomes. Over 6,600 participants with and without diabetes, with a wide range of GFR declines, from mild to severe, and with normal or high albuminuria, were randomized to empagliflozin versus placebo in addition to standard-of-care therapies. Over 2 years’ median duration, empagliflozin reduced the risk of ESKD progression or CV death by 28% compared with placebo (hazard ratio [HR] 0.72; 95% CI 0.64–0.82; P < 0.001), findings that generalized to patients with and without diabetes. Additionally, the empagliflozin group evidenced a 14% risk reduction in hospitalization from any cause compared with placebo (HR 0.86; 95% CI 0.78–0.95; P = 0.003) (84). It should further be noted that there were many people with normal and high albuminuria in this trial.
To put this in perspective, when all the SGLT2 inhibitor data are evaluated in concert with ACEI/ARB use, the declines seen in kidney function are diminished by nearly 30–40% above those of ACEI/ARB use alone (85). Hence, SGLT2 inhibitors serve as a solid second pillar to slow DKD progression (21).
Pillar 3: NS-MRAs
Mechanistically, blocking angiotensin II generation or action should partially inhibit aldosterone secretion, yet this is not long-standing due to “aldosterone escape,” which is the upstream accumulation of renin in the setting of long-term ACEI/ARB therapy, resulting in plasma aldosterone rise by overcoming RAS inhibition or by alternative pathways that bypass RAS. In one study using UACR as an indicator of the renoprotective effects of trandolapril (the longest-acting ACEI), aldosterone breakthrough was observed in 40% of patients at 40 weeks, when UACR started to increase during a relatively long period of ACEI use (86).
The hazardous sequela of MR activation by aldosterone shifted attention to developing therapies downstream of the RAS target, which ultimately addresses the inflammatory and ensuing profibrotic processes that contribute to DKD progression and heart failure. MR antagonism is not new. Steroid-based MRAs, including first- and second-generation spironolactone and eplerenone, respectively, continue to be used extensively in symptomatic heart failure patients (87). Use in DKD has been much more limited given the scarcity of supporting data and concern about hyperkalemia and further declines in kidney function, particularly if given in addition to ACEI/ARBs.
Data from outcome trials inform us that a sustained reduction in UACR of at least 30% is associated with slowing of DKD and decreased overall mortality (88,89). This has been adopted by the U.S. Food and Drug Administration as evidence of benefit and as a guideline by the American Diabetes Association. The exception is when a dual RAS blockade is used. In a meta-analysis of 829 patients on dialysis, MRA achieved a 66% reduction in CV mortality but a threefold increase in hyperkalemia risk compared with the control group (90). Therefore, MRAs have been generally contraindicated in advanced kidney disease (91).
Finerenone, apararenone, esaxerenone, and ocedurenone are members of a new class of NS-MRAs with pharmacologic properties distinct from those of their distant cousins, the steroidal agents. Finerenone is the only one developed and approved for cardiorenal risk reduction, whereas the others are approved only for blood pressure control, with no outcome data supporting use in DKD. Finerenone demonstrates superior anti-inflammatory and antifibrotic outcomes compared with its steroidal counterparts in preclinical studies (92–95). Unlike the steroidal MRAs, the NS-MRAs achieve balanced tissue distribution between the heart and kidney rather than affecting the kidney alone (22). Finerenone was studied in two complementary phase 3 randomized, double-blinded, placebo-controlled clinical trials that included over 13,000 participants with type 2 diabetes optimized on maximally tolerated RAS blockade before randomization to the NS-MRA or placebo (96,97). These trials were developed with the same protocol but different inclusion criteria, which allowed for an individual pooled patient analysis in the Finerenone in Chronic Kidney Disease and Type 2 Diabetes: Combined FIDELIO-DKD and FIGARO-DKD Trial Programme Analysis (FIDELITY) (98).
The Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) trial demonstrated that finerenone significantly reduced the kidney composite outcome of ESKD, sustained reduction in eGFR of >40%, or sustained reduction in renal death of 18% compared with placebo as well as the prespecified secondary CV end point of death from CV causes (nonfatal myocardial infarction, nonfatal stroke, or heart failure hospitalization) by 14% (96). Despite the similar frequency of adverse effects in both groups, the incidence of trial discontinuation related to hyperkalemia was slightly higher with finerenone than with placebo (2.3% and 0.9%, respectively). In Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD), which included patients with higher CV risk and less advanced DKD than patients in FIDELIO-DKD, finerenone reduced the primary CV outcome (composite death from CV causes, nonfatal myocardial infarction, nonfatal stroke, or heart failure hospitalization) by 13%, a benefit attributed largely to a lower incidence of heart failure hospitalizations. The frequency of reported adverse effects was similar to that for placebo, and the incidence of trial discontinuation related to hyperkalemia was slightly higher with finerenone than with placebo (1.2% and 0.4%, respectively).
The FIDELITY individual pooled patient analysis included 13,026 patients with type 2 diabetes and a wide range of CKD stages, from 1 to 4, and high to very high albuminuria. In this analysis, there was a 23% reduction in doubling of creatinine, ESKD, and renal death, with a significant 20% reduction in ESKD alone. There was also a 14% decreased risk in the composite CV outcomes that was largely driven by a significant reduction in heart failure (98). This is a very important finding given the notably higher risk of heart failure and premature death associated with having both diabetes and CKD (4,99,100). Studies of finerenone in type 1 diabetes are currently underway.
