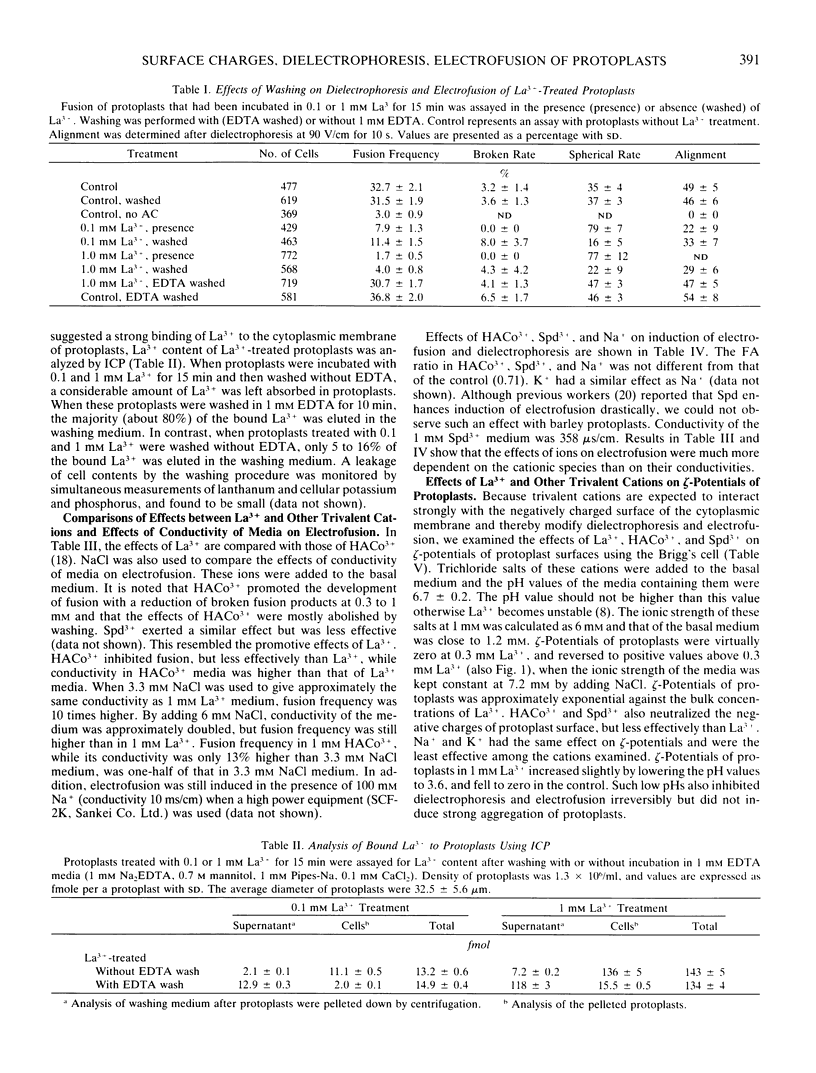
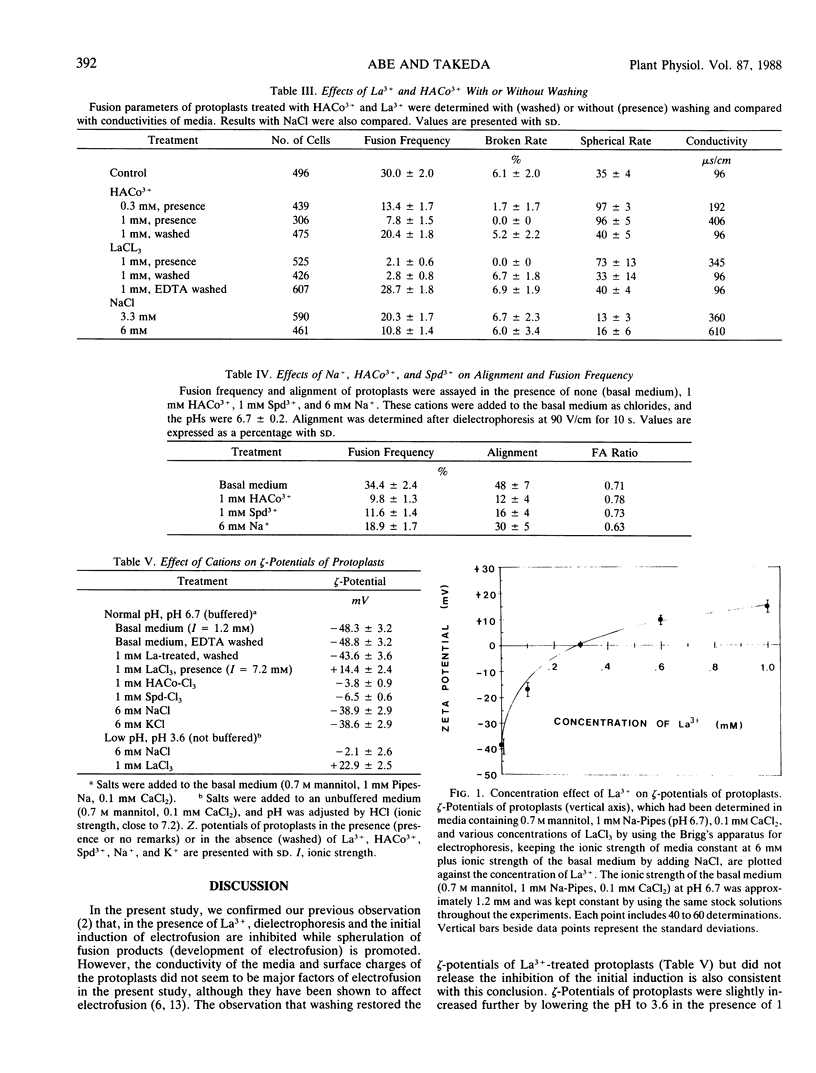
Abstract
When dielectrophoresis and electrofusion of barley (Hordeum vulgare var Moor) leaf protoplasts were assayed in the presence of 0.1 to 1 millimolar lanthanum ion (La3+) in the basal medium (0.7 molar mannitol, 1 millimolar piperazine-N, N-bis[2-ethanesulfonic acid]-Na [pH 6.7], 0.1 millimolar CaCl2), dielectrophoresis and induction of electrofusion were strongly inhibited. The latter remained inhibited and the former recovered by about 60% after washing the La3+ -treated protoplasts without EDTA. These inhibitions were almost completely abolished by washing the La3+ -treated protoplasts with 1 millimolar EDTA. Inductively coupled plasma atomic emission spectroscopic analysis revealed that protoplasts retained a considerable amount of La3+ after washing without EDTA and released most of the bound La3+ by washing with 1 millimolar EDTA. This tightly bound La3+ seemed responsible for the inhibition of electrofusion and dielectrophoresis that was observed in the La3+ -treated protoplasts after washing. ζ-potentials of protoplasts were -39.0±3.2 millivolts, -16.7 ± 2.6 millivolts, and virtually zero in media containing 0, 0.1, and 0.3 millimolar La3+ (I = 7.2 millimolar), respectively, and had a positive value (+ 14.2 ± 2.2 millivolts) in the presence of 1 millimolar La3+. These effects of La3+ on ζ-potentials were easily abolished by washing without EDTA. This indicates that charged species located at the surface of plasma membrane of protoplasts cannot account for the sites at which La3+ exerts its inhibition of dielectrophoresis and electrofusion. In contrast, the promotion of spherical fusion and the reduction of broken fusion products observed in the presence of La3+ were almost completely abolished by washing without EDTA. Our results also indicate that the initial induction and development of electrofusion can be studied independently.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe S., Takeda J. Possible involvement of calmodulin and the cytoskeleton in electrofusion of plant protoplasts. Plant Physiol. 1986 Aug;81(4):1151–1155. doi: 10.1104/pp.81.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Duerr A., Solomon F., Penman S. The outer boundary of the cytoskeleton: a lamina derived from plasma membrane proteins. Cell. 1979 Aug;17(4):859–865. doi: 10.1016/0092-8674(79)90326-x. [DOI] [PubMed] [Google Scholar]
