Abstract
Our thought states shift from one state to another from moment to moment. The relationship between the thought shifting and bodily responses is yet to be directly examined. This exploratory study examined the influence of cardiovascular reactivity and interoception—sensing an internal bodily state—on the shifting of thought states. Participants (N = 100, 70 women) completed two tasks: the heartbeat counting task (HCT) and the vigilance task (VT). We assessed their interoceptive accuracy through their performance on the HCT. The VT was a simple sustained attention task in which participants pressed a key when the target stimulus appeared and were asked to report their thoughts. We presented subliminal vibration stimuli to induce alterations in heart rate (i.e., vibration block). Results showed that participants with higher interoceptive accuracy reported more continuation of self-referential thought (about past episodes and future plans regarding themselves) during the vibration block than did those with lower interoceptive accuracy. These results suggest that individuals with higher interoceptive accuracy are more likely to be influenced by their subliminal bodily response, resulting in divergent attention from the task and intermittent self-referential thought.
Subject terms: Psychology, Human behaviour
Introduction
In our daily lives, we experience various states of thought, such as when something comes to mind unintentionally (spontaneous thought), when the content of our thoughts shifts from one thought to another (mind-wandering; MW), or when we continue to think deeply about a certain matter (goal-directed thought;1). What triggers such thought shifting has primarily been pursued in MW research. MW refers to the phenomenon of one’s thoughts wandering to things unrelated to the present context2. It has been estimated to occupy between 20 and 50% of our daily lives and mainly involves involuntary autobiographical memories3, spontaneous future thinking4, prospective memories5, and intrusive memories6. Various factors have been reported to contribute to the occurrence of MW. The difficulty and proficiency of action7,8, personal interest in tasks8, differences in individual working memory capacity (attentional resources needed to temporarily hold the information necessary to act)9, fatigue, boredom, and mood state during a task10,11 are factors that could contribute to the occurrence of MW. Moreover, the perception of external stimuli has received much attention as a trigger for thought shifting before and after MW. For example, most MW during simple cognitive tasks is triggered by irrelevant cue phrases presented on a monitor3,12–15. In these studies, task-irrelevant cue phrases representing positive, neutral, or negative things or actions were presented during the task while participants engaged in a simple attention task. Among MW contents reported during the task, involuntary memory recall was more likely to be triggered by negative phrases3. This suggests that negative cue phrases could serve some adaptive function by acting as a warning sign of what has happened before and protecting the individual from potentially harmful or unpleasant events. Contrastingly, cue stimuli strongly related to the task they were originally intended to address sometimes promote a return from MW. For example, during a lecture, students often recover from MW when they notice peripheral clues related to the class16. However, a major challenge in studying MW triggers is that MW often occurs unconsciously17. Indeed, more than half of all MW in daily life is prompted by unknown triggers18, suggesting that treating only consciously perceived external stimuli as triggers of thought transitions may be insufficient.
Recently, the impact of interoception—the sensing of the internal bodily state—on thought shifting has also been studied. Interoception is the overarching mechanism by which the nervous system detects and integrates signals originating from within the body, primarily from internal organs, and functions at both conscious and unconscious levels19,20. Most of our interoception, such as monitoring our own psychophysiological state, unfolds at an unconscious level and often only reaches consciousness when there is a deviation in the system (i.e., pain, thirst)21. However, subconsciously processed interoceptive signals amplify or attenuate specific perceptual and cognitive processes22. For example, the detection rate of visual, tactile, and facial expression stimuli varies depending on the phase (systole/diastole) of the cardiac cycle during which the stimuli are presented21,23,24. It is also associated with physiological functions, such as ingestion and elimination25, as well as cognitive functions such as memory, emotion26, decision-making27, and time perception28. In addition, individuals with more accurate cardiac interoception; that is, more accurate detection of cardiac activity, may be better able to detect stimulus-induced autonomic activity and apply it to subsequent adaptive cognition and behavior29. For example, interoceptive accuracy is associated with sensitivity to specific emotional expressions of others30 and the magnitude of the effect of reappraisal on the control of disgust31. Moreover, individuals with more accurate interoception are less susceptible to the adverse effects of the cardiac cycle on memory32. Also, they can better recall prospective memory at the appropriate timing in response to cue stimuli33.
Several studies have shown a correlation between MW and interoception. First, brain regions activated during MW include the posterior insular cortex, in addition to the default mode network associated with resting state, and the fronto-parietal network related to executive function34. As this region is associated with sensing interoceptive information35,36, interoception could be related to the specific contents of MW, such as body representations. The self-relevance of thoughts during MW is also known to covary with the information processing of heartbeat signals in the default mode network and right insular cortex37,38. Both regions monitor body signals and autonomic regulation, suggesting that neural responses to body signals may encode specific contents in MW. Furthermore, interoception might also play a role in awareness of thought shifting. For example, the salience network, which includes insula, is activated during awareness of MW during respiratory focus tasks39,40. The salience network is involved in autonomic processing and in detecting the saliency of external/internal events41–43, and these functions could be important for noticing changes in one’s own thoughts. However, whether changes in interoception cause thought transitions in the same way as exteroception, or what individual differences exist in the influence of interoception on thought transitions, remains unknown.
To directly examine the relationship between interoception and thought shifting in a controlled experimental environment, we considered it necessary to manipulate participants’ bodily responses and interoception through external manipulation. The presentation of supraliminal visual, auditory, and vibration stimuli to body parts can alter participants’ heart rate44–47; their interoception as the ability to detect heart rate48,49; or cognitive functions related to interoception such as time perception50, emotion evaluation51,52, and decision-making53. However, in these studies, participants could consciously perceive the stimuli for manipulating heart rate, or received explicit feedback about their own interoception. In experiments using such threshold stimuli or explicit instructions about bodily sensations, it is impossible to isolate the effect of the change in internal bodily state on thought shifting since awareness of the stimuli could cause the thought state to shift in the first place. Therefore, we attempted to determine whether subliminal tactile stimuli could induce changes in heart rate at an unconscious level during the task. Subliminal tactile stimuli can modulate the detection rate immediately following suprathreshold tactile stimuli54,55 or facilitate the perception of subsequent visual stimuli or motor responses56,57. Such effects have been applied to techniques for optimizing driver attention by presenting subthreshold or subthreshold-like minute vibration stimuli during driving58–61. In addition, the detection rate of subliminal tactile stimuli varies depending on the relationship between the cardiac or respiratory cycles and the timing of stimulus presentation62,63. However, it is unclear how subliminal tactile stimuli affect physiological responses such as cardiac activity and higher-order cognitive functions such as memory, emotion, and thought.
This exploratory study examined the possibility that unconscious interoceptive information processing could cause changes in thought, which has yet to be clarified in previous studies. We also examined the influence of the ability to detect fluctuations in cardiovascular reactivity occurring in real time on the transition of thought states. Therefore, among the subcategories of interoception, we focused on interoceptive accuracy, which is objective exactness in detecting internal bodily sensations64. To measure this indicator, participants completed the heartbeat counting task (HCT)65. Performance on this task correlates with the strength of functional coupling in areas related to interoception processing at rest66 and the amplitude of afferent signals associated with cardiac activity67. This suggests that the interoceptive accuracy measured by the HCT is also reflected in the processing of heart rate changes when attention is not focused on bodily responses. Participants also completed the vigilance task (VT). The VT is a simple attention task and is one of several used to measure spontaneous shifting of thought states in the laboratory2. Participants reported their thought contents, contemplation (how deeply they thought), and consistency (coherence of thought contents) while performing a task in which they responded by pressing the keyboard only when the target stimulus was presented. The thought probe we used to record participants’ thought contents included six categories based on the semantic relevance of the thought contents to the task or the stimuli presented during the task68. Based on our daily experience of thought states, transition, in which the content of reported thought changes between trials, and continuation, in which the same content is reported between trials, were the subjects of the investigation. Additionally, during the VT, we presented subliminal vibration stimuli to induce implicit changes in participants’ heart rates. The reasons we used subliminal vibration stimuli were (1) they are suitable for subliminal presentation because they are less invasive, and (2) supraliminal slight vibration stimuli can change the heartbeat itself and emotional responses44–46. We examined how thought shifting (i.e., transition and continuation) varies depending on the interoceptive accuracy of the individual, as measured by the performance of the HCT, and the presence or absence of heart rate change induction by vibration presentation. The hypotheses were that (1) changes in heart rate by vibration would induce thought shifting, and (2) the effect of (1) would depend on one’s interoceptive accuracy.
Material and methods
Participants
One hundred university and graduate students (70 women, 30 men, Mage = 21 ± 1.46 years) were recruited. All participants had normal or corrected-to-normal vision. This study was approved by the Keio University Research Ethics Committee (no. 210300000) and was conducted per the Declaration of Helsinki. All participants provided written informed consent before participation. To examine the effect of subliminal vibration presentation on heart rate, we did not explain the presentation of vibration during VT in our pre-experiment briefing. After the experiment, we disclosed that we were presenting subliminal vibration during the VT and the purpose of it, and again acquired written informed consent for the use of the data obtained in the experiment.
Apparatus
We used a pulse oximeter (NONIN, 8600) and a cuff (NONIN, 8000SM), attached to the tip of the left index finger, to measure participants’ pulse rates. The measured signal was recorded by POWER1401mk2, and Spike2 (Cambridge Electronic Design). Participants could not know their own real-time pulse rate by viewing the waveform of their own pulse or listening to sounds linked to their pulse rate. The heart rate index used in this study is the temporal variation in the interval between the apexes of participant's pulse wave at rest (see “Heart Rate Variability (HRV)”), which can be measured accurately enough with a pulse oximeter. Therefore, considering the burden of attaching a measurement device such as an electrocardiogram (ECG) to participants’ bodies, we used a pulse oximeter, which is relatively simple compared to an ECG.
A vibration device (Soundbrenner_Pulse_615867123689), connected via Bluetooth to an iPad, was used to present the subliminal vibration stimuli. The experimenter operated the device from behind a partition, out of sight of participants, using the Soundbrenner application on the iPad. The vibration stimuli presented during the experiment were the smallest of the three levels of vibration intensity and duration that could be set for the vibration device. Soundbrenner was designed for music performance, and the values for vibration intensity, frequency, and stimulus presentation time could not be set as exact values. We used two cushions as armrests (Cushion L and R). Both were made by Seria Co., Ltd., in Japan, whose surface cover was made of polyester, and the inner stuffing was made of polypropylene, measuring 17 cm in width and 37 cm in length. Participants placed their left forearm on Cushion L and their right elbow on the left half of Cushion R. To keep the device out of sight, it was inserted into the right half of Cushion R. The vibration stimuli were presented to the right elbow through a Cushion R. We placed Cushion L to secure the left hand, which was wearing a pulse oximeter and to prevent participants from being suspicious of Cushion R, which was used to hide the vibration device. The arrangement of the apparatus used during the experiment is shown in Fig. 1.