The incidence of clinically important adverse effects related to hyperkalemia was slightly higher for finerenone, with 1.7% treatment discontinuation versus 0.6% for the placebo, in a cohort of >6,500 patients per arm. However, unlike many other trials, all participants were required to be on maximally tolerated RAS blockade. Additionally, many participants at randomization had potassium levels that had increased to 5 mEq/L but were still randomized (98). Hence, the Food and Drug Administration label allows the use of finerenone with potassium levels up to 5 mEq/L.
Differences in hyperkalemia risk between the NS-MRA finerenone and its steroidal counterpart, spironolactone, are exemplified by a comparative outcome of a subgroup with resistant hypertension from the FIDELITY analysis and the Spironolactone With Patiromer in the Treatment of Resistant Hypertension in Chronic Kidney Disease (AMBER) trial (101). In this analysis, both groups had eGFR of 37 mL/min/1.73 m2, documented resistant hypertension, and the need for a fourth drug. In the FIDELITY study, the incidence of hyperkalemia was 11.2% with finerenone and 64.1% in the spironolactone group without a potassium binder (102).
Evidence for Potential Addition of Pillar 4: GLP-1 RAs
GLP-1 RAs are recommended for patients with DKD who have not met their glycemic targets despite optimization with metformin and SGLT2 inhibitors (103). A wave of CV outcome trials (CVOT) have demonstrated a significant reduction in atherosclerotic CV events by GLP-1 RAs and led to guideline revisions in 2020 that recommend the integration of GLP-1 RAs in the setting of type 2 diabetes, atherosclerotic disease, and/or high risk for CV events (104–106).
Post hoc analyses of these CVOT, like analyses of SGLT2 inhibitors, also demonstrated possible benefits in delaying DKD progression (107). Additionally, a large analysis of more than 12,500 participants pooled from the earliest trials of GLP-1 RAs, Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) (108) and Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) (109), evaluated changes in albuminuria, rate of annual eGFR change, and time to persistent eGFR declines. Compared with placebo, semaglutide and liraglutide evidenced a 24% reduction in albuminuria from baseline to 2 years (95% CI 20–27%; P < 0.001). Semaglutide and liraglutide were associated with significant slowing of annual eGFR decline, 0.87 and 0.26 mL/min/1.73 m2/year (P < 0.0001 and P < 0.001), respectively, compared with placebo, with benefits being more pronounced in patients with baseline eGFR <60 mL/min/1.73 m2. Semaglutide and liraglutide also lowered the risk of persistent eGFR declines to 40% and 50% (HR 0.86 [95% CI 0.75–0.99], P = 0.039, and HR 0.80 [95% CI 0.66–0.97], P = 0.023), respectively (110).
Effect of Efpeglenatide on Cardiovascular Outcomes (AMPLITUDE-O) is the latest published trial as of this writing to examine the efficacy of efpeglenatide compared with that of placebo on time to first major adverse CV event and secondary composite kidney outcomes (incident UACR of >300 mg/g, >30% UACR from baseline, sustained reduction in eGFR >40% for >30 days, sustained eGFR <15 mL/min/1.73 m2 for >30 days, or need for RRT >30 days) (111). This trial enrolled 4,076 patients with known type 2 diabetes and either current CKD (eGFR 25.0–60 mL/min/1.73 m2) or a history of CVD plus at least one other CV risk factor. Notably, there was a higher prevalence of CKD in the trial cohort than in the seven CVOT studies completed to date. Additionally, 21.8% of the participants had both DKD and CVD. Participants were on guideline-recommended cardiorenal protective therapies, but only 80% were on RAS blockade. Since 15.2% of participants were using SGLT2 inhibitors at study entry, stratified randomization was performed based on the current or potential future use of SGLT2 inhibitors to ensure group balance. Efpeglenatide achieved a 32% relative risk reduction in the composite kidney outcome compared with placebo (HR 0.68; 95% CI 0.57–0.79), independent of baseline SGLT2 inhibitor use (112).
The substantial and sustained weight loss, reaching 20% with some GLP-1 RAs, is important to note (113). Abdominal obesity is independently associated with albuminuria despite normoglycemia and normotension (114) and addresses an important subgroup of patients who have obesity-related glomerulopathy and who, by conventional screening, may be classified as metabolically healthy yet are still at risk for developing ESKD (115). This is not surprising given the inflammatory milieu engendered by excess adiposity (Fig. 5).
FLOW (A Research Study to See How Semaglutide Works Compared to Placebo in People With Type 2 Diabetes and Chronic Kidney Disease) is an ongoing randomized placebo-controlled trial that assesses the efficacy of semaglutide in type 2 diabetes and CKD and the first designed with primary renal end points (116). The renoprotective properties associated with GLP-1 RAs reviewed here were drawn from post hoc analyses of major CVOT, the evidence of which has been compelling, nonetheless.
Clinical Application
DKD management should start with maximally dosed RAS blockade; this is based on the doses used in the original RAS blocking trials, wherein dose reduction to avoid hyperkalemia resulted in markedly reduced protection against DKD decline. This was observed in a large analysis (N = 205,108) showing that submaximal ACEI/ARB dosing was associated with worse cardiorenal outcomes (117).
Each drug class that provided improved outcomes was coupled with RAS blockade and independently showed benefits on kidney and heart outcomes. These findings provide impetus to adopt a pillared approach for reducing cardiorenal events similar to how heart failure cardiologists have approached heart failure management. Notably, there has never been a trial that evaluated the simultaneous use of all four agents in heart failure or that compared different drug combinations against each other. Each trial was assessed on its merits within each drug class and then combined in retrospect to provide the best results. We currently have three agents with additive effects on albuminuria and heart failure outcomes (118) when combined individually with RAS blockade. An adequately statistically powered study that evaluates the three, or possibly soon-to-be four, different agents together would require well over 100,000 participants; therefore, extrapolation, which the cardiologists have done successfully, uses all three agents together over a short time.
