Abstract
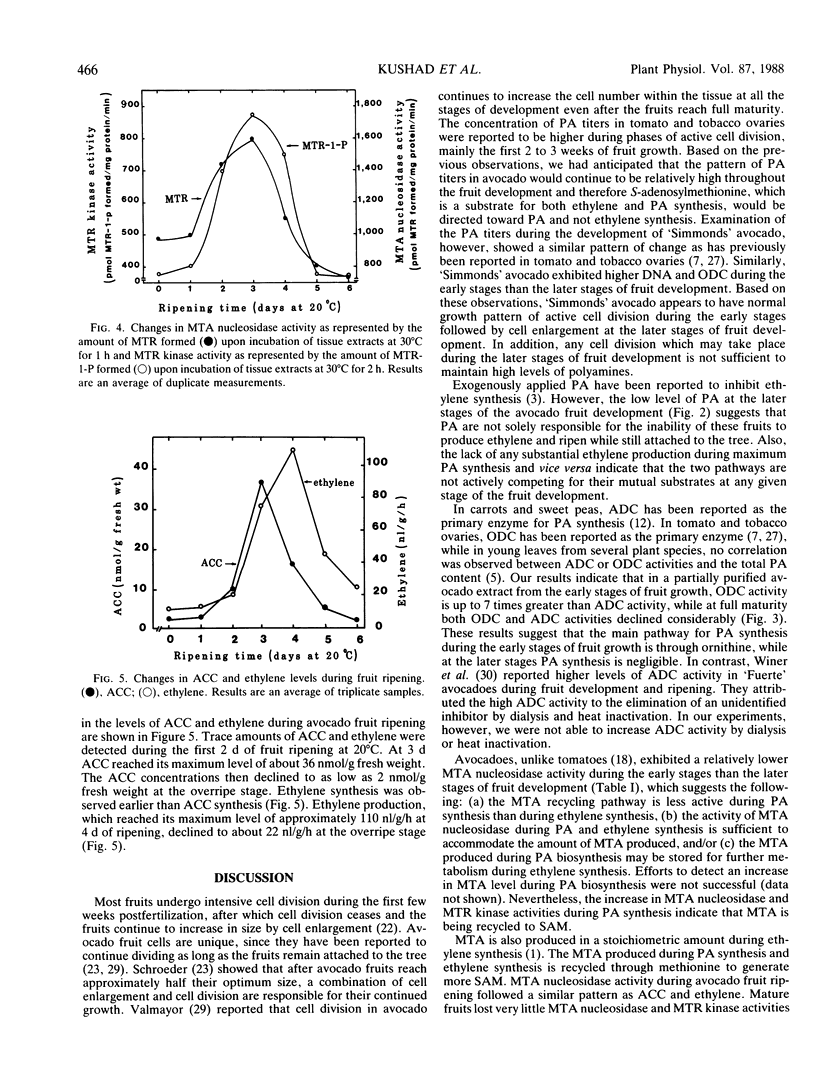
Concentrations of polyamines (PA) and the activities of the PA-synthesizing enzymes ornithine decarboxylase (ODC) and arginine decarboxylase (ADC) extracted from the mesocarp tissue of avocado (Persea americana Mill, cv `Simmonds') fruits at different stages of development were compared with DNA content and the activities of 5′-methylthioadenosine (MTA) nucleosidase and 5-methylthioribose (MTR) kinase. Putrescine, spermidine, and spermine were at their peak concentrations during the early stages of fruit development (362, 201, and 165 nanomoles per gram fresh weight, respectively, at 15 days from full bloom), then declined to 30% or less at full maturity. Agmatine showed only a slight change in concentration throughout the fruit development. The activity of ODC, which was low during flowering (8 nmoles per milligram protein per hour), increased more than threefold during the first 2 months then declined at the later stages of fruit development, while ADC activity showed only a slight increase. DNA content followed a similar pattern of change as that of PA and ODC. The decline in DNA and ODC activity suggest a lack of correlation between cell proliferation and PA at the later stages of the avocado fruit development. It is also possible that any cell division which may take place during the latter stages of the fruit development is not sufficient to alter the pattern of PA biosynthesis. MTA nucleosidase and MTR kinase activities increased during the first 15 days of fruit development followed by a slight decline at 60 and 90 days from full bloom. At 120 days (1 month before full maturity) both MTA nucleosidase and MTR kinase activities increased significantly. During maximum ethylene synthesis, MTA nucleosidase and MTR kinase activities were approximately fivefold and eightfold, respectively, higher than during maximum PA synthesis. The data indicate that the MTA molecules produced during PA and ethylene synthesis are actively metabolized to MTR and MTR-1-P, the two intermediates involved in the regeneration of S-adenosylmethionine from MTA. The data also suggest that the PA and ethylene biosynthetic pathways are not actively competing for the same substrates at any given stage of the avocado fruit development and ripening.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A., Burgoon A. C., Anderson J. D., Lieberman M. Polyamines inhibit biosynthesis of ethylene in higher plant tissue and fruit protoplasts. Plant Physiol. 1981 Aug;68(2):453–456. doi: 10.1104/pp.68.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer G. R., Meyers S. P., Molin W. T., Schrader L. E. A simple and sensitive DNA assay for plant extracts. Plant Physiol. 1982 Oct;70(4):999–1003. doi: 10.1104/pp.70.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birecka H., Bitonti A. J., McCann P. P. Activities of arginine and ornithine decarboxylases in various plant species. Plant Physiol. 1985 Oct;79(2):515–519. doi: 10.1104/pp.79.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cohen E., Arad S. M., Heimer Y. M., Mizrahi Y. Participation of ornithine decarboxylase in early stages of tomato fruit development. Plant Physiol. 1982 Aug;70(2):540–543. doi: 10.1104/pp.70.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feirer R. P., Mignon G., Litvay J. D. Arginine decarboxylase and polyamines required for embryogenesis in the wild carrot. Science. 1984 Mar 30;223(4643):1433–1435. doi: 10.1126/science.223.4643.1433. [DOI] [PubMed] [Google Scholar]
