Abstract