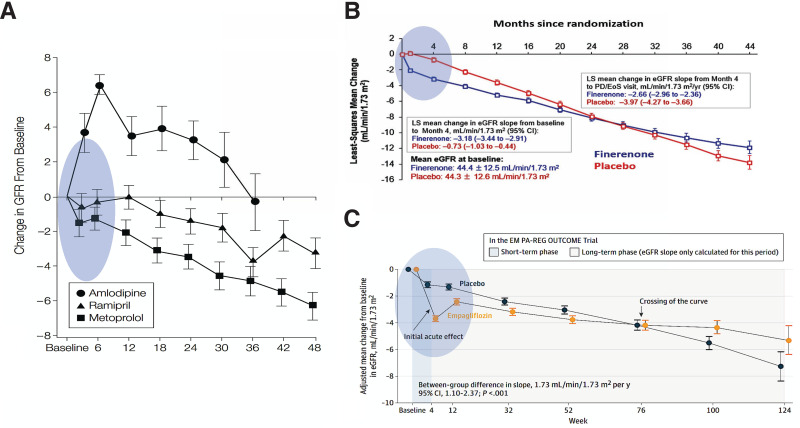
Practice guidelines articulate that clinicians should start first by titrating to maximally tolerated RAS blockade before introducing these medications (SGLT2 inhibitors, NS-MRAs, and GLP-1 RAs), as was done in pivotal clinical trials (119). This is challenging for many clinicians due to eGFR dipping observed at treatment initiation, reaching 10–30% (120,121) depending on hydration status and duration of suboptimally controlled hypertension, but this response is reassuring (Fig. 6). In a study examining long-term renal disease progression among patients with preexisting CKD (creatinine >1.4 mg/dL) who received ACEI, a strong correlation emerged between acute serum creatinine elevations of up to 30% and long-term renal function preservation (120), a pattern consistently observed across other studies (122,123). Initial changes in eGFR and associated long-term amelioration of renal function decline have also been reported with SGLT2 inhibitors (124) and finerenone, albeit to a lesser degree than with RAS blockers (96,120,125–128).
Figure 6.
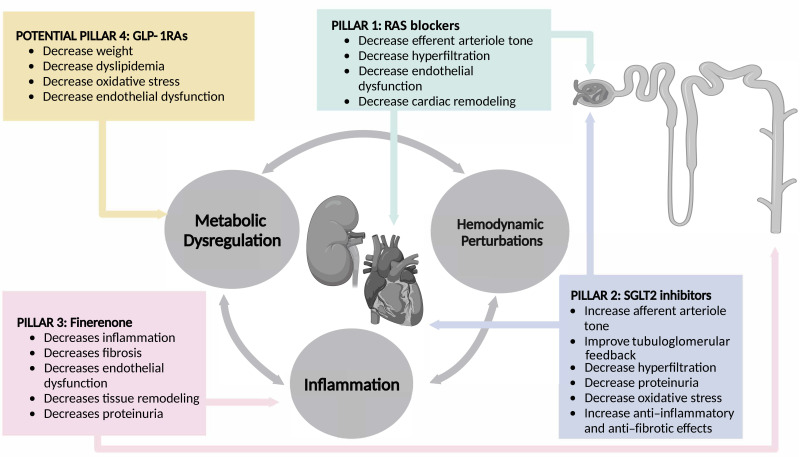
The manifold pathophysiological mechanisms involved in end-organ damage argue for a pillared approach with targeted therapies that have distinct pharmacodynamic actions (135).
The Additive Value of Drug Combination
An elegant study that used an animal model of preclinical hypertension–induced cardiorenal disease with a low-dose combination therapy of finerenone and empagliflozin revealed additive cardiorenal benefit above that of the respective dose-dependent monotherapy, as measured by reductions in blood pressure, proteinuria, cardiac fibrosis, vasculopathy, and mortality (129). These findings further argue strongly for distinct pharmacodynamic actions that counteract the manifold pathophysiological mechanisms involved in end-organ damage (Fig. 7).
Figure 7.
Acute changes in GFR slopes with three distinct classes of drugs with unique mechanisms that slow kidney disease progression associated diabetes. A: Estimated mean changes (SE) in GFR (mL/min/1.73 m2) from baseline through follow-up in three drug interventions (127). B: Rate of eGFR decline between finerenone and placebo. Despite equivalent GFR at baseline, the finerenone group shows stabilization of slowed GFR declines (96) and higher GFR declines at 4 months, which are associated with better long-term outcomes. C: Initial decline in GFR decline in the empagliflozin group compared with placebo at month 4 (128). The slopes eventually cross over at week 76, and analyses of the slopes in the long-term phase shows higher GFR decline in the placebo group than the empagliflozin group, P < 0.05. EMPA-REG Outcome, BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; LS, least squares.
Additionally, in a small open-label randomized crossover clinical study, the efficacy and safety of dapagliflozin and a low dose of the steroidal MRA eplerenone were evaluated in a cohort of patients with CKD. The combination of the two drugs was associated with an additive effect on albuminuria reduction compared with the use of either drug alone. Importantly, the incidence of hyperkalemia was significantly less in the combination group than in the group that received eplerenone alone (130). This is consistent with data from the larger FIDELIO-DKD trial that demonstrated greater protection from hyperkalemia when an SGLT2 inhibitor was combined with finerenone (131).