- Blangero C., Teissié J. Ionic modulation of electrically induced fusion of mammalian cells. J Membr Biol. 1985;86(3):247–253. doi: 10.1007/BF01870604. [DOI] [PubMed] [Google Scholar]
- Christov A. M., Vaklinova S. G. Effects of prostaglandins e(2) and f(2alpha) on the electrofusion of pea mesophyll protoplasts. Plant Physiol. 1987 Mar;83(3):500–504. doi: 10.1104/pp.83.3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. B., Richardson F. S. Lanthanides as probes for calcium in biological systems. Q Rev Biophys. 1979 May;12(2):181–209. doi: 10.1017/s0033583500002754. [DOI] [PubMed] [Google Scholar]
- Morita H., Shimomura S., Okagawa K., Saito S., Sakigawa C., Sato H. Determination of germanium and some other elements in hair, nail, and toenail from persons exposed and unexposed to germanium. Sci Total Environ. 1986 Dec 31;58(3):237–242. doi: 10.1016/0048-9697(86)90203-2. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Okada Y. Electric pulse-induced fusion of mouse lymphoma cells: roles of divalent cations and membrane lipid domains. J Membr Biol. 1985;85(3):269–280. doi: 10.1007/BF01871522. [DOI] [PubMed] [Google Scholar]
- Saunders J. A., Roskos L. A., Mischke S., Aly M. A., Owens L. D. Behavior and viability of tobacco protoplasts in response to electrofusion parameters. Plant Physiol. 1986 Jan;80(1):117–121. doi: 10.1104/pp.80.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen D., Crothers D. M. Condensation of chromatin: role of multivalent cations. Biochemistry. 1986 Apr 8;25(7):1495–1503. doi: 10.1021/bi00355a004. [DOI] [PubMed] [Google Scholar]
- Theoharides T. C. Polyamines spermidine and spermine as modulators of calcium-dependent immune processes. Life Sci. 1980 Sep 1;27(9):703–713. doi: 10.1016/0024-3205(80)90323-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann U. Electrical breakdown, electropermeabilization and electrofusion. Rev Physiol Biochem Pharmacol. 1986;105:176–256. [PubMed] [Google Scholar]


