Abstract
Macrolide resistance is an emerging problem in AIDS patients who receive these agents for treatment or prophylaxis against Mycobacterium avium (MAC) infection. We compared the emergence of resistant MAC strains during therapy with clarithromycin (clarithromycin resistance was defined as MIC ≥ 32 μg/ml) and azithromycin (azithromycin resistance was defined as MIC ≥ 128 μg/ml) in C57BL/6 beige mice. Treatment with clarithromycin and azithromycin resulted in a decrease of 98.5% in the number of viable bacteria in spleens at week 8 and 99% at week 12 compared with the number of bacteria present in spleen before the initiation of therapy (P < 0.001). Splenic homogenates were also plated onto 7H11 agar plus clarithromycin at 32 μg/ml or azithromycin at 128 μg/ml. Resistance emerged significantly more often in mice treated with clarithromycin (100% of treated mice at both 8 and 12 weeks) than in those receiving azithromycin (0% at week 8 and 14% at week 12). The frequencies of resistance of the MAC population in the spleen to clarithromycin were 2.1 × 10−3 at week 8 and 1.1 × 10−2 at week 12, whereas resistance to azithromycin was absent at week 8 (all mice) and was ∼3.5 × 10−5 (mean for the three positive animals) at week 12. Clarithromycin was more effective in initial reduction of MAC burden in tissue after 8 and 12 weeks of treatment, but resistant strains emerged significantly more frequently after treatment with clarithromycin than after treatment with azithromycin.
Disseminated Mycobacterium avium complex (MAC) infection is a common late-stage opportunistic infection in patients with AIDS (11, 18). While MAC is characteristically resistant to the conventional antituberculosis agents, macrolides such as clarithromycin and azithromycin were shown to be active against MAC in vitro (7, 10, 12), in experimental animals (3, 4, 13, 15), and in human clinical trials (3, 5, 23).
Macrolide resistance readily occurs after the initiation of clarithromycin monotherapy in humans (3). The incidence of clarithromycin resistance in “breakthrough” MAC isolates between 8 and 10 weeks of therapy has been as high as 46% (3). Although almost all organisms are susceptible to clarithromycin prior to therapy, resistant strains are eventually isolated from one-half of patients with breakthrough after 16 weeks of therapy (3).
Prophylaxis against opportunistic infections is critical to the management of AIDS. Clarithromycin, azithromycin, and rifabutin are effective as single agents in preventing MAC disease (8, 19, 20).
Resistance to clarithromycin prophylaxis, however, emerges in 58% of the MAC isolates from patients with relapse (20). In contrast, MAC resistance to azithromycin is reported in 11% of breakthrough isolates from individuals taking azithromycin as prophylaxis (8).
Development of clarithromycin resistance has been shown to result in cross-resistance to azithromycin (9) and has been shown to be primarily due to single-base mutations in the 23S rRNA gene (16, 17). The objective of our study was to compare the incidence of MAC strains resistant to clarithromycin and azithromycin in MAC-infected beige mice and the frequency of the resistance.
MAC strain 101 (serovar 1) was originally isolated from the blood of an AIDS patient with disseminated MAC infection. In order to maintain virulence, the strain has been periodically passed through C57BL/6 black mice. MAC microorganisms were grown at 37°C for 10 days on Middlebrook 7H11 agar (Difco Laboratories, Detroit, Mich.) supplemented with oleic acid, albumin, dextrose, and catalase (OADC; Difco Laboratories). After MAC cultures had been examined for purity, transparent colonies were harvested and suspended in Hanks’ balanced salt solution. The suspension was adjusted to approximately 4 × 108 CFU/ml by using a McFarland turbidity standard, and the number of CFU per milliliter of the final inoculum was confirmed by plating serial dilutions of the inoculum onto 7H11 agar (1, 13).
Female beige mice (C57BL/6J/bgj/bgj) were purchased from Jackson Laboratories (Bar Harbor, Maine). Eight- to 10-week-old female beige mice (14 to 18 g) were inoculated intravenously with 4 × 107 CFU of MAC 101.
Clarithromycin was provided by Abbott Laboratories, North Chicago, Ill., and prepared as a suspension in a sterile saturated sucrose solution. Azithromycin was provided by Pfizer (Groton, Conn.) and prepared as a suspension similarly to clarithromycin.