The use of combination therapies with NS-MRAs and SGLT2 inhibitors was further explored in the FIDELITY subgroup analysis, which revealed that, compared with placebo, cardiorenal benefits of finerenone were appreciably higher irrespective of concomitant GLP-1RA or SGLT2 inhibitor use at baseline or anytime during the trial. An important caveat is that GLP-1 RA and SGLT2 inhibitor users comprised 6.9% and 4.6% of the study population, respectively, precluding meaningful conclusions due to limited statistical power. More importantly, there was no sign that drug coadministration with finerenone increased any risk of kidney injury (132).
When combined with finerenone, SGLT2 inhibitors reduced hyperkalemic events compared with levels found in nonusers (8.1% vs. 18.7%). These are separate retrospective analyses of renal outcome trials clearly showing a protective effect of SGLT2 inhibitors from hyperkalemia in the setting of NS-MRA and MRA use (98,133). The Study to Learn How Well the Treatment Combination of Finerenone and Empagliflozin Works and How Safe It Is Compared to Each Treatment Alone in Adult Participants With Long-term Kidney Disease (Chronic Kidney Disease) and Type 2 Diabetes (CONFIDENCE) trial is an ongoing three-arm, double-blinded trial that will compare the efficacy of combined therapy with empagliflozin and finerenone versus that of each drug alone on the relative change in UACR over 8 months using a patient pool with type 2 diabetes and CKD stages 2 and 3 and UACR in the range of 300–5,000 mg/g (134).
Conclusions
Since the institution of the RAS blockade in the 1990s, we have witnessed significant strides in addressing the unmitigated risk associated with DKD progression. We now have two additional drug classes to add to the RAS blockers, SGLT2 inhibitors and NS-MRAs, bolstered by a robust body of outcome data, and a possible third class. The efficacy of GLP-1 RAs is supported by retrospective analyses but needs to be proven in the ongoing FLOW randomized clinical trial. The safety and tolerability of these two drug classes, when given together against a backdrop of maximal RAS blockade, are very encouraging and reflect the complexity of the underlying pathophysiology that drives DKD progression. Moreover, the protective role of SGLT2 inhibitors in preventing hyperkalemia with finerenone use is very heartening. In closing, endocrinologists, nephrologists, and cardiologists are strongly encouraged to use a pillared approach to DKD using the framework described, irrespective of the degree of kidney impairment, down to an eGFR of 25 mL/min/1.73 m2.
Article Information
Funding. S.C.N. was supported in part by a National Institutes of Health T32 award.
Duality of Interest. G.L.B. reports being a consultant to and on steering committees of several outcome trials of companies, including KBP Biosciences, Janssen, Novo Nordisk, Ionis, Alnylam, ESTAR, Bayer, and AstraZeneca. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.C.N. and G.L.B. researched the data and shared writing and editing responsibilities for the manuscript.
Footnotes
This article is featured in a podcast available at diabetesjournals.org/care/pages/diabetes_care_on_air.
References
- 1. Garg AX, Kiberd BA, Clark WF, Haynes RB, Clase CM. Albuminuria and renal insufficiency prevalence guides population screening: results from the NHANES III. Kidney Int 2002;61:2165–2175 [DOI] [PubMed] [Google Scholar]
- 2. Duru OK, Middleton T, Tewari MK, Norris K. The landscape of diabetic kidney disease in the United States. Curr Diab Rep 2018;18:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng HT, Xu X, Lim PS, Hung KY. Worldwide epidemiology of diabetes-related end-stage renal disease, 2000-2015. Diabetes Care 2021;44:89–97 [DOI] [PubMed] [Google Scholar]
- 4. National Institute of Diabetes and Digestive and Kidney Diseases . CKD in the General Population. Accessed 14 May 2023. Available from https://usrds-adr.niddk.nih.gov/2022/chronic-kidney-disease/1-ckd-in-the-general-population
- 5. NCD Risk Factor Collaboration (NCD-RisC) . Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 2022;183:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015;385:1975–1982 [DOI] [PubMed] [Google Scholar]
- 8. Kidney Disease: Improving Global Outcomes Diabetes Working Group . KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2022;102:S1–S127 [DOI] [PubMed] [Google Scholar]
- 9. Jin Q, Luk AO, Lau ESH, et al.; Hong Kong Diabetes Biobank Study Group . Nonalbuminuric diabetic kidney disease and risk of all-cause mortality and cardiovascular and kidney outcomes in type 2 diabetes: findings from the Hong Kong Diabetes Biobank. Am J Kidney Dis 2022;80:196–206.e1 [DOI] [PubMed] [Google Scholar]
- 10. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 2021;143:1157–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–1305 [DOI] [PubMed] [Google Scholar]
- 12. Abbott K, Basta E, Bakris GL. Blood pressure control and nephroprotection in diabetes. J Clin Pharmacol 2004;44:431–438 [DOI] [PubMed] [Google Scholar]
- 13. U.S. Renal Data System . Chronic Kidney Disease in the General Population. Accessed 14 May 2023. Available from https://usrds-adr.niddk.nih.gov/2022/chronic-kidney-disease/1-ckd-in-the-general-population
- 14. Astor BC, Hallan SI, Miller ER 3rd, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol 2008;167:1226–1234 [DOI] [PubMed] [Google Scholar]
- 15. Bello AK, Hemmelgarn B, Lloyd A, et al.; Alberta Kidney Disease Network . Associations among estimated glomerular filtration rate, proteinuria, and adverse cardiovascular outcomes. Clin J Am Soc Nephrol 2011;6:1418–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. ElSayed NA, Aleppo G, Aroda VR, et al.; American Diabetes Association . 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes–2023. Diabetes Care 2023;46:S191–S201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sarnak MJ, Levey AS, Schoolwerth AC, et al.; American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention . Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003;108:2154–2169 [DOI] [PubMed] [Google Scholar]