- Ferro A. J., Barrett A., Shapiro S. K. 5-Methylthioribose kinase. A new enzyme involved in the formation of methionine from 5-methylthioribose. J Biol Chem. 1978 Sep 10;253(17):6021–6025. [PubMed] [Google Scholar]
- Ferro A. J., Barrett A., Shapiro S. K. Kinetic properties and the effect of substrate analogues on 5'-methylthioadenosine nucleosidase from Escherichia coli. Biochim Biophys Acta. 1976 Jul 8;438(2):487–494. doi: 10.1016/0005-2744(76)90264-3. [DOI] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 1982 Mar;69(3):701–706. doi: 10.1104/pp.69.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer Y. M., Mizrahi Y., Bachrach U. Ornithine decarboxylase activity in rapidly proliferating plant cells. FEBS Lett. 1979 Aug 1;104(1):146–148. doi: 10.1016/0014-5793(79)81102-3. [DOI] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Shih L. M., Galston A. W. Relation of polyamine biosynthesis to the initiation of sprouting in potato tubers. Plant Physiol. 1982 Feb;69(2):411–415. doi: 10.1104/pp.69.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushad M. M., Richardson D. G., Ferro A. J. 5'-Methylthioadenosine Nucleosidase and 5-Methylthioribose Kinase Activities and Ethylene Production during Tomato Fruit Development and Ripening. Plant Physiol. 1985 Oct;79(2):525–529. doi: 10.1104/pp.79.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushad M. M., Richardson D. G., Ferro A. J. Intermediates in the recycling of 5-methylthioribose to methionine in fruits. Plant Physiol. 1983 Oct;73(2):257–261. doi: 10.1104/pp.73.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. P. Polyamine metabolism and its relation to response of the aleurone layers of barley seeds to gibberellic Acid. Plant Physiol. 1984 Apr;74(4):975–983. doi: 10.1104/pp.74.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- McDonald R. E., Kushad M. M. Accumulation of Putrescine during Chilling Injury of Fruits. Plant Physiol. 1986 Sep;82(1):324–326. doi: 10.1104/pp.82.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih L. M., Kaur-Sawhney R., Fuhrer J., Samanta S., Galston A. W. Effects of exogenous 1,3-diaminopropane and spermidine on senescence of oat leaves : I. Inhibition of protease activity, ethylene production, and chlorophyll loss as related to polyamine content. Plant Physiol. 1982 Dec;70(6):1592–1596. doi: 10.1104/pp.70.6.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum R. D., Galston A. W. Changes in polyamine biosynthesis associated with postfertilization growth and development in tobacco ovary tissues. Plant Physiol. 1985;79:336–343. doi: 10.1104/pp.79.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum R. D., Kaur-Sawhney R., Galston A. W. The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys. 1984 Dec;235(2):283–303. doi: 10.1016/0003-9861(84)90201-7. [DOI] [PubMed] [Google Scholar]
- Winer L., Vinkler C., Apelbaum A. Partial purification and characterization of arginine decarboxylase from avocado fruit, a thermostable enzyme. Plant Physiol. 1984 Sep;76(1):233–237. doi: 10.1104/pp.76.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]