Mice were infected with 4 × 107 CFU intravenously (1, 2), and after 7 days 24 mice per experiment were harvested to establish the baseline level of infection. Clarithromycin or azithromycin (200 mg/kg of body weight/day) was administered by gavage without sedation over the entire period (up to 12 weeks). Control mice received saturated sucrose solution without drug. Twenty-four mice were harvested per experimental group at 0, 8, and 12 weeks after the start of therapy, and the spleens were removed and cultured in a quantitative manner. To decrease the possibility of drug carryover, mice were killed 48 h after receiving the last dose of drug (1, 2).
Mice were killed, and the spleens were removed by aseptic dissection. The organs were weighed and homogenized in Middlebrook 7H9 broth. Aliquots of the suspensions were plated onto 7H11 agar with OADC and onto 7H11 agar with OADC and either clarithromycin at 32 μg/ml or azithromycin at 128 μg/ml. The frequency of resistance was determined by counting the number of CFU per gram growing on plates with antibiotics in comparison with that growing on plates without antibiotics. The plates were incubated for 8 to 10 days at 37°C, and CFU were counted as previously described and expressed as means per gram of tissue ± standard errors (1, 2). Both the total number of colonies and the number of resistant colonies per mouse were taken into consideration to calculate the frequency of resistance.
MAC strains isolated from mice that developed microbiological evidence of clarithromycin resistance were tested in vitro to determine the MIC of clarithromycin by the radiometric broth macrodilution procedure as previously described (13). If more than one colony was isolated from a culture, a sweep of all colonies was taken for testing.
Resistance to clarithromycin was defined as MIC greater than 32 μg/ml, while resistance of azithromycin was defined as 128 μg/ml, based on the observation that clinical isolates which break through during therapy with clarithromycin or azithromycin have MICs of ≥32 and ≥128 μg/ml, respectively (3, 8, 20, 23).
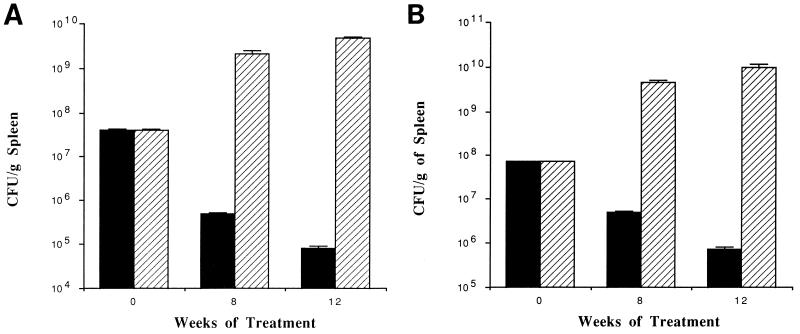
The differences between control and experimental groups at the same time point were analyzed by Student’s t test and analysis of variance. P < 0.05 was considered statistically significant. Figure 1 shows the reductions in MAC CFU per gram of spleen for mice receiving either clarithromycin or azithromycin in comparison with the untreated control group over time. Treatment with either clarithromycin or azithromycin resulted in a significant reduction in the number of MAC organisms in spleen compared with untreated mice. In comparison with the number of bacteria in control mice, mice treated with clarithromycin for 8 weeks had 7.2 × 103-fold fewer bacteria in the spleen (99.9% reduction), while mice receiving azithromycin therapy had 3.2 × 102-fold fewer bacteria in the spleen than controls (99.9% reduction). After 12 weeks of therapy, use of clarithromycin was associated with 5.9 × 104-fold fewer bacteria in the spleen (99.9% reduction) than in control mice at the same time point, while the use of azithromycin was associated with 1.7 × 103-fold fewer bacteria in the spleen than in untreated control mice (99.9% reduction). The difference between the number of bacteria in mice treated with either clarithromycin or azithromycin and the number of bacteria present before the initiation of therapy was also significant (P < 0.05).
FIG. 1.
Comparison of CFU per gram of spleen in mice receiving clarithromycin (A) or azithromycin (B) (200 mg/kg) (solid bars) after 8 and 12 weeks of therapy. Spleens were harvested, homogenized, and plated onto 7H10 agar as described in the text. Matched bars, control mice.
Table 1 shows the number of mice from which macrolide-resistant MAC was isolated over time. Treatment with clarithromycin led to the isolation of resistant strains from all 24 mice both at week 8 and at week 12 of therapy. In contrast, azithromycin-resistant MAC was isolated only from 2 of 24 mice by 8 weeks and 4 of 24 mice after 12 weeks. One of 12 mice in the untreated control group had a macrolide-resistant natural mutant.