- 18. Lewis EJ, Hunsicker LG, Bain RP; The Collaborative Study Group . The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 1993;329:1456–1462 [DOI] [PubMed] [Google Scholar]
- 19. Lewis EJ, Hunsicker LG, Clarke WR, et al.; Collaborative Study Group . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–860 [DOI] [PubMed] [Google Scholar]
- 20. Brenner BM, Cooper ME, de Zeeuw D, et al.; RENAAL Study Investigators . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–869 [DOI] [PubMed] [Google Scholar]
- 21. Ma Y, Lin C, Cai X, et al. Baseline eGFR, albuminuria and renal outcomes in patients with SGLT2 inhibitor treatment: an updated meta-analysis. Acta Diabetol 2023;60:435–445 [DOI] [PubMed] [Google Scholar]
- 22. Kintscher U, Bakris GL, Kolkhof P. Novel non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Br J Pharmacol 2022;179:3220–3234 [DOI] [PubMed] [Google Scholar]
- 23. Watanabe K, Sato E, Mishima E, Miyazaki M, Tanaka T. What’s new in the molecular mechanisms of diabetic kidney disease: recent advances. Int J Mol Sci 2022;24:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tuttle KR, Agarwal R, Alpers CE, et al. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int 2022;102:248–260 [DOI] [PubMed] [Google Scholar]
- 25. Timmermans PB, Wong PC, Chiu AT, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev 1993;45:205–251 [PubMed] [Google Scholar]
- 26. Griendling KK, Lassègue B, Alexander RW. Angiotensin receptors and their therapeutic implications. Annu Rev Pharmacol Toxicol 1996;36:281–306 [DOI] [PubMed] [Google Scholar]
- 27. Goodfriend TL, Elliott ME, Catt KJ. Angiotensin receptors and their antagonists. N Engl J Med 1996;334:1649–1654 [DOI] [PubMed] [Google Scholar]
- 28. Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension 2000;35:155–163 [DOI] [PubMed] [Google Scholar]
- 29. Ames MK, Atkins CE, Pitt B. The renin-angiotensin-aldosterone system and its suppression. J Vet Intern Med 2019;33:363–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest 1996;98:1063–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruiz-Ortega M, Lorenzo O, Suzuki Y, Rupérez M, Egido J. Proinflammatory actions of angiotensins. Curr Opin Nephrol Hypertens 2001;10:321–329 [DOI] [PubMed] [Google Scholar]
- 32. Premaratne E, Verma S, Ekinci EI, Theverkalam G, Jerums G, MacIsaac RJ. The impact of hyperfiltration on the diabetic kidney. Diabetes Metab 2015;41:5–17 [DOI] [PubMed] [Google Scholar]
- 33. Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 2009;361:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bakris GL, Re RN. Endothelin modulates angiotensin II-induced mitogenesis of human mesangial cells. Am J Physiol 1993;264:F937–F942 [DOI] [PubMed] [Google Scholar]
- 35. Gaber L, Walton C, Brown S, Bakris G. Effects of different antihypertensive treatments on morphologic progression of diabetic nephropathy in uninephrectomized dogs. Kidney Int 1994;46:161–169 [DOI] [PubMed] [Google Scholar]
- 36. Bakris GL, Fairbanks R, Traish AM. Arginine vasopressin stimulates human mesangial cell production of endothelin. J Clin Invest 1991;87:1158–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hostetter TH. Hyperfiltration and glomerulosclerosis. Semin Nephrol 2003;23:194–199 [DOI] [PubMed] [Google Scholar]
- 38. Barutta F, Bellini S, Gruden G. Mechanisms of podocyte injury and implications for diabetic nephropathy. Clin Sci (Lond) 2022;136:493–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith AC, Toto R, Bakris GL. Differential effects of calcium channel blockers on size selectivity of proteinuria in diabetic glomerulopathy. Kidney Int 1998;54:889–896 [DOI] [PubMed] [Google Scholar]
- 40. Poursharif S, Hamza S, Braam B. Changes in proximal tubular reabsorption modulate microvascular regulation via the TGF system. Int J Mol Sci 2022;23:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tuttle KR. Back to the future: glomerular hyperfiltration and the diabetic kidney. Diabetes 2017;66:14–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016;134:752–772 [DOI] [PubMed] [Google Scholar]
- 43. Grabias BM, Konstantopoulos K. The physical basis of renal fibrosis: effects of altered hydrodynamic forces on kidney homeostasis. Am J Physiol Renal Physiol 2014;306:F473–F485 [DOI] [PubMed] [Google Scholar]
- 44. Tsimihodimos V, Filippatos TD, Filippas-Ntekouan S, Elisaf M. Renoprotective effects of SGLT2 inhibitors: beyond glucose reabsorption inhibition. Curr Vasc Pharmacol 2017;15:96–102 [DOI] [PubMed] [Google Scholar]
- 45. Ferrannini E. Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab 2017;26:27–38 [DOI] [PubMed] [Google Scholar]
- 46. Picken M, Long J, Williamson GA, Polichnowski AJ. Progression of chronic kidney disease after acute kidney injury: role of self-perpetuating versus hemodynamic-induced fibrosis. Hypertension 2016;68:921–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pichler R, Afkarian M, Dieter BP, Tuttle KR. Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am J Physiol Renal Physiol 2017;312:F716–F731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hojs R, Ekart R, Bevc S, Hojs N. Markers of inflammation and oxidative stress in the development and progression of renal disease in diabetic patients. Nephron 2016;133:159–162 [DOI] [PubMed] [Google Scholar]
- 49. Dou L, Jourde-Chiche N. Endothelial toxicity of high glucose and its by-products in diabetic kidney disease. Toxins (Basel) 2019;11:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res 1990;67:1355–1364 [DOI] [PubMed] [Google Scholar]