Figure 1.
The arrangement of the apparatus during the experiment. Participants placed their left and right arms on Cushions L and R, respectively, and a pulse oximeter was attached to the left index finger. We set the vibration device in the right half of Cushion R.
Procedure
Participants came to the laboratory and performed two tasks: the HCT and the VT. In both tasks, we measured participants’ pulse rates with a pulse oximeter (Fig. 2).
Figure 2.
(A) and (B) Flow of the tasks in this experiment. Participants performed two tasks: the heartbeat counting task (A; the HCT) and the vigilance task (B; the VT). We used the former task to measure participants’ interoceptive accuracy and the latter to measure the shifting of thought states during the task.
The HCT
Participants first completed the HCT65 to measure their interoceptive accuracy (Fig. 2A). This task comprised three processes: resting heart rate measurement, the HCT, and the time estimation task (TET). In both the HCT and the TET, all cues for the start and end of the task were provided by the experimenter, and all participant responses were given verbally.
First, participants put a pulse oximeter on their left index finger and measured their resting heart rate for three minutes. They placed their entire left arm on Cushion L on the desk at that time.
In the HCT, they silently counted their own heartbeats over a series of time intervals (2 × 25 s, 2 × 35 s, and 2 × 45 s) and then reported the number of heartbeats counted for each interval. At that time, we instructed participants as follows:
In this task, you are asked to count your heartbeats at various time intervals. You will be asked to count your heartbeat when I say “Start.” Then, until I say “Stop,” count your own heartbeat without saying it aloud. Please do not touch any part of your body, press your body hard against a backrest or desk, or hold your breath to count your actual heartbeat. Do not count heartbeats that you did not actually feel by assuming that they are probably beating based on the sensations you felt before and after the heartbeats, or by estimating the number of heartbeats that occurred based on your knowledge of it and your sense of time. Please answer only the number of heartbeats you actually felt. If you could not feel any heartbeats, please answer “0.”
This instruction conforms to the adapted version, which asks participants to avoid reliance on signals that are not interoceptive and to report only the perceived heartbeat69.
Lastly, they estimated the length of the time they felt had elapsed at a series of time intervals (2 × 23 s, 2 × 49 s, and 2 × 56 s) and then reported that length (TET). In the HCT and TET, each interval was measured randomly.
The VT
Task flows
After the HCT, the experimenter verified that the vibration device and iPad were properly connected via Bluetooth. The experimenter then placed Cushion R containing the vibration device on the desk, and participants placed their right elbows on it to begin the VT (Fig. 2B). We did not place Cushion R until the beginning of the VT because participants had not used their right hand until the VT. We explained to participants that the purpose of using Cushion R was to prevent the pulse data from introducing noise from body movements when pressing the keyboard with the right hand during the task. Participants wore silicone earplugs in both ears to prevent loud noises from interfering with their thoughts.
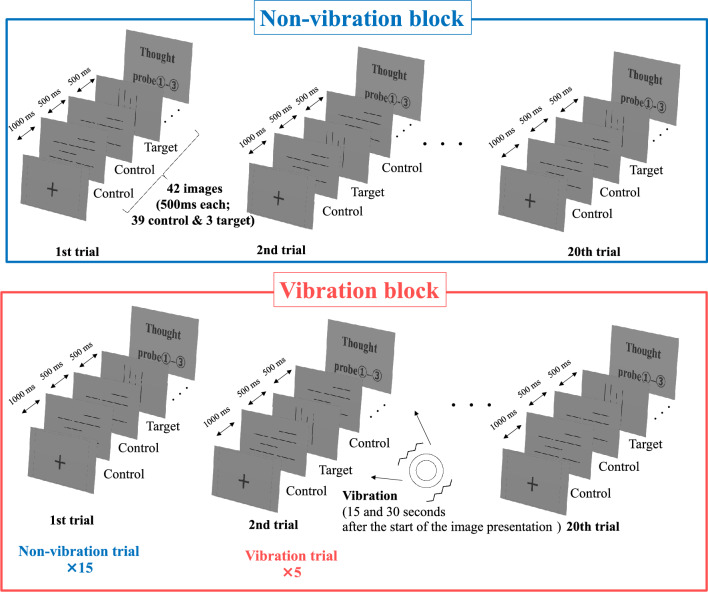
Participants performed a simple attention task for a sustained period, during which the shifting of their thought states was measured. First, a gazing cross was presented for 1s, and then 42 images (39 × control stimulus, 3 × target stimulus) and thought probes ①–③ were presented in one trial (Fig. 3). Examples of image stimuli are presented in Appendix Fig. A.1. The image stimulus presentation and inter-stimulus durations were 500 ms each. Participants responded by pressing the space key as soon as the target stimulus appeared on the screen. The time required for all image stimuli to be presented in one trial was 42 s, and no time limit was placed on responses to the thought probes. The order in which the target and control stimuli were presented in the trials was randomized trial-by-trial. The VT consisted of non-vibration block in the first half and vibration block in the second half, each block containing 20 trials. Participants took a 5-min break between the two blocks. We did not randomize the order of the blocks across participants to prevent the effects of the vibration presentation on heart rate from remaining in the later block (described in detail in “Heart rate variability (HRV)”). The vibration block consisted of 5 vibration trials and 15 non-vibration trials. In the vibration trials, a subthreshold vibration stimulus was presented 15 s and 30 s after the start of the image presentation. The order of vibration and non-vibration trials was random among participants, but the vibration trials were always arranged nonconsecutive. This was done to prevent participants from becoming aware of the vibration stimulus itself or their physical response to the stimulus, as the effect of the vibration stimulus is amplified by its successive presentation.
Figure 3.
Timeline of the VT. Participants were asked to respond by pressing the space key only when presented with the target stimulus. We presented 42 image stimuli (3 of which were target stimuli) and thought probes per trial. If participants selected 1–5 in probe ①, probes ② and ③ were presented. If participants selected 6 in probe ①, probes ② and ③ were not presented, and participants proceeded to the next trial. Non-vibration block/vibration block included 20 trials each, and subliminal vibration stimuli were presented in five random trials of vibration block.
After the VT, the experimenter clarified that the vibration stimulus was presented later in the task and asked whether participants perceived the vibration stimuli themselves or a change in heart rate owing to some external manipulation. Participants who answered “yes” to this question were excluded from the analysis.
Thought categories
Three thought probes (Thought probe ①–③) were presented once per trial: thought content, contemplation (how deeply they thought), and consistency (coherence of thought contents). Since constraints on the transition from one mental state to another are important indicators in the framework of variation in thought states over time1, we estimated this from measures of how deep participants were stuck in a particular thought state (contemplation) and how scattered the content of their thoughts was (consistency). This frequency of probe presentation is generally consistent with the average duration of MW that has been self-caught in previous studies70. Because one block consisted of 20 trials, 20 combinations of data from thought probes ①–③ were obtained for each vibration block (non-vibration/vibration block). The number of thought probes presented per block was based on previous studies that measured thought content and its transition by probe-caught method, as in this study71–74.
In thought probe ①, participants reported their contents of thoughts immediately before the probe was displayed. We prepared the following six categories for participants to categorize their thoughts: Category 1, task-focused; Category 2, task-relevant (score of the task/elapsed time); Category 3, peripheral stimuli (changes in surrounding stimuli, sound/flash); Category 4, internal-body states (changes in bodily state, hunger/pain/heartbeat); Category 5, task-unrelated (past episodes, plans for the future); and Category 6, absent-mindedness (nothing). This classification was based on Stawarczyk et al.68, who distinguished the thoughts during the task in terms of task-relatedness (whether the thought contents were semantically related to the task itself) and stimulus-dependency (whether the thought contents were semantically related to the external and internal stimuli presented during the task). Category 1 was task-related and stimulus-dependent, Category 2 was task-related but stimulus-independent, Category 3 and 4 were task-unrelated but stimulus-dependent and Category 5 was task-unrelated and stimulus-independent. We set Category 6 as the option for participants to report if they could not monitor their thoughts. Category 1: task-focused is a state in which participants focused their thoughts on the performance improvement required in the VT (pressing the space key as soon as possible after the target stimulus is presented), and Category 2: task-relevant is a state in which their thoughts were related to the task such as elapsed time during the task or their own performance on the task, but not directly to the responses required in the VT. Since the purpose of this study was to discuss the role of interoception in thought shifting, as distinguished from exteroception (i.e., the perception of peripheral stimuli), we separated Categories 3 and 4. Additionally, we provided past episodes and future plans about oneself as specific examples of Category 5. This is based on previous research that showed that during MW, varius self-referential thoughts are reported, including simulations of one’s own past episodes, and future plans and goals2,75.
When participants pressed one of the number keys from 1 to 5 in thought probe ①, they responded to the contemplation of thoughts they answered in thought probe ① in thought probe ②, and to the consistency of thoughts in thought probe ③. However, for participants who answered 6 in thought probe ①, the next probes ② and ③ were not presented. In thought probe ②, participants responded by selecting one of the options ranging from “1. not very much” to “3. very deeply” to indicate how deeply they thought about the thoughts they responded to in probe ①. In thought probe ③, participants rated the degree to which they felt coherence between the contents of their immediate previous thoughts. Participants responded by pressing the number key 1, if they were thinking about a single matter, 2, if they were thinking about multiple contents and their semantic relevance was high, or 3, if their semantic relevance was low. We described in the Appendix the instructions we gave participants before the VT.
Pre-processing
The HCT and the TET
We first performed pre-processing on the first task, the error rates for the HCT and the TET. We excluded data from two participants in our analysis of the HCT and the TET error rates. One participant was excluded because the heart rate data was not accurately measured in all tasks, and the other participant reported the same number of heartbeats in all conditions of the HCT and was considered to have not performed the task properly. Consequently, we used data from 98 university and graduate students (70 women, 28 men, Mage = 21 ± 1.50 years) to analyze both tasks. The HCT error rates for each participant were calculated using the following formula: 76. The TET error rates for each participant were calculated using the following formula:. To confirm that the HCT results were not affected by inferences based on time estimates, we calculated Pearson’s product moment correlation coefficient, for the HCT and the TET error rates.