TABLE 1.
Incidence of macrolide-resistant MAC in splenic homogenates
| Treatment | No. of mice with resistant bacteriaa
|
|
|---|---|---|
| 8 wk | 12 wk | |
| Clarithromycin | 24 | 24 |
| Azithromycin | 2 | 4 |
| Control | 1 | 1 |
n = 24.
Table 2 shows the frequencies of macrolide resistance among MAC strains in spleen homogenates in treated and control mice.
TABLE 2.
Frequencies of macrolide-resistant MAC in splenic homogenates
| Treatment | Frequency of resistancea
|
||
|---|---|---|---|
| 0 wk | 8 wk | 12 wk | |
| Clarithromycin | 1 × 10−7 | 1 × 10−3 | 1.1 × 10−2 |
| Azithromycin | 4 × 10−8 | 1 × 10−8 | 3.5 × 10−5 |
| P | <0.04 | <0.001 | <0.001 |
Based on the number of bacteria per gram of tissue, determined by plating spleen homogenates onto a 7H10 plate containing 32 μg of clarithromycin or 128 μg of azithromycin per ml and comparison with plates without antibiotic.
While the frequency of resistance to clarithromycin was 1 × 10−7 prior to therapy, the frequency of natural resistance to azithromycin was 4 × 10−8 (P < 0.05).
At both 8 and 12 weeks there were significantly more organisms isolated that were resistant to clarithromycin than organisms resistant to azithromycin (P < 0.001). Clarithromycin-resistant strains obtained from some mice were plated onto 7H10 agar with 128 μg of clarithromycin per ml to rule out the possibility that the concentration used to determine resistance to azithromycin (128 μg/ml) represented a different standard of resistance. All the clarithromycin-resistant M. avium organisms were able to grow in the presence of 128 μg of the antibiotic per ml (data not shown).
Macrolide susceptibilities of MAC strains obtained from spleens of macrolide-treated mice at 8 and 12 weeks of therapy were determined. All the isolates that were obtained from either clarithromycin- or azithromycin-treated mice were resistant to both antibiotics (≥32 μg of clarithromycin and ≥128 μg of azithromycin per ml).
Either clarithromycin or azithromycin given to MAC-infected beige mice caused a significant reduction in the number of viable bacteria in the spleen compared with untreated control mice. Although this does not represent a new observation, the finding that the use of clarithromycin led to a significantly greater reduction in bacterial load after 8 and 12 weeks than the administration of azithromycin with the challenge strain MAC 101 has not been previously described. The fact that our study reports on the comparison of separate experiments does not question the merit of the observation. In fact, it would be expected that mice with greater bacterial load (such as the mice that received azithromycin) would have more organisms resistant to the macrolide used, but our finding shows the opposite, which strengthens the value of the observation. A recent report comparing the clinical response to treatment with azithromycin or clarithromycin also demonstrated that administration of clarithromycin was associated with a significantly greater reduction in bacteremia than the administration of azithromycin (22). In contrast, Cynamon and Klemens reported that treatment of MAC-infected mice in which clarithromycin and azithromycin (200 mg/kg/day for 10 days) were simultaneously compared, showed that azithromycin was significantly more active than clarithromycin in the liver and spleen but not in the lung (4). The difference in findings between that study and ours could be explained, at least partially, by the different challenge strains used and by the duration of therapy—10 days in the study by Cynamon and Klemens versus 84 days in this study.
The incidence of resistance to clarithromycin was greater than that of resistance to azithromycin. These findings are in agreement with previous studies, both ours (2) and that by Ji and colleagues (14), showing that resistance to clarithromycin in mice with disseminated MAC infection develops after approximately 4 weeks of therapy. In addition, recent reports of human clinical trials showed that MAC resistance to clarithromycin therapy emerged in 29 to 58% of the patients (3). In contrast, resistance to azithromycin was reported in 11% of breakthrough isolates from individuals receiving prophylactic therapy (8). A previous in vitro study by Heifets and colleagues determined that the frequency of resistance of MAC isolates to clarithromycin was 1.1 × 10−7 compared with 1.2 × 10−6 for azithromycin (9). The discrepancy between that study and ours could be related to the fact that those assays were performed in vitro while ours were based on an in vivo model. Nonetheless, the clinical trials using clarithromycin and azithromycin to treat or prevent M. avium infection support our findings.