- 51. Brilla CG, Reams GP, Maisch B, Weber KT. Renin-angiotensin system and myocardial fibrosis in hypertension: regulation of the myocardial collagen matrix. Eur Heart J 1993;14(Suppl J):57–61 [PubMed] [Google Scholar]
- 52. Tostes RC, Touyz RM, He G, Chen X, Schiffrin EL. Contribution of endothelin-1 to renal activator protein-1 activation and macrophage infiltration in aldosterone-induced hypertension. Clin Sci (Lond) 2002;103(Suppl. 48):25S–30S [DOI] [PubMed] [Google Scholar]
- 53. Rocha R, Rudolph AE, Frierdich GE, et al. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol 2002;283:H1802–H1810 [DOI] [PubMed] [Google Scholar]
- 54. Nakamura T, Girerd S, Jaisser F, Barrera-Chimal J. Nonepithelial mineralocorticoid receptor activation as a determinant of kidney disease. Kidney Int Suppl (2011) 2022;12:12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barrera-Chimal J, Girerd S, Jaisser F. Mineralocorticoid receptor antagonists and kidney diseases: pathophysiological basis. Kidney Int 2019;96:302–319 [DOI] [PubMed] [Google Scholar]
- 56. Tesch GH, Young MJ. Mineralocorticoid receptor signaling as a therapeutic target for renal and cardiac fibrosis. Front Pharmacol 2017;8:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cantero-Navarro E, Rayego-Mateos S, Orejudo M, et al. Role of macrophages and related cytokines in kidney disease. Front Med (Lausanne) 2021;8:688060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tesch GH. Macrophages and diabetic nephropathy. Semin Nephrol 2010;30:290–301 [DOI] [PubMed] [Google Scholar]
- 59. Usher MG, Duan SZ, Ivaschenko CY, et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest 2010;120:3350–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Barrera-Chimal J, Estrela GR, Lechner SM, et al. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int 2018;93:1344–1355 [DOI] [PubMed] [Google Scholar]
- 61. Miric G, Dallemagne C, Endre Z, Margolin S, Taylor SM, Brown L. Reversal of cardiac and renal fibrosis by pirfenidone and spironolactone in streptozotocin-diabetic rats. Br J Pharmacol 2001;133:687–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ruilope LM, Agarwal R, Chan JC, et al. Rationale, design, and baseline characteristics of ARTS-DN: a randomized study to assess the safety and efficacy of finerenone in patients with type 2 diabetes mellitus and a clinical diagnosis of diabetic nephropathy. Am J Nephrol 2014;40:572–581 [DOI] [PubMed] [Google Scholar]
- 63. Katayama S, Yamada D, Nakayama M, et al.; ARTS-DN Japan Study Group . A randomized controlled study of finerenone versus placebo in Japanese patients with type 2 diabetes mellitus and diabetic nephropathy. J Diabetes Complications 2017;31:758–765 [DOI] [PubMed] [Google Scholar]
- 64. Bakris GL, Agarwal R, Chan JC, et al.; Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group . Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA 2015;314:884–894 [DOI] [PubMed] [Google Scholar]
- 65. Wada T, Inagaki M, Yoshinari T, et al. Apararenone in patients with diabetic nephropathy: results of a randomized, double-blind, placebo-controlled phase 2 dose-response study and open-label extension study. Clin Exp Nephrol 2021;25:120–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ito S, Shikata K, Nangaku M, Okuda Y, Sawanobori T. Efficacy and safety of esaxerenone (CS-3150) for the treatment of type 2 diabetes with microalbuminuria: a randomized, double-blind, placebo-controlled, phase II trial. Clin J Am Soc Nephrol 2019;14:1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bakris GL, Molitch M. Microalbuminuria as a risk predictor in diabetes: the continuing saga. Diabetes Care 2014;37:867–875 [DOI] [PubMed] [Google Scholar]
- 68. Patel RB, Colangelo LA, Reis JP, Lima JAC, Shah SJ, Lloyd-Jones DM. Association of longitudinal trajectory of albuminuria in young adulthood with myocardial structure and function in later life: Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA Cardiol 2020;5:184–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004;110:921–927 [DOI] [PubMed] [Google Scholar]
- 70. Holtkamp FA, de Zeeuw D, de Graeff PA, et al. Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J 2011;32:1493–1499 [DOI] [PubMed] [Google Scholar]
- 71. Ninomiya T, Perkovic V, de Galan BE, et al.; ADVANCE Collaborative Group . Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009;20:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schmieder RE, Schutte R, Schumacher H, et al.; ONTARGET/TRANSCEND Investigators . Mortality and morbidity in relation to changes in albuminuria, glucose status and systolic blood pressure: an analysis of the ONTARGET and TRANSCEND studies. Diabetologia 2014;57:2019–2029 [DOI] [PubMed] [Google Scholar]
- 73. Tuttle KR, McGill JB, Haney DJ, Lin TE, Anderson PW; PKC-DRS, PKC-DMES, and PKC-DRS 2 Study Groups . Kidney outcomes in long-term studies of ruboxistaurin for diabetic eye disease. Clin J Am Soc Nephrol 2007;2:631–636 [DOI] [PubMed] [Google Scholar]
- 74. Mann JF, Green D, Jamerson K, et al.; ASCEND Study Group . Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 2010;21:527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sharma K, Ix JH, Mathew AV, et al. Pirfenidone for diabetic nephropathy. J Am Soc Nephrol 2011;22:1144–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Packham DK, Wolfe R, Reutens AT, et al.; Collaborative Study Group . Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J Am Soc Nephrol 2012;23:123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Parving HH, Brenner BM, McMurray JJ, et al.; ALTITUDE Investigators . Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012;367:2204–2213 [DOI] [PubMed] [Google Scholar]