Effect on heart rate changes by vibration
We pre-processed the heart rate data before and after the presentation of vibration stimuli during the VT. At that time, data from 18 participants were excluded from the analysis, in addition to the two participants indicated in “The HCT and the TET”. Nine reported noticing vibration stimuli presented during the VT; three did not respond correctly on more than half of the trials of the VT; and six never reported any of 1, 2, 4, or 5 of thought probes ① in the entire VT. Consequently, we used data from 80 university and graduate students (54 women, 26 men, Mage = 21 ± 1.50 years) for the following analysis.
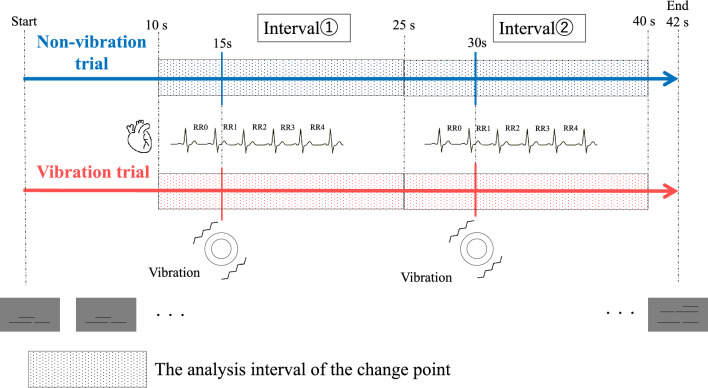
We examined whether the vibration presentation changed participants’ heart rate. The time interval at the apex of participant’s pulse wave (hereafter referred to as RR) was used as an index to track changes in participant’s heart rate. We presented the vibration stimuli 15 and 30 s after the start of the trial in the vibration trials (Fig. 4). The RR interval immediately before vibration presentation was defined as RR0, and the RR intervals at the first, second, third, and fourth times after vibration presentation were defined as RR1–4. The difference between RR1–4 respectively, and RR0 was calculated. Based on the trend of the difference between each time point and RR0, we considered that the vibration-induced changes in heart rate converged up to RR3, and after that, RR1–3 were considered in the analysis as the time points affected by the vibration presentation (Appendix Fig. A.2).
Figure 4.
The timing of vibration presentation and the analysis interval of change point. We presented the vibration stimuli 15 and 30 s after the start of the trial in the vibration blocks. RR here is the time interval between the apexes of the pulse wave. RR0 is the RR interval immediately before the vibration, and RR1–4 are the RR intervals of the first, second, third, and fourth times after the vibration, respectively. In the non-vibration trials, which are just before the vibration trials, we defined RR0–4 based on 15 and 30 s after the start of the trial adjusting to the vibration trials. We analyzed change point detection for the RR interval from 5 s before to 10 s after the two vibration presentations in one trial (interval①, ②) and calculated the percentage of change points detected at RR1–3.
A change point analysis was performed using the R package “changepoint” (version 2.2.3) to compare the change in RR intervals when vibration stimuli were presented and when they were not77. We detected change points on vibration trials and on non-vibration trials that immediately preceded vibration trials. For the non-vibration trials, RR0–3 was defined as 15 and 30 s after the start of the trial. These two time points were when the vibration stimuli were presented in the vibration trial. The change points of the mean and variance of the RR intervals at 10–25 s (interval ①) and 25–40 s (interval ②) after the start of a trial were detected (Fig. 4). The reason we set the time window from 5 s before to 10 s after the stimulus presentation, is to examine the deviation from the vibration stimulus based on the trend of the heart rate immediately before and after the vibration presentation. The heart rate change rate for each block was calculated using the following equation:
Thought probes
Thought probe ①–③
First, for each vibration/non-vibration block, we calculated the number of times each of the six categories of thought probe ① was reported (Appendix Fig. A.3). Because responses to Category 3: peripheral stimuli were significantly fewer than those to the other thoughts, we excluded them from subsequent analyses. We also excluded responses to Category 6: absent-mindedness from the analysis because they differed from the state of thought targeted in this study, in that the contents of the thoughts were not reported verbally.
Next, we calculated the rate of trials that responded with each of the options in thought probes ② and ③ out of the number of trials that responded with a response other than Category 6 in thought probe ① (Appendix Figs. A.4, A.5). Based on the distribution of responses and the expression of each option in thought probes ② and ③ (see “The VT”), we defined responses of 2 and 3 to probe ② as high contemplation and responses of 1 to probe ③ as high consistency. The specific distribution of responses to each option and their interpretation are described in the Appendix.
Indicators of thought states
We used two indices, transition, and continuation, as kinds of thought shifting. Transition refers to reporting different thought categories in thought probe ① on successive trials, and continuation refers to reporting the same thought category on successive trials. Because there is always interference by thought probes between trials (Fig. 3), continuation can be interpreted as a return to the same state of thought as before the external interference, rather than a state in which one continues to think about a particular matter all the time. Since one’s thoughts wandering to things unrelated to the task at hand is the general definition of MW2, we focused on the transition of thought between Category 1 in probe ① and the other categories (Categories 2, 4, and 5). We calculated transitions in two ways; one is to group all thoughts, except Category 1, into a single “MW state” and the other is to distinguish these states according to the content of the thoughts (see the Appendix). We calculated the number of times each transition/continuation pattern occurred for Categories 1, 2, 4, and 5. Moreover, additional analyses were conducted on contemplation and consistency in Category 5 because, as discussed in “Patterns of transition and continuation of thought”, specific effects of vibration presentation and interoceptive accuracy on thought shifting between trials were found only in Category 5. When Category 5 was reported in thought probe ①, we counted the number of times it met the high contemplation (selecting 2 or 3 in thought probe ②) or high consistency (selecting 1 in thought probe ③). We defined them as highly contemplated 5 and highly consistent 5, respectively.
Heart rate variability (HRV)
In this experiment, the vibration stimuli were presented in the latter block for all participants to avoid the possibility that the effect of the vibration-inducing heart rate change might remain in the subsequent block without the vibration presentation, and the “baseline” heart rate state unaffected by vibration might not be reproduced. However, since MW tends to increase with time11 and physical fatigue associated with the continuation of the task amplifies the frequency of MW78,79 and gives rise the conditions that can cause MW, such as depletion of attentional resources, negative emotions, and rumination80, it is possible that MW was more likely to occur in the second half of the task than in the first half. Therefore, by estimating autonomic nervous activity through heart rate data, we examined whether there was a significant difference in the physical condition between the first and second blocks. Using the fast Fourier transformation method, we quantified cardiovascular autonomic control from HRV. HRV data were collected and analyzed using Kubios HRV Standard 3.6.581. The following parameters are mainly used; very low frequency, < 0.003–0.04 Hz (VLF), low-frequency power, 0.04–0.15 Hz (LF), high-frequency power, 0.15–0.4 Hz (HF), and the ratio LF/HF. We featured HF and LF/HF, the former is often understood as a representation of the parasympathetic nervous system, and the latter might reflect the global sympathetic/vagal balance82. We calculated HF and LF/HF power values for each participant for the last 3/4 of each block and compared these values across blocks.
Modeling
We used Bayesian approach for estimating parameters in the statistical model. This is because we would like to stabilize convergence in estimating models with many parameters. There has been an increasing number of attempts to analyze self-reported data on thought using Bayesian statistical modeling83,84. We used a generalized linear mixed model to account for the distributional characteristics of each acquired dataset. The brms R package was used for the analysis85,86, and parameters were estimated with four Markov Chain Monte Carlo chains. Each chain contained 1000 burn-in samples and 2000 additional samples with a thinning parameter of 1, resulting in 1000 posterior samples per chain, that were combined into one posterior sample consisting of 4000 samples for each model parameter. Regression weights had Cauchy distributed priors, bi>0 ∼ N (0, 3), and intercepts, while standard deviations of random effects had Student’s t-distributed priors, b0 ∼t (3, 0, 2.5). Model convergence was evaluated based on the Gelman–Rubin convergence statistic R̂87, with it close to 1 indicating negligible differences between within- and between-chain variances. We report the mean and 95% credible interval (CI) as an equally tailed interval, to describe the posterior distributions of sampled regression weights. To account for individual differences of each index, they were set as a random intercept for all modeling.
Induction of heart rate changes
To investigate whether there is a difference in the heart rate change rate between the non-vibration trials and the vibration trials, we used Bayesian generalized mixed models, assuming a Gaussian distribution with an identity link function, to accommodate for the distributional properties of the heart rate change rate. We included the main effect of vibration (0 = non-vibration, 1 = vibration), participants’ HCT error rates (= score), and participants’ sex (0 = men, 1 = women). To examine the effect of vibration on heart rate, we focused on the coefficient of the main effect of vibration.
Thought characteristics
To investigate how the patterns of thought transition and continuation, as well as the contemplation and consistency of Category 5, differ depending on vibration and the accuracy of interoception, we used Bayesian generalized mixed models assuming a Poisson distribution with a logit link function, to accommodate for the distributional properties of the number of the reports of each thought conditions. For all modeling, we included the main effects of participants’ error rates of the HCT (= score), vibration (0 = non-vibration, 1 = vibration), and participants’ sex (0 = men, 1 = women). Moreover, we included interaction of participants’ HCT and vibration error rates (= score: vibration). To examine how the relationship between interoceptive accuracy and the presence or absence of vibration affects the characteristics of thought, we focused on the coefficient of interaction between the HCT error rates and vibration.
HRV
To investigate whether there is a difference in the power value of HRV parameters between the non-vibration block and the vibration block, we used Bayesian generalized mixed models assuming a Gaussian distribution with an identity link function, to accommodate for the distributional properties of the power value of HF and LF/HF. We included the main effects of vibration (0 = non-vibration, 1 = vibration), and participants’ sex (0 = men, 1 = women). To examine the effect of different blocks of the VT; that is, vibration conditions, on the autonomic function of participants, we focused on the coefficient of the main effect of vibration.