It is possible that differences in the emergence of resistance between macrolides, with more resistance associated with clarithromycin than azithromycin, can be explained by the pharmacokinetic properties of clarithromycin (a shorter half-life and lower tissue concentration) in mice receiving one daily dose. However, we found that MAC resistance to clarithromycin was proportionally greater than resistance to azithromycin prior to therapy. Initially, clarithromycin has significant anti-MAC effect, but its shorter tissue half-life may allow for the “regrowth” of resistant bacteria and, hence, more resistant organisms by 8 to 12 weeks. Azithromycin’s initial anti-MAC effect is less pronounced, but it is more tissue concentrated for longer intervals, thereby allowing less opportunity for growth of a resistant subpopulation.
Clinically significant resistance to macrolides in MAC is primarily due to single-base mutations in the 23S rRNA gene (16, 17). Our findings that the frequencies of resistance to the two macrolides prior to treatment are not equal cannot be completely explained by the assumption that MAC mechanisms of resistance to clarithromycin and azithromycin are the same. Mechanisms such as mutation of the ribosomal binding sites for the drug and efflux have been reported for clarithromycin (6) and erythromycin (21), respectively, and could explain the difference observed between the two macrolides. Since we did not test cross-resistance in all resistant isolates from macrolide-treated mice, it is possible that a percentage of clarithromycin-resistant MAC organisms would be susceptible to azithromycin.
In conclusion, we found that clarithromycin is more potent than azithromycin against MAC in experimental infection in beige mice, but its use was associated with a significantly greater emergence of resistant MAC strains. Direct comparisons of the rates of the emergence of resistance in clinical trials with AIDS patients are needed to validate this observation.
Acknowledgments
We thank Karen Allen for preparing the manuscript and Baohong Ji and Clark Inderlied for helpful discussions.
This work was supported by contract AI-25140 of the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Bermudez L E, Inderlied C B, Kolonoski P, Petrofsky M, Young L S. Clarithromycin, dapsone and their combination to treat or prevent disseminated Mycobacterium avium complex in beige mice. Antimicrob Agents Chemother. 1994;38:2717–2722. doi: 10.1128/aac.38.12.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez L E, Nash K A, Petrofsky M, Young L S, Inderlied C B. Effect of ethambutol on emergence of clarithromycin-resistant Mycobacterium avium complex in the beige mouse model. J Infect Dis. 1996;174:1218–1222. doi: 10.1093/infdis/174.6.1218. [DOI] [PubMed] [Google Scholar]
- 3.Chaisson R E, Benson C A, Dube M P, Heifets L B, Korvick J A, Elkin S, Smith T, Craft J C, Sattler F R AIDS Clinical Trials Group. Clarithromycin therapy for bacteremic Mycobacterium avium complex. Ann Intern Med. 1994;121:905–911. doi: 10.7326/0003-4819-121-12-199412150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Cynamon M H, Klemens S P. Activity of azithromycin against Mycobacterium avium infection in beige mice. Antimicrob Agents Chemother. 1992;36:1611–1618. doi: 10.1128/aac.36.8.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dautzenberg B, Truffot C, Legris S, Meyohas M C, Berlie H C, Mercat A, Chevret S, Grosset J. Activity of clarithromycin against Mycobacterium avium infection in patients with the acquired immune deficiency syndrome. Am Rev Respir Dis. 1991;144:564–569. doi: 10.1164/ajrccm/144.3_Pt_1.564. [DOI] [PubMed] [Google Scholar]
- 6.Doucet-Populaire F, Goldman R, Grosset J, Jarlier V. Program and abstracts of 35th International Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Molecular basis of clarithromycin activity against Mycobacterium avium, abstr. C93; p. 56. [Google Scholar]