- 78. Fried LF, Emanuele N, Zhang JH, et al.; VA NEPHRON-D Investigators . Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013;369:1892–1903 [DOI] [PubMed] [Google Scholar]
- 79. de Zeeuw D, Akizawa T, Audhya P, et al.; BEACON Trial Investigators . Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 2013;369:2492–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bakris GL. Major advancements in slowing diabetic kidney disease progression: focus on SGLT2 inhibitors. Am J Kidney Dis 2019;74:573–575 [DOI] [PubMed] [Google Scholar]
- 81. McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021;6:148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 83. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al.; DAPA-CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446 [DOI] [PubMed] [Google Scholar]
- 84. EMPA-KIDNEY Collaborative Group . Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023;388:117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Al Dhaybi O, Bakris GL. Non-steroidal mineralocorticoid antagonists: prospects for renoprotection in diabetic kidney disease. Diabetes Obes Metab 2020;22(Suppl. 1):69–76 [DOI] [PubMed] [Google Scholar]
- 86. Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension 2003;41:64–68 [DOI] [PubMed] [Google Scholar]
- 87. McDonagh TA, Metra M, Adamo M, et al.; ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726 [DOI] [PubMed] [Google Scholar]
- 88. Schmieder RE, Mann JF, Schumacher H, et al.; ONTARGET Investigators . Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 2011;22:1353–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lea J, Greene T, Hebert L, et al. The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: results of the African American study of kidney disease and hypertension. Arch Intern Med 2005;165:947–953 [DOI] [PubMed] [Google Scholar]
- 90. Quach K, Lvtvyn L, Baigent C, et al. The safety and efficacy of mineralocorticoid receptor antagonists in patients who require dialysis: a systematic review and meta-analysis. Am J Kidney Dis 2016;68:591–598 [DOI] [PubMed] [Google Scholar]
- 91. Charytan DM, Himmelfarb J, Ikizler TA, et al.; Hemodialysis Novel Therapies Consortium . Safety and cardiovascular efficacy of spironolactone in dialysis-dependent ESRD (SPin-D): a randomized, placebo-controlled, multiple dosage trial. Kidney Int 2019;95:973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Grune J, Beyhoff N, Smeir E, et al. Selective mineralocorticoid receptor cofactor modulation as molecular basis for finerenone’s antifibrotic activity. Hypertension 2018;71:599–608 [DOI] [PubMed] [Google Scholar]
- 93. Kolkhof P, Delbeck M, Kretschmer A, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol 2014;64:69–78 [DOI] [PubMed] [Google Scholar]
- 94. Kolkhof P, Jaisser F, Kim SY, Filippatos G, Nowack C, Pitt B. Steroidal and novel non-steroidal mineralocorticoid receptor antagonists in heart failure and cardiorenal diseases: comparison at bench and bedside. Handb Exp Pharmacol 2017;243:271–305 [DOI] [PubMed] [Google Scholar]
- 95. Agarwal R, Kolkhof P, Bakris G, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J 2021;42:152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bakris GL, Agarwal R, Anker SD, et al.; FIDELIO-DKD Investigators . Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–2229 [DOI] [PubMed] [Google Scholar]
- 97. Filippatos G, Anker SD, Agarwal R, et al.; FIGARO-DKD Investigators . Finerenone reduces risk of incident heart failure in patients with chronic kidney disease and type 2 diabetes: analyses from the FIGARO-DKD trial. Circulation 2022;145:437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Agarwal R, Filippatos G, Pitt B, et al.; FIDELIO-DKD and FIGARO-DKD Investigators . Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022;43:474–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zareini B, Blanche P, D’Souza M, et al. Type 2 diabetes mellitus and impact of heart failure on prognosis compared to other cardiovascular diseases: a nationwide study. Circ Cardiovasc Qual Outcomes 2020;13:e006260. [DOI] [PubMed] [Google Scholar]
- 100. Lawson CA, Seidu S, Zaccardi F, et al. Outcome trends in people with heart failure, type 2 diabetes mellitus and chronic kidney disease in the UK over twenty years. EClinicalMedicine 2021;32:100739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Agarwal R, Rossignol P, Romero A, et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019;394:1540–1550 [DOI] [PubMed] [Google Scholar]
- 102. Agarwal R, Pitt B, Palmer BF, et al. A comparative post hoc analysis of finerenone and spironolactone in resistant hypertension in moderate-to-advanced chronic kidney disease. Clin Kidney J 2022;16:293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes–2021. Diabetes Care 2021;44(Suppl. 1):S111–S124 [DOI] [PubMed] [Google Scholar]
- 104. American Diabetes Association . 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes–2021. Diabetes Care 2021;44(Suppl. 1):S125–S150 [DOI] [PubMed] [Google Scholar]
- 105. Cosentino F, Grant PJ, Aboyans V, et al.; ESC Scientific Document Group . 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255–323 [DOI] [PubMed] [Google Scholar]
- 106. Kidney Disease: Improving Global Outcomes Diabetes Working Group . KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int 2020;98:S1–S115 [DOI] [PubMed] [Google Scholar]
- 107. Mann JFE, Buse JB, Idorn T, et al. Potential kidney protection with liraglutide and semaglutide: exploratory mediation analysis. Diabetes Obes Metab 2021;23:2058–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 110. Shaman AM, Bain SC, Bakris GL, et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation 2022;145:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lam CSP, Ramasundarahettige C, Branch KRH, et al. Efpeglenatide and clinical outcomes with and without concomitant sodium-glucose cotransporter-2 inhibition use in type 2 diabetes: exploratory analysis of the AMPLITUDE-O trial. Circulation 2022;145:565–574 [DOI] [PubMed] [Google Scholar]