Results
The HCT and the TET
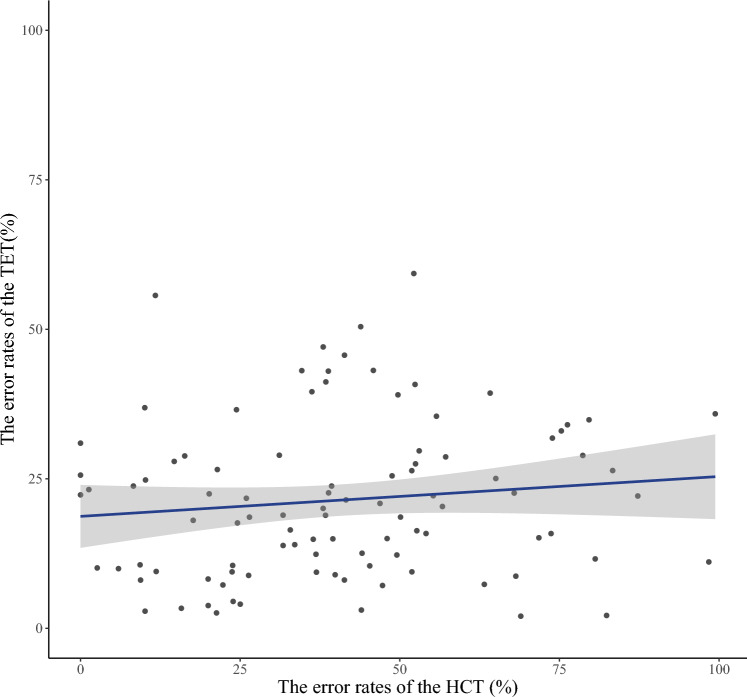
The mean error rate for the HCT was 40.69 with a standard deviation of 23.48. The mean error rate for the TET was 21.44 with a standard deviation of 13.10. There was little correlation between the error rates of the HCT and that of the TET (Fig. 5, r98 = 0.11, p = 0.24). Because these results suggest that the performance of HCT was not affected by the time-estimated inferences, the error rates of the HCT were used as a measure of interoceptive accuracy in this later analysis.
Figure 5.
The relationship between the error rates of the HCT and the TET. The vertical and horizontal axes represent the HCT and the TET error rates respectively. The dots indicate the measured error rates. The solid line is the regression line. There was little correlation between the two error rates.
Induction of heart rate changes
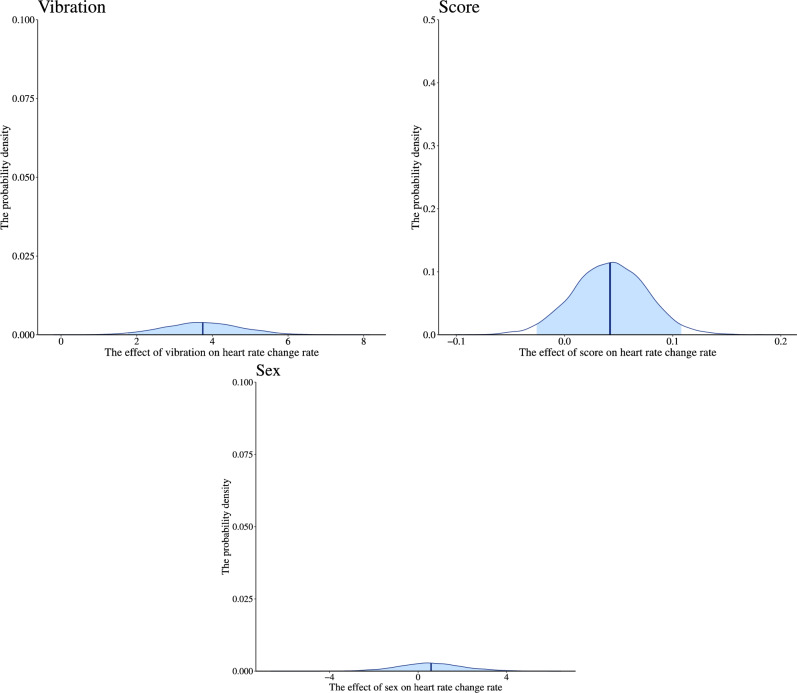
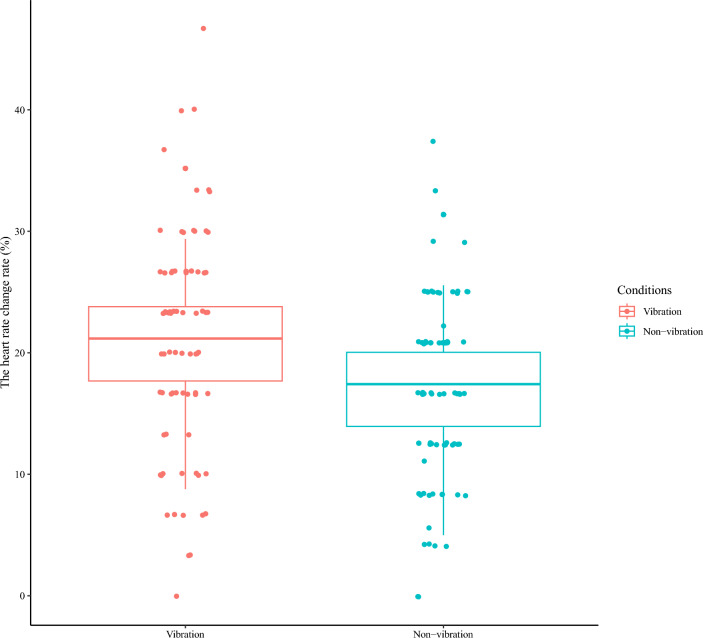
To investigate whether subliminal vibration increases heart rate change rate in the vibration block, we performed the modeling described in “Induction of heart rate changes”. All chains converged for the model, as indicated by R̂ equal to 1. We found the main effect of vibration, b = 3.734, 95% CI [1766; 5.644]. Contrastingly, there was little effect of score and sex (Table 1, and Fig. 6). The heart rate change rate was higher for the vibration block (Fig. 7). The results show that more changes in heart rate occurred immediately after the presentation of the subliminal vibration stimuli compared to the same time point in the condition without it, and that this effect was independent of participants’ interoceptive accuracy or sex. We concluded that the heart rate change induction in this experiment was successful.
Table 1.
Summary of estimated parameters of heart rate changes.
| Parameters | Estimate | Est. error | l-95% CI | u-95% CI | R̂ | Bulk_ESS | Tail_ESS |
|---|---|---|---|---|---|---|---|
| Intercept | 14.905 | 1.900 | 11.216 | 18.733 | 1 | 3034 | 3001 |
| Vibration | 3.734 | 0.992 | 1.766 | 5.644 | 1 | 8677 | 2640 |
| Score | 0.042 | 0.034 | − 0.026 | 0.108 | 1 | 2830 | 2696 |
| Sex | 0.836 | 1.533 | − 2.104 | 3.943 | 1 | 3638 | 2971 |
Notes:
Since R̂ = 1 for all parameters, the model has converged; Estimate: the estimated value of each parameter; Est. Error: the error in the estimated value of each parameter; l-95% CI: the lower limit of the 95% credible interval (CI); u-95% CI: the upper limit of the 95% CI; R̂: indicators for determining convergence of Markov Chain Monte Carlo (MCMC) chain; Usually, the model is considered to have converged at R̂ ≤ 1.1.; Bulk_ESS: the effective sample size calculated by normal rank; Tail_ESS: the effective sample size for the 5% and 95% quantile points; Vibration: the main effect of with or without vibration presentation (0 = non-vibration, 1 = vibration); Score: the main effect of participants’ error rates of the HCT; Sex: participants’ biological sex.
Figure 6.
Distribution of the estimated parameters of heart rate change rate. We indicated the influence of each factor on the heart rate change rate on the horizontal axis and the probability density on the vertical axis. The dark blue line shows the estimated coefficient, and the light blue range shows the 95% credible interval (CI). The lower limit of the 95% CI in main effect of vibration (0 = non-vibration, 1 = vibration) is higher than 0, indicating that this factor influences the heart rate change rate. The 95% CI of main effect of score and sex includes 0, indicating that this factor has little effect on the change rates of heart rate.
Figure 7.
The posterior predictive distribution of the probability of detecting a change point at RR1–3 for each condition. We show the condition name (vibration/non-vibration) on the horizontal axis and the estimated heart rate change rate on the vertical axis. The points on the graph indicate measured values. RR0 is the RR interval immediately before vibration; RR1–3 are the first, second, and third RR intervals after vibration. Change point detection was analyzed for the RR interval from 5 s before to 10 s after each vibration presentation, and the percentage of change points detected at RR1, 2, or 3 was calculated. The probability of detecting the change points at RR1–3 was higher in the vibration block than in the non-vibration block, suggesting that this experiment's heart rate change induction was successful.
Patterns of transition and continuation of thought
First, to examine whether participants’ interoceptive accuracy and the presence or absence of unconscious deviation of heart rate would affect thought transition, we performed the modeling described in “Thought characteristics” for the transition of thought (see “Indicators of thought states”). There was no interaction between score and vibration in any of these transition patterns (Appendix Table A.1–8 and Fig. A.6–8). The results indicate that the presence or absence of unconscious heartbeat deviation, interoceptive accuracy, or their interaction do not affect the transition between the task-focused state and other thoughts as defined in this experiment.
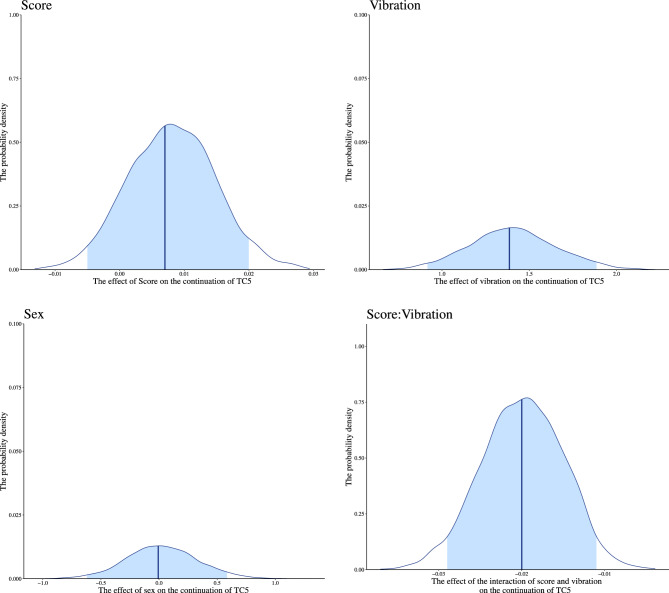
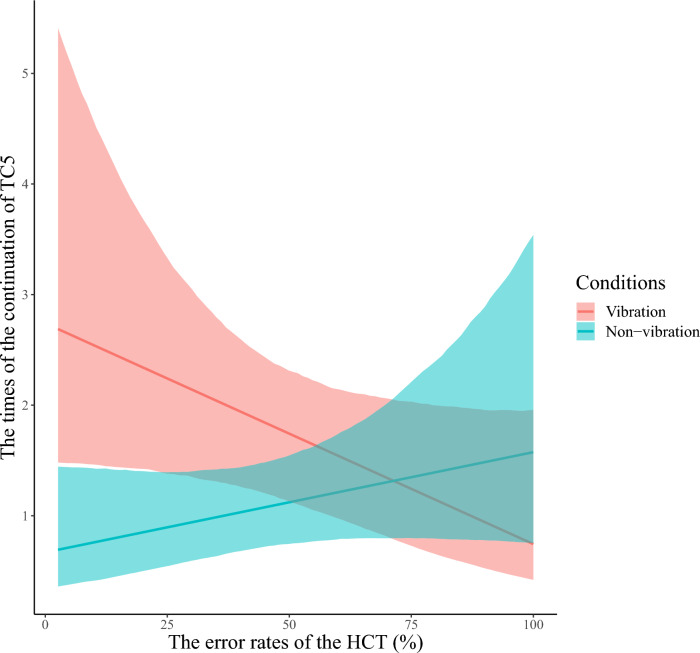
Second, to examine whether participants’ interoceptive accuracy and the presence or absence of unconscious deviation of heart rate would cause a difference in the continuation of thought, we performed the modeling described in “Thought characteristics” for the continuation of thought Category 1, 2, 4, and 5 (see “Indicators of thought states”). For the continuation of thought Category 5: task-unrelated (hereafter, referred to as TC5), all chains converged for the model, as indicated by R̂ equal to 1. We found the main effect of vibration and interaction between score and vibration b = -0.020, 95% CI [− 0.029; − 0.011], for the continuation of TC5. Contrastingly, there was little effect of sex and score (Table 2 and Fig. 8). The higher the accuracy of interoception, the higher the number of reported continuation of TC5 in the vibration block (Fig. 9). There was no interaction between interoceptive accuracy and vibration for the continuation of thoughts other than TC5 (Appendix Table A.9–11, and Fig. A.9). When unconscious deviations in heartbeat occur, the greater the ability to accurately capture them, the more likely the TC5; that is, task-unrelated and self-referential thoughts (see “Thought categories”), would continue. No similar results were found for the other categories of thought, suggesting that changes in bodily response and perception of them specifically affect TC5.
Table 2.
Summary of estimated parameters of the continuation of TC5.
| Parameters | Estimate | Est. error | l-95% CI | u-95% CI | R̂ | Bulk_ESS | Tail_ESS |
|---|---|---|---|---|---|---|---|
| Intercept | − 0.240 | 0.351 | − 0.932 | 0.436 | 1 | 1068 | 1791 |
| Score | 0.007 | 0.006 | − 0.005 | 0.020 | 1 | 1156 | 1774 |
| Vibration | 1.387 | 0.246 | 0.916 | 1.886 | 1 | 2493 | 2795 |
| Sex | − 0.007 | 0.300 | − 0.621 | 0.586 | 1 | 863 | 1568 |
| Score: Vibration | − 0.020 | 0.005 | − 0.029 | − 0.011 | 1 | 2602 | 2941 |
Notes:
Since R̂ = 1 for all parameters, the model has converged; Estimate: the estimated value of each parameter; Est. error: the error in the estimated value of each parameter; l-95% CI: the lower limit of the 95% credible interval (CI); u-95% CI: the upper limit of the 95% CI; R̂: indicators for determining convergence of Markov Chain Monte Carlo (MCMC) chains; Usually, the model is considered to have converged at R̂ ≤ 1.1.; Bulk_ESS: the effective sample size calculated by normal rank; Tail_ESS: the effective sample size for the 5% and 95% quantile points; Score: main effect of participants’ error rates of the HCT; Vibration: main effect of with or without vibration presentation (0 = non-vibration, 1 = vibration). Sex: participants’ biological sex; Score/Vibration: interaction of participants’ error rates of the HCT and with or without vibration presentation.
Figure 8.
Distribution of each estimated parameter in the continuation of TC5. We indicated the influence of each factor on the continuation of TC5 on the horizontal axis and the probability density on the vertical axis. The dark blue line shows the estimated coefficient, and the light blue range shows the 95% credible interval (CI). The lower limit of the 95% CI in main effect of vibration is higher than 0, and the higher limit in interaction of score and vibration is lower than 0. This indicates that these factors influence the continuation of TC5. The 95% CI of main effect of the HCT error rates and sex includes 0, indicating that these factors have little effect on the continuation of TC5.
Figure 9.
The posterior predictive distribution of the relationship between the HCT error rates and the number of reported continuation of TC5 for each vibration condition. TC5 are reports of self-referential thoughts, such as past episodes and future plans regarding oneself. We show the HCT error rates on the horizontal axis and the estimated number of the continuation of TC5 reports on the vertical axis. The colors indicate the conditions (vibration/non-vibration). The solid line on the graph shows the regression line based on the estimated values. Each colored range indicates 95% CI. Participants with the lower HCT error rates; that is, more accurate interoception, reported a greater number of the continuation of TC5 in the vibration block compared to the non-vibration block. Contrastingly, the lower the interoceptive accuracy, the smaller the difference in the frequency of reports of the continuation of TC5 between vibration conditions.
Post-hoc analysis of contemplation and consistency of category 5
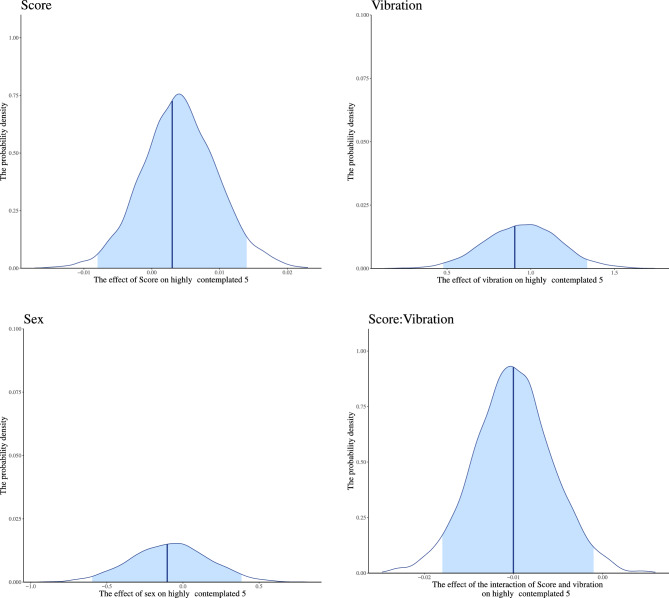
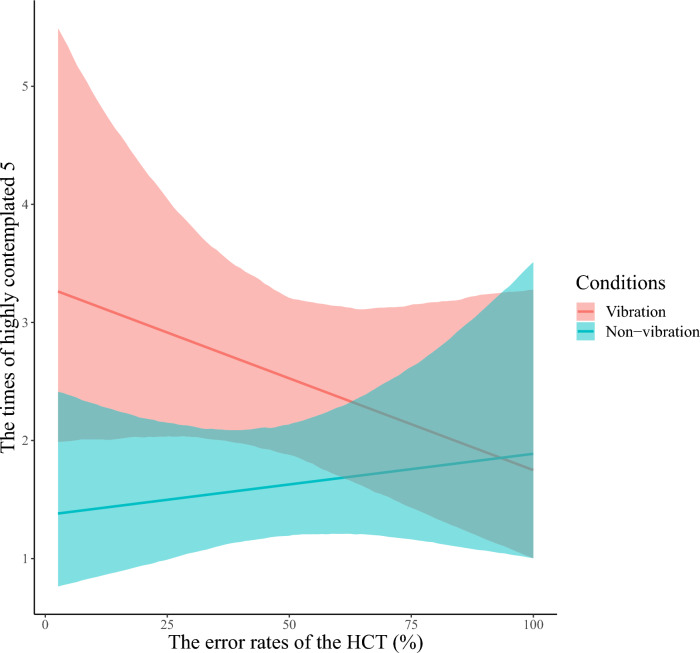
Since we found an interaction between interoceptive accuracy and vibration in the continuation of TC5 in section “Patterns of Transition and Continuation of Thought”, we examined whether there is an interaction between interoceptive accuracy and vibration in contemplation and consistency of TC5. We performed the modeling described in “Thought characteristics” for highly contemplated and highly consistent 5 (see “Indicators of thought states”). For highly contemplated 5, all chains converged for the model, as indicated by R̂ equal to 1. We found the main effect of vibration, and interaction between score and vibration b = − 0.010, 95% CI [− 0.018; − 0.001], for highly contemplated 5 (Table 3, and Fig. 10). The higher the interoceptive accuracy, the more frequently a highly contemplated TC5 is reported in the vibration block (Fig. 11). There was no interaction between interoceptive accuracy and vibration for highly consistent 5 (Appendix Table A.12 and Fig. A.10). These results indicate that the greater the ability to capture unconscious deviations in bodily response accurately, the more likely one is to contemplate TC5.
Table 3.
Summary of estimated parameters of highly contemplated 5.
| Parameters | Estimate | Est. error | l-95% CI | u-95% CI | R̂ | Bulk_ESS | Tail_ESS |
|---|---|---|---|---|---|---|---|
| Intercept | 0.316 | 0.304 | − 0.306 | 0.896 | 1 | 1060 | 1700 |
| Score | 0.003 | 0.006 | − 0.008 | 0.014 | 1 | 1124 | 2206 |
| Vibration | 0.904 | 0.219 | 0.476 | 1.338 | 1 | 2977 | 2902 |
| Sex | -0.103 | 0.253 | − 0.596 | 0.385 | 1 | 957 | 1925 |
| Score: Vibration | -0.010 | 0.004 | − 0.018 | − 0.001 | 1 | 2788 | 3073 |
Notes:
Since R̂ = 1 for all parameters, the model has converged; Estimate: the estimated value of each parameter; Est. error: the error in the estimated value of each parameter; l-95% CI: the lower limit of the 95% credible interval (CI); u-95% CI: the upper limit of the 95% CI; R̂: indicators for determining convergence of Markov Chain Monte Carlo (MCMC) chains; Usually, the model is considered to have converged at R̂ ≤ 1.1.; Bulk_ESS: the effective sample size calculated by normal rank; Tail_ESS: the effective sample size for the 5% and 95% quantile points; Score: main effect of participants’ error rates of the HCT; Vibration: main effect of with or without vibration presentation (0 = non-vibration, 1 = vibration); Sex: participants’ biological sex; Score/Vibration: interaction of participants’ error rates of the HCT and with or without vibration presentation.
Figure 10.
Distribution of each estimated parameter in highly contemplated 5. We indicated the influence of each factor on highly contemplated 5 on the horizontal axis and the probability density on the vertical axis. The dark blue line shows the estimated coefficient, and the light blue range shows the 95% credible interval (CI). The lower limit of the 95% CI in main effect of vibration is higher than 0, and the higher limit in interaction of the error rates of the HCT and vibration is lower than 0. This indicates that these factors influence highly contemplated 5. The 95% CI of main effect of the HCT error rates and sex includes 0, indicating that these factors have little effect on highly contemplated 5.
Figure 11.
The posterior predictive distribution of the relationship between the HCT error rates and the number of reported highly contemplated 5 for each vibration condition. We show the HCT error rates on the horizontal axis and the estimated number of highly contemplated 5 reports on the vertical axis. The colors indicate the conditions (vibration/non-vibration). The solid line on the graph shows the regression line based on the estimated values. Each colored range indicates 95% CI. Participants with the lower HCT error rates; that is, more accurate interoception, reported more highly contemplated 5 in the vibration block compared to the non-vibration block. Contrastingly, the lower the interoceptive accuracy, the smaller the difference in the frequency of reports of highly contemplated 5 between vibration conditions.
HRV
To examine whether there were any differences in the physical condition of participants between the first and second halves of the experiment, we performed the modeling described in “HRV” for HF and LF/HF values. For both values, all chains converged for the models, as indicated by R̂ equal to 1. We found no main effects of block for both models (Appendix Table A.13 and 14 and Fig. A.11). Therefore, it can be considered that the passage of time owing to the continuation of the task did not affect participants’ physical condition.
Discussion
The study aimed to examine the effects of unconscious changes in bodily response and the individual’s interoceptive accuracy on thought shifting. This study hypothesized that (1) changes in heart rate by vibration would induce thought shifting, and (2) the effect of (1) would depend on one’s interoceptive accuracy. We measured participants’ interoceptive accuracy by the HCT and their thought state by presenting thought probes during the VT. Additionally, we induced unconscious changes in participants’ heart rate by presenting subliminal vibration stimuli during the VT.
To test the above hypothesis, we examined participants’ interoceptive accuracy and the effect of the heart rate change induction by vibration on the characteristics of their thought states (see “Indicators of thought states” and “Thought characteristics”). We found an interaction between interoceptive accuracy and vibration, in addition to the main effect of vibration, for the continuation and contemplation of self-referential MW (TC5). Continuation in this experiment refers to reporting the same category of thought content on successive trials, and contemplation measures how deeply participants thought about the reported thought content. Self-referential MW is a state of thinking about past episodes or future plans concerning the self, among MW that is thought unrelated to the task at hand. Moreover, there was little effect of interoceptive accuracy alone for either continuation or contemplation of self-referential MW (TC5; Tables 2 and 3; Figs. 8 and 10). Posterior predictive distribution showed that when the vibration-induced unconscious heart rate changes occurred, the more accurate one’s interoception was, the more self-referential MW (TC5) was continued and the higher its contemplation was. (Figs. 9 and 11). These results suggest that people with accurate interoception are not unique in how self-referential MW (TC5) arises; rather, they accurately capture larger-than-normal changes in heart rate, enhancing the continuation of self-referential MW (TC5) and its contemplation.
Notably, the “continuation” of thought as measured in this experiment is not necessarily the same as goal-directed thought—the state of continuing to think about a particular matter1. We referred to the continuation of thought when participants reported that they were in the same thought state in successive trials. We collected these thoughts using the probe-caught method, in which probe stimuli are randomly presented during the task and participants respond to their immediate prior conscious experience88. Because participants were asked to retrospectively evaluate not only the content of their immediate previous thought but also its contemplation and consistency during the probe response, it is likely that the presentation of the probe interrupted their thought and shifted them into a state of meta-consideration of it. Although the same thought appears to be continuing in the data, what was actually occurring in the continuation was a process in which a certain thought was interrupted by the probe and a meta-evaluation of that thought was made, and then a return to the same state of thought as before the probe was presented. That is to say, cardiovascular responses to subliminal vibration stimuli specifically produce intermittent regression to self-referential MW (TC5). These results suggest that for specific MW whose content is related to the self, the interoceptive information processed subconsciously triggers MW as well as the exteroceptive stimuli3,12–15, and this effect is stronger in individuals with accurate interoception.
In these terms, the present results partially support the hypothesis. The contribution of interoception to MW awareness has been elucidated39,40; however, the current results suggest that interoception may also be involved in the process of shifting to a specific thought. This view is consistent with previous studies that suggested that limbic areas, including the posterior insula, involved in the representation of interoceptive information35,36 are activated during the generation of spontaneous thought, and that this might be owing to changes in the saliency of the internal environment, such as memory recall and the perception of emotional/physical states89. The sensory and emotional saliency associated with such somatosensory processing could automatically limit thought states independent of one’s intentions and increase the contemplation of thought1,90. The higher the accuracy of interoception based on the HCT scores, the higher the connectivity between posterior insular cortex, which is responsible for processing such saliency, and the saliency network66. Consequently, it is possible that participants with accurate interoception in this study detected some degree of saliency, even in the case of weak, unconscious changes in their bodily response, resulting in a higher frequency of highly contemplative self-referential MW (TC5).
The interoceptive inference theory could explain why this effect of interoception on shifting thoughts is found only specifically in self-referential MW (TC5;91). This theory states that our brains have top-down predictions about interoceptive inputs, and that the process of minimizing the prediction error between the actual interoceptive input and the predicted one gives rise to concepts of self-related to the perception of emotion and the experience of body ownership. In our experiment, the subliminal vibration stimulation could have caused a change in heart rate, resulting in a prediction error between the prediction of interoceptive input and the actual input. This theory suggests that the prediction error minimization of interoception could be related not only to the low-level self-concept of the experience of body ownership but also to higher-level self-concepts such as first-person perspective, intention, and subjectivity91,92, and that self-related thought could have occurred intermittently in the process of minimizing the prediction error caused by the heartbeat change. Contrastingly, some body information always influences neural processing even without specific body changes, and this information could generate self-related thoughts37. In the present experiment, self-referential MW (TC5) was more likely to continue when vibration-induced heart rate changes occurred; however, it is possible that the default state of body information processing also had an influence here. In the future, we plan to develop a method of heartbeat change induction so that the degree of heartbeat deviation can be manipulated in more detail, and then measure neural activity during heartbeat manipulation to determine (1) what level of body change triggers the prediction error minimization process of interoception centered in the anterior insular cortex91, and (2) whether the subjective nature (content, emotional valence, vividness, etc.) of the self-related thoughts differ between cases in which the prediction error minimization process is triggered by a heartbeat change and cases in which no specific physical change is present.
One of the most novel aspects of this study was the attempt to induce changes in heart rate by presenting subliminal vibration stimuli. We found the main effect of with or without vibration on the heart rate change rate (Table 1 and Fig. 6). The distribution of the posterior predicted detection rate of change points showed that the heart rate change rate was higher for trials in which vibration was presented (Fig. 7). In this experiment, participants who noticed the vibration presentation itself or the changes in heart rate caused by it were excluded from the analysis (9 out of 100 participants). Given this, the results indicate that presenting subliminal vibration stimuli to a part of the body can produce larger-than-normal heart rate fluctuations in unconsciousness. To our knowledge, studies have yet to demonstrate that changes in heart rate can be induced without disclosing the possibility of stimulus presentation to participants. While previous studies found that the cardiac cycle influences the detection of near-threshold tactile stimuli62,63, the current results suggest that subliminal tactile stimulation alters cardiac activity. Future studies should examine changes in stimulus detectability owing to the interaction between subliminal vibration stimulus presentation and cardiac activity. We find it very interesting that the heart rate change rate in this experiment was not affected by the error rates of the HCT (i.e., one’s interoceptive accuracy). This suggests that interoception is only involved in signal processing from inside the body19,20, and that different factors, such as individual stimulus thresholds and body size, are relevant to how external stimuli affect bodily responses.
This study had some limitations. First, we could not measure the threshold for each participant in the subliminal vibration presentation. The “subliminal” in this study is merely that participants reported that they did not feel any vibration in their retrospective verbal reports after the experiment. We are currently conducting an experiment in which we measure participants’ thresholds using other stimulus devices, present subliminal stimuli, and measure changes in physiological responses. Examining whether more accurate induction of heart rate changes is possible based on the results of this experiment is necessary. Second, the thought measurement paradigm used in this experiment may not capture minute changes in thought. We used a probe-caught method, in which participants reported their thought states only at timings set by the experimenter (once every 42 s). In studies in which thought probes were presented more frequently than in the present experiment (93 (once every 25 s to 175 s)83, (once every 30 s or 60 s)), the rate of MW reporting decreases as the frequency of probe presentation increases. However, since the frequency of probing did not affect the performance of the sequential reaction tasks performed in parallel (this is known to decrease with MW), it is suggested that the frequency of probing, rather than the actual frequency of MW occurrence itself or the reaction to probing, could be causing participants' resistance to reporting MW by prompting frequent awareness of MW94. Contrastingly, as already mentioned, in a study using the self-caught method, MW was reported at a frequency similar to the probe interval in this experiment70. Consequently, the current experiment may capture natural thought shifting, and we believe that changing the frequency of presentation of the thought probes in this experiment would not affect the results reported here. However, when considering individual thought states, there is a possibility that transitions in thought occur between the previous thought probe and the current one, and it is assumed that our method does not reflect all these transitions. Developing a paradigm that can reproduce more natural transitions in thought, such as manipulating the timing of probe presentation based on objective indices that are thought to reflect transitions in thought states, (e.g., reaction time to a task and eye movements), is necessary.
This study was an exploratory investigation of the direct relationship between interoceptive processing and thought shifting. The novelty of this study is that it showed the following three points: (1) the presentation of subliminal vibration can induce changes in physiological responses, (2) changes in interoception specifically trigger MW related to the self, and (3) the effect of interoception on thought transitions depends on interoceptive accuracy based on cardiac perception. The paradigm contributes to future research on thought by presenting a methodology for examining the effects of internal-body responses induced by subliminal external stimuli on thought states. Researchers should examine the relationship between the processing of bodily response and thought in more detail by strictly controlling subliminal stimuli, comprehensively examining factors other than interoception that influence thought shifting, and measuring brain activity during the task.
Conclusion
This study examined the effects of unconscious changes in bodily response and individuals’ interoceptive accuracy on thought shifting. For people with high interoceptive accuracy, when subliminal vibration stimuli induced unconscious changes in heart rate, self-referential thought occurred intermittently, and the contemplation of these thoughts was enhanced. This study is significant because it showed the possibility that bodily response and accurate perception of it could influence shifting toward a particular thought state.
Supplementary Information
Acknowledgements
We are grateful to all participants.
Author contributions
M.S. and S.U. collaborated to design the study. M.S. programmed the computer tasks, collected the data, and analyzed them. All authors interpreted the results. M.S. drafted the manuscript. All other authors provided critical revisions. All authors approved the final version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Numbers JP18H05525 and JP20H00108, by the Ministry of Education, Culture, Sports, Science and Technology, Japan. The authors received no other funding for the research.
Data availability
The data that support the current findings are available on request from the corresponding author (M.S.). The data are not publicly available because we did not obtain consent from participants.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-43861-w.
References
- 1.Christoff K, Irving ZC, Fox KC, Spreng RN, Andrews-Hanna JR. Mind-wandering as spontaneous thought: A dynamic framework. Nat. Rev. Neurosci. 2016;17:718–731. doi: 10.1038/nrn.2016.113. [DOI] [PubMed] [Google Scholar]
- 2.Kvavilashvili L, Niedźwieńska A, Gilbert SJ, Markostamou I. Deficits in spontaneous cognition as an early marker of Alzheimer’s disease. Tren. Cog. Sci. 2020;24:285–301. doi: 10.1016/j.tics.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Schlagman S, Kvavilashvili L. Involuntary autobiographical memories in and outside the laboratory: How different are they from voluntary autobiographical memories? Mem. Cog. 2008;36:920–932. doi: 10.3758/mc.36.5.920. [DOI] [PubMed] [Google Scholar]
- 4.Cole S, Kvavilashvili L. Spontaneous future cognition: The past, present and future of an emerging topic. Psychol. Res. 2019;83:631–650. doi: 10.1007/s00426-019-01193-3. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert SJ, Hadjipavlou N, Raoelison M. Automaticity and control in prospective memory: A computational model. PLos One. 2013;8:e59852. doi: 10.1371/journal.pone.0059852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleim B, Graham B, Bryant RA, Ehlers A. Capturing intrusive re-experiencing in trauma survivors’ daily lives using ecological momentary assessment. J. Aborm. Psychol. 2013;122:998–1009. doi: 10.1037/a0034957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng S, D’Mello S, Graesser AC. Mind wandering while reading easy and difficult texts. Psychonom. Bull. Rev. 2013;20:586–592. doi: 10.3758/s13423-012-0367-y. [DOI] [PubMed] [Google Scholar]
- 8.Giambra, L. M. & Grodsky, A. Task-unrelated images and thoughts while reading. In Imagery (eds. Shorr, J. E. et al.) (Springer, 1989). 10.1007/978-1-4899-0876-6_3.
- 9.Kane MJ, et al. For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychol. Sci. 2007;18:614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- 10.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smallwood J, Obonsawin M, Reid H. The effects of block duration and tasks demands on the experience of task unrelated thought. Imag. Cog. Person. 2003;22:13–31. doi: 10.2190/TBML-N8JN-W5YB-4L9R. [DOI] [Google Scholar]
- 12.Pelagatti C, Binda P, Vannucci M. Tracking the dynamics of mind wandering: Insights from Pupillometry. J. Cogn. 2018;1:38. doi: 10.5334/joc.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelagatti C, Binda P, Vannucci M. A closer look at the timecourse of mind wandering: Pupillary responses and behaviour. PLos One. 2020;15:e0226792. doi: 10.1371/journal.pone.0226792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plimpton B, Patel P, Kvavilashvili L. Role of triggers and dysphoria in mind-wandering about past, present and future: A laboratory study. Conscious. Cogn. 2015;33:261–276. doi: 10.1016/j.concog.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Vannucci M, Pelagatti C, Marchetti I. Manipulating cues in mind wandering: Verbal cues affect the frequency and the temporal focus of mind wandering. Conscious. Cogn. 2017;53:61–69. doi: 10.1016/j.concog.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Morita T, Kawasaki M. Daigaku jugyou bamenn ni okeru maindowandaringu kara no hukki no keiki. Jpn. Soc. Educ. Tech. 2018;42:149–152. doi: 10.15077/jjet.S42079. [DOI] [Google Scholar]
- 17.Smallwood J, Schooler JW. The science of mind wandering: Empirically navigating the stream of consciousness. Annu. Rev. Psychol. 2015;66:487–518. doi: 10.1146/annurev-psych-010814-015331. [DOI] [PubMed] [Google Scholar]
- 18.Faber M, D’Mello SK. How the stimulus influences mind wandering in semantically rich task contexts. Cogn. Res. Princ. Implic. 2018;3:35. doi: 10.1186/s41235-018-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalsa SS, et al. Interoception and mental health: A roadmap. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2018;3:501–513. doi: 10.1016/j.bpsc.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherrington CS. The Integrative Action of the Nervous System. Yale University Press; 1906. [Google Scholar]
- 21.Galvez-Pol, A., Munar, E. & Kilner, J. M. The influence of interoceptive signals on the processing of external sensory stimuli. In The Routledge International Handbook of Neuroaesthetics (eds. Skov, M. & Nadal, M.) 89–102 (Routledge, 2022).
- 22.Critchley HD, Garfinkel SN. The influence of physiological signals on cognition. Curr. Opin. Behav. Sci. 2018;19:13–18. doi: 10.1016/j.cobeha.2017.08.014. [DOI] [Google Scholar]
- 23.Azevedo RT, Garfinkel SN, Critchley HD, Tsakiris M. Cardiac afferent activity modulates the expression of racial stereotypes. Nat. Commun. 2017;8:13854. doi: 10.1038/ncomms13854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pramme L, Larra MF, Schächinger H, Frings C. Cardiac cycle time effects on selection efficiency in vision. Psychophysiology. 2016;53:1702–1711. doi: 10.1111/psyp.12728. [DOI] [PubMed] [Google Scholar]
- 25.Quigley KS, Kanoski S, Grill WM, Barrett LF, Tsakiris M. Functions of interoception: From energy regulation to experience of the self. Trend. Neurosci. 2021;44:29–38. doi: 10.1016/j.tins.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terasawa Y, Fukushima H, Umeda S. How does interoceptive awareness interact with the subjective experience of emotion? An fMRI study. Human Brain Mapp. 2013;34:598–612. doi: 10.1002/hbm.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirk U, Downar J, Montague PR. Interoception drives increased rational decision-making in meditators playing the ultimatum game. Front. Neurosci. 2011;5:49. doi: 10.3389/fnins.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meissner K, Wittman M. Body signals, cardiac awareness, and the perception of time. Biol. Psychol. 2011;86:289–297. doi: 10.1016/j.biopsycho.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Garfinkel, S., Critchley, H. & Pollatos, O. The interoceptive system: Implications for cognition, emotion, and health. In Handbook of Psychophysiology (Cambridge Handbooks in Psychology, pp. 427–443) (eds. Cacioppo, J. et al.) (Cambridge University press, 2016). 10.1017/9781107415782.019.
- 30.Terasawa Y, Moriguchi Y, Tochizawa S, Umeda S. Interoceptive sensitivity predicts sensitivity to the emotions of others. Cogn. Emot. 2014;28:1435–1448. doi: 10.1080/02699931.2014.888988. [DOI] [PubMed] [Google Scholar]
- 31.Füstös J, Gramann K, Herbert BM, Pollatos O. On the embodiment of emotion regulation: Interoceptive awareness facilitates reappraisal. Soc. Cogn. Affect. Neurosci. 2013;8:911–917. doi: 10.1093/scan/nss089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garfinkel SN, et al. What the heart forgets: Cardiac timing influences memory for words and is modulated by metacognition and interoceptive sensitivity. Psychophysiology. 2013;50:505–512. doi: 10.1111/psyp.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umeda S, Tochizawa S, Shibata M, Terasawa Y. Prospective memory mediated by interoceptive accuracy: A psychophysiological approach. Phil. Transact. R. Soc. Lond. Ser. B Biol. Sci. 2016;371:20160005. doi: 10.1098/rstb.2016.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrews-Hanna, J. R., Irving, Z. C., Fox, K. C. R., Spreng, N. & Christoff, K. The Neuroscience of Spontaneous Thought: An Evolving Interdisciplinary Field. The Oxford Handbook of Spontaneous Thought: Mind-Wandering, Creativity, and Dreaming (Oxford University, 2018).
- 35.Craig AD. How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 36.Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 37.Babo-Rebelo M, Richter CG, Tallon-Baudry C. Neural responses to heartbeats in the default network encode the self in spontaneous thoughts. J. Neurosci. 2016;36:7829–7840. doi: 10.1523/JNEUROSCI.0262-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babo-Rebelo M, Wolpert N, Adam C, Hasboun D, Tallon-Baudry C. Is the cardiac monitoring function related to the self in both the default network and right anterior insula? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371:20160004. doi: 10.1098/rstb.2016.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasenkamp W, Barsalou LW. Effects of meditation experience on functional connectivity of distributed brain networks. Front. Hum. Neurosci. 2012;6:38. doi: 10.3389/fnhum.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasenkamp W, Wilson-Mendenhall CD, Duncan E, Barsalou LW. Mind wandering and attention during focused meditation: A fine-grained temporal analysis of fluctuating cognitive states. NeuroImage. 2012;59:750–760. doi: 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Critchley HD, Nagai Y, Gray MA, Mathias CJ. Dissecting axes of autonomic control in humans: Insights from neuroimaging. Auton. Neurosci. 2011;161:34–42. doi: 10.1016/j.autneu.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Seeley WW. The salience network: A neural system for perceiving and responding to homeostatic demands. J. Neurosci. 2019;39:9878–9882. doi: 10.1523/JNEUROSCI.1138-17.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azevedo RT, et al. The calming effect of a new wearable device during the anticipation of public speech. Sci. Rep. 2017;7:2285. doi: 10.1038/s41598-017-02274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi, K. Y. & Ishii, H. AmbienBeat: Wrist-worn mobile tactile biofeedback for heart rate rhythmic regulation. In Proceedings of the Fourteenth International Conference on Tangible, Embedded, and Embodied Interaction (TEI ‘20) 17–30 (Assoc. for Computing Machinery, 2020). 10.1145/3374920.3374938.
- 46.Xu M, et al. The effect of haptic stimulation simulating heartbeats on the regulation of physiological responses and prosocial behavior under stress: The influence of interoceptive accuracy. Biol. Psychol. 2021;164:108172. doi: 10.1016/j.biopsycho.2021.108172. [DOI] [PubMed] [Google Scholar]
- 47.Mollakazemi MJ, Biswal D, Elayi SC, Thyagarajan S, Evans J. Synchronization of autonomic and cerebral rhythms during listening to music: Effects of tempo and cognition of songs. Physiol. Res. 2019;68:1005–1019. doi: 10.33549/physiolres.934163. [DOI] [PubMed] [Google Scholar]
- 48.Meyerholz L, Irzinger J, Witthöft M, Gerlach AL, Pohl A. Contingent biofeedback outperforms other methods to enhance the accuracy of cardiac interoception: A comparison of short interventions. J. Behav. Ther. Experiment. Psychiatry. 2019;63:12–20. doi: 10.1016/j.jbtep.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Rominger C, Graßmann TM, Weber B, Schwerdtfeger AR. Does contingent biofeedback improve cardiac interoception? A preregistered replication of Meyerholz, Irzinger, Withöft, Gerlach, and Pohl (2019) using the heartbeat discrimination task in a randomised control trial. PLos One. 2021;16:e0248246. doi: 10.1371/journal.pone.0248246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di-Lernia D, Serino S, Pezzulo G, Pedroli E, Cipresso P, Riva G. Feel the time. Time perception as a function of interoceptive processing. Front. Hum. Neurosci. 2018;12:74. doi: 10.3389/fnhum.2018.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costa J, Adams AT, Jung MF, Guimbretière F, Choudhury T. EmotionCheck: A wearable device to regulate anxiety through false heart rate feedback. GetMobile. 2017;21:22–25. doi: 10.1145/3131214.3131222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ueoka R, AlMutawa A. Emotion hacking VR: Amplifying the VR fear experience using false vibrotactile heartbeat feedback. Transact. Virtual Reality Soc. Jpn. 2019;24:231–240. doi: 10.18974/tvrsj.24.3_231. [DOI] [Google Scholar]
- 53.Łukowska M, Sznajder M, Wierzchoń M. Error-related cardiac response as information for visibility judgements. Sci. Rep. 2018;8:1131. doi: 10.1038/s41598-018-19144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baumgarten TJ, Königs S, Schnitzler A, Lange J. Subliminal stimuli modulate somatosensory perception rhythmically and provide evidence for discrete perception. Sci. Rep. 2017;7:43937. doi: 10.1038/srep43937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrè ER, Sahani M, Haggard P. Subliminal stimulation and somatosensory signal detection. Acta Psychol. 2016;170:103–111. doi: 10.1016/j.actpsy.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Imanaka K, Kita I, Suzuki K. Effects of nonconscious perception on motor response. Hum. Mov. Sci. 2002;21:541–561. doi: 10.1016/s0167-9457(02)00175-6. [DOI] [PubMed] [Google Scholar]
- 57.Lang Y, et al. Tactile priming accelerates conscious access to continuous flash-suppressed characters. Exp. Physiol. 2019;104:1711–1716. doi: 10.1113/EP087944. [DOI] [PubMed] [Google Scholar]
- 58.Riener, A., Ferscha, A., Frech, P., Hackl, M. & Kaltenberger, M. Subliminal vibro-tactile based notification of CO2 economy while driving. In Proceedings of the 2nd International Conference on Automotive User Interfaces and Interactive Vehicular Applications 92–101 (2010).
- 59.Riener, A. Information injection below conscious awareness: Potential of sensory channels. In 3rd International Conference on Automotive User Interfaces and Interactive Vehicular Applications Automotive UI’11 Vol. 6 (2011).
- 60.Riener A. Subliminal persuasion and its potential for driver behavior adaptation. IEEE Trans. Intell. Transp. Syst. 2012;13:71–80. doi: 10.1109/TITS.2011.2178838. [DOI] [Google Scholar]
- 61.Riener A, Chalfoun P, Frasson C. The potential of subliminal information displays to change driver behavior. Presence Teleoper. Virtual Environ. 2014;23:51–70. doi: 10.1162/PRES_a_00170. [DOI] [Google Scholar]
- 62.Al E, Iliopoulos F, Forschack N, Nierhaus T, Grund M, Motyka P, Gaebler M, Nikulin VV, Villringer A. Heart-brain interactions shape somatosensory perception and evoked potentials. Proc. Nat. Acad. Sci. U.S.A. 2020;117:10575–10584. doi: 10.1073/pnas.1915629117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grund M, Al E, Pabst M, Dabbagh A, Stephani T, Nierhaus T, Gaebler M, Villringer A. Respiration, heartbeat, and conscious tactile perception. J. Neurosci. 2022;42:643–656. doi: 10.1523/JNEUROSCI.0592-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garfinkel SN, Critchley HD. Interoception, emotion and brain: New insights link internal physiology to social behavior. Soc. Cog. Affect. Neurosc. 2013;8:231–234. doi: 10.1093/scan/nss140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schandry R. Heartbeat perception and emotional experience. Psychophysiology. 1981;18:483–488. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- 66.Chong JSX, Ng GJP, Lee SC, Zhou J. Salience network connectivity in the insula is associated with individual differences in interoceptive accuracy. Brain Struct. Funct. 2017;222:1635–1644. doi: 10.1007/s00429-016-1297-7. [DOI] [PubMed] [Google Scholar]
- 67.Coll MP, Hobson H, Bird G, Murphy J. Systematic review and meta-analysis of the relationship between the heartbeat-evoked potential and interoception. Neurosc. Biobehav. Rev. 2021;122:190–200. doi: 10.1016/j.neubiorev.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 68.Stawarczyk D, Majerus S, Maquet P, D’Argembeau A. Neural correlates of ongoing conscious experience: Both task-unrelatedness and stimulus-independence are related to default network activity. PLOS ONE. 2011;6:e16997. doi: 10.1371/journal.pone.0016997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Desmedt O, Luminet O, Corneille O. The heartbeat counting task largely involves non-interoceptive processes: Evidence from both the original and an adapted counting task. Biol. Psychol. 2018;138:185–188. doi: 10.1016/j.biopsycho.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 70.Voss MJ, Zukosky M, Wang RF. A new approach to differentiate states of mind wandering: Effects of working memory capacity. Cognition. 2018;179:202–212. doi: 10.1016/j.cognition.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 71.Welhaf MS, et al. An exploratory analysis of individual differences in mind wandering content and consistency. Psychol. Conscious. Theor. Res. Pract. 2020;7:103–125. doi: 10.1037/cns0000180. [DOI] [Google Scholar]
- 72.Zanesco AP, Denkova E, Witkin JE, Jha AP. Experience sampling of the degree of mind wandering distinguishes hidden attentional states. Cognition. 2020;205:104380. doi: 10.1016/j.cognition.2020.104380. [DOI] [PubMed] [Google Scholar]
- 73.Kane MJ, et al. Individual differences in the executive control of attention, memory, and thought, and their associations with schizotypy. J. Exper. Psychol. Gen. 2016;145:1017–1048. doi: 10.1037/xge0000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kane MJ, et al. For whom the mind wanders, and when, varies across laboratory and daily-life settings. Psychol. Sci. 2017;28:1271–1289. doi: 10.1177/0956797617706086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.D’Argembeau, A. Mind-wandering and self-referential thought. In The Oxford Handbook of Spontaneous Thought: Mind-Wandering, Creativity, and Dreaming, 1st ed. 181–191 (Oxford University Press, 2018).
- 76.Ehlers A, Breuer P. Increased cardiac awareness in panic disorder. J. Abnorm. Psychol. 1992;101(3):371–382. doi: 10.1037//0021-843x.101.3.371. [DOI] [PubMed] [Google Scholar]
- 77.Killick R, Eckley IA. changepoint: An R package for changepoint analysis. J. Stat. Soft. 2014;58:1–19. doi: 10.3389/fnins.2011.00049. [DOI] [Google Scholar]
- 78.Hablani, S., Higgins, C. & Walsh, D. & Reilly, R. Neural based assessment of mind wandering during a fatigue inducing motor task: is task failure due to fatigue or distraction? In IEEE/EMBS Conference on Neural Engineering 45–48 (2019). 10.1109/NER.2019.87170.
- 79.Smallwood J, Mrazek MD, Schooler JW. Medicine for the wandering mind: Mind wandering in medical practice. Med. Educ. 2011;45:1072–1080. doi: 10.1111/j.1365-2923.2011.04074.x. [DOI] [PubMed] [Google Scholar]
- 80.Walker HEK, Trick LM. Mind-wandering while driving: The impact of fatigue, task length, and sustained attention abilities. Transp. Res. F. 2018;59:81–97. doi: 10.1016/j.trf.2018.08.009. [DOI] [Google Scholar]
- 81.Tarvainen, M. P., Lipponen, J. A., Niskanen, J-P. & Ranta-aho, P. O. Kubios HRV software user’s guide. https://www.kubios.com/downloads/Kubios_HRV_Users_Guide.pdf (2021). [DOI] [PubMed]
- 82.Ernst G. Heart-rate variability-more than heart beats? Front. Public Health. 2017;5:240. doi: 10.3389/fpubh.2017.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schubert AL, Frischkorn GT, Rummel J. The validity of the online thought-probing procedure of mind wandering is not threatened by variations of probe rate and probe framing. Psychol. Res. 2020;84:1846–1856. doi: 10.1007/s00426-019-01194-2. [DOI] [PubMed] [Google Scholar]
- 84.Webb CA, et al. Spontaneous thought characteristics are differentially related to heightened negative affect versus blunted positive affect in adolescents: An experience sampling study. J. Child. Psychol. Pychiatry Advanc. 2022;2:e12110. doi: 10.1002/jcv2.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bürkner P-C. brms: An R package for Bayesian multilevel models using Stan. J. Stat. Soft. 2017;80:1–28. doi: 10.18637/jss.v080.i01. [DOI] [Google Scholar]
- 86.Bürkner P-C. Advanced Bayesian multilevel modeling with the R package brms. R J. 2018;10:395–411. doi: 10.32614/RJ-2018-01. [DOI] [Google Scholar]
- 87.Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat. Sci. 1992;7:457–472. doi: 10.1214/ss/1177011136. [DOI] [Google Scholar]
- 88.Smallwood J, Schooler JW. The restless mind. Psychol. Bull. 2006;132:946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- 89.Ellamil M, et al. Dynamics of neural recruitment surrounding the spontaneous arising of thoughts in experienced mindfulness practitioners. NeuroImage. 2016;136:186–196. doi: 10.1016/j.neuroimage.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 90.van Vugt MK, Broers N. Self-reported stickiness of mind-wandering affects task performance. Front. Psychol. 2016;7:732. doi: 10.3389/fpsyg.2016.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seth AK. Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 2013;17:565–573. doi: 10.1016/j.tics.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 92.Seth AK, Friston KJ. Active interoceptive inference and the emotional brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371:20160007. doi: 10.1098/rstb.2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seli P, Carriere JS, Levene M, Smilek D. How few and far between? Examining the effects of probe rate on self-reported mind wandering. Front. Psychol. 2013;4:430. doi: 10.3389/fpsyg.2013.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Robinson MK, Miller AL, Unsworth N. Examining the effects of probe frequency, response options, and framing within the thought-probe method. Behav. Res. Methods. 2019;51:398–408. doi: 10.3758/s13428-019-01212-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the current findings are available on request from the corresponding author (M.S.). The data are not publicly available because we did not obtain consent from participants.