- 7.Fernandes P B, Hardy D J, McDaniel D, Hanson C W, Swanson R N. In vitro and in vivo activities of clarithromycin against Mycobacterium avium. Antimicrob Agents Chemother. 1989;33:1531–1536. doi: 10.1128/aac.33.9.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havlir D V, Dube M P, Sattler F R, Fortal D N, Demper C A, Dunne M W, Parenti D M, Lavelle J P, White A C, Jr, Witt M D, Bozzette S A, McCutchan J A California Clinical Trials Group. Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. N Engl J Med. 1996;335:392–398. doi: 10.1056/NEJM199608083350604. [DOI] [PubMed] [Google Scholar]
- 9.Heifets L, Mor N, Vanderkolk J. Mycobacterium avium strains resistant to clarithromycin and azithromycin. Antimicrob Agents Chemother. 1993;37:2364–2370. doi: 10.1128/aac.37.11.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heifets L B, Lindholm-Levy P J, Comstock R D. Clarithromycin minimal inhibitory and bactericidal concentrations against Mycobacterium avium. Am Rev Respir Dis. 1992;145:856–858. doi: 10.1164/ajrccm/145.4_Pt_1.856. [DOI] [PubMed] [Google Scholar]
- 11.Horsburgh C R., Jr Mycobacterium avium complex in the acquired immunodeficiency syndrome (AIDS) N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 12.Inderlied C B, Kemper C A, Bermudez L E. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inderlied C B, Kolonoski P T, Wu M, Young L S. In vitro and in vivo activity of azithromycin (CP 62, 993) against the Mycobacterium avium complex. J Infect Dis. 1989;159:994–997. doi: 10.1093/infdis/159.5.994. [DOI] [PubMed] [Google Scholar]
- 14.Ji B, Lounis N, Truffot-Pernot C, Grosset J. Selection of resistant mutants of Mycobacterium avium in beige mice by clarithromycin monotherapy. Antimicrob Agents Chemother. 1992;36:2839–2840. doi: 10.1128/aac.36.12.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klemens S P, DeStefano M S, Cynamon M H. Activity of clarithromycin against Mycobacterium avium complex infection in beige mice. Antimicrob Agents Chemother. 1992;36:2413–2417. doi: 10.1128/aac.36.11.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier A, Kirschner P, Springer B, Steingrube V A, Brown B A, Wallace R J, Jr, Böttger E C. Identification of mutations in 23S rRNA gene of clarithromycin-resistant Mycobacterium intracellulare. Antimicrob Agents Chemother. 1994;38:381–384. doi: 10.1128/aac.38.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nash K A, Inderlied C B. The genetic basis of macrolide resistance in Mycobacterium avium. Antimicrob Agents Chemother. 1995;39:2625–2630. doi: 10.1128/aac.39.12.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nightingale S D, Byrd L T, Southern P M, Jockusch J D, Cal S X, Wynne B. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992;165:1082–1085. doi: 10.1093/infdis/165.6.1082. [DOI] [PubMed] [Google Scholar]
- 19.Nightingale S D, Cameron D W, Gordin F M, Sullam P M, Cohn D L, Chaisson R, Eron L J, Sparti P D, Bihari B, Kaufman D L, Stern J J, Pearce D D, Winberg W G, LaMarca A, Siegal F P. Two placebo controlled trials of rifabutin prophylaxis against Mycobacterium avium complex infection in AIDS. N Engl J Med. 1993;329:828–833. doi: 10.1056/NEJM199309163291202. [DOI] [PubMed] [Google Scholar]
- 20.Pierce M, Crampton S, Henry D, Heifets L, LaMarca A, Montecalvo M, Wormser G P, Jablonowski H, Cynamon J J, M, Yangco B G, Notario G, Craft J C. A randomized trial of clarithromycin as prophylaxis against disseminated Mycobacterium avium complex infection in patients with advanced acquired immunodeficiency syndrome. N Engl J Med. 1996;335:384–391. doi: 10.1056/NEJM199608083350603. [DOI] [PubMed] [Google Scholar]
- 21.Sutcliffe J, Tait-Kamradt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996;40:1817–1824. doi: 10.1128/aac.40.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward T, Rimland D, Huycke M, Kauffman C. Program and abstracts of 34th Infectious Diseases Society of America Meeting, Alexandria, Va. 1996. Randomized open label trial of azithromycin plus ethambutol versus clarithromycin plus ethambutol therapy of MAC bacteremia in AIDS; p. 21. [DOI] [PubMed] [Google Scholar]
- 23.Young L S, Wiviott L, Wu M, Kolonoski P T, Bolan R, Inderlied C B. Azithromycin reduces Mycobacterium avium complex bacteremia and relieves the symptoms of disseminated disease in patients with AIDS. Lancet. 1991;338:1107–1109. doi: 10.1016/0140-6736(91)91965-w. [DOI] [PubMed] [Google Scholar]