- 112. Gerstein HC, Khurmi NS. Efpeglenatide and heart and kidney outcomes in type 2 diabetes. N Engl J Med 2021;385:2107 [Reply] [DOI] [PubMed] [Google Scholar]
- 113. Jastreboff AM, Aronne LJ, Ahmad NN, et al.; SURMOUNT-1 Investigators . Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022;387:205–216 [DOI] [PubMed] [Google Scholar]
- 114. Sarathy H, Henriquez G, Abramowitz MK, et al. Abdominal obesity, race and chronic kidney disease in young adults: results from NHANES 1999-2010. PLoS One 2016;11:e0153588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol 2006;17:1695–1702 [DOI] [PubMed] [Google Scholar]
- 116. Novo Nordisk A/S . A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW). In: ClinicalTrials.gov. Bethesda, MD, National Library of Medicine, 2019. Accessed 5 May 2023. Available from https://clinicaltrials.gov/ct2/show/NCT03819153
- 117. Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015;21(Suppl.):S212–S220 [PubMed] [Google Scholar]
- 118. Rossing P, Anker SD, Filippatos G, et al.; FIDELIO-DKD and FIGARO-DKD Investigators . Finerenone in patients with chronic kidney disease and type 2 diabetes by sodium-glucose cotransporter 2 inhibitor treatment: the FIDELITY analysis. Diabetes Care 2022;45:2991–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. de Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 2022;45:3075–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med 2000;160:685–693 [DOI] [PubMed] [Google Scholar]
- 121. Holtkamp FA, de Zeeuw D, Thomas MC, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 2011;80:282–287 [DOI] [PubMed] [Google Scholar]
- 122. Collard D, Brouwer TF, Olde Engberink RHG, Zwinderman AH, Vogt L, van den Born BH. Initial estimated glomerular filtration rate decline and long-term renal function during intensive antihypertensive therapy: a post hoc analysis of the SPRINT and ACCORD-BP randomized controlled trials. Hypertension 2020;75:1205–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Appel LJ, Wright JT Jr, Greene T, et al.; AASK Collaborative Research Group . Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010;363:918–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cherney DZI, Cosentino F, Dagogo-Jack S, et al.; VERTIS CV Investigators . Initial eGFR changes with ertugliflozin and associations with clinical parameters: analyses from the VERTIS CV Trial. Am J Nephrol 2022;53:516–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Oshima M, Jardine MJ, Agarwal R, et al. Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int 2021;99:999–1009 [DOI] [PubMed] [Google Scholar]
- 126. Kraus BJ, Weir MR, Bakris GL, et al. Characterization and implications of the initial estimated glomerular filtration rate “dip” upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int 2021;99:750–762 [DOI] [PubMed] [Google Scholar]
- 127. Wright JT Jr, Bakris G, Greene T, et al.; African American Study of Kidney Disease and Hypertension Study Group . Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002;288:2421–2431 [DOI] [PubMed] [Google Scholar]
- 128. Khan MS, Bakris GL, Shahid I, Weir MR, Butler J. Potential role and limitations of estimated glomerular filtration rate slope assessment in cardiovascular trials: a review. JAMA Cardiol 2022;7:549–555 [DOI] [PubMed] [Google Scholar]
- 129. Kolkhof P, Hartmann E, Freyberger A, et al. Effects of finerenone combined with empagliflozin in a model of hypertension-induced end-organ damage. Am J Nephrol 2021;52:642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Provenzano M, Puchades MJ, Garofalo C, et al.; ROTATE-3 Study Group; ROTATE-3 Study Group Members . Albuminuria-lowering effect of dapagliflozin, eplerenone, and their combination in patients with chronic kidney disease: a randomized crossover clinical trial. J Am Soc Nephrol 2022;33:1569–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Agarwal R, Joseph A, Anker SD, et al.; FIDELIO-DKD Investigators . Hyperkalemia risk with finerenone: results from the FIDELIO-DKD trial. J Am Soc Nephrol 2022;33:225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Rossing P, Agarwal R, Anker SD, et al.; FIDELIO-DKD Investigators . Efficacy and safety of finerenone in patients with chronic kidney disease and type 2 diabetes by GLP-1RA treatment: a subgroup analysis from the FIDELIO-DKD trial. Diabetes Obes Metab 2022;24:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Neuen BL, Oshima M, Perkovic V, et al. Effects of canagliflozin on serum potassium in people with diabetes and chronic kidney disease: the CREDENCE trial. Eur Heart J 2021;42:4891–4901 [DOI] [PubMed] [Google Scholar]
- 134. Green JB, Mottl AK, Bakris G, et al. Design of the combination effect of finerenone and empagliflozin in participants with chronic kidney disease and type 2 diabetes using an UACR endpoint study (CONFIDENCE). Nephrol Dial Transplant 2022;38:894–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011;80:17–28 [DOI] [PubMed] [Google Scholar]
- 136. Jaisser F, Farman N. Emerging roles of the mineralocorticoid receptor in pathology: toward new paradigms in clinical pharmacology. Pharmacol Rev 2016;68:49–75 [DOI] [PubMed] [Google Scholar]
- 137. Zou J, Yang J, Zhu X, et al. Stabilization of hypoxia-inducible factor ameliorates glomerular injury sensitization after tubulointerstitial injury. Kidney Int 2021;99:620–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Tonneijck L, Muskiet MH, Smits MM, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol 2017;28:1023–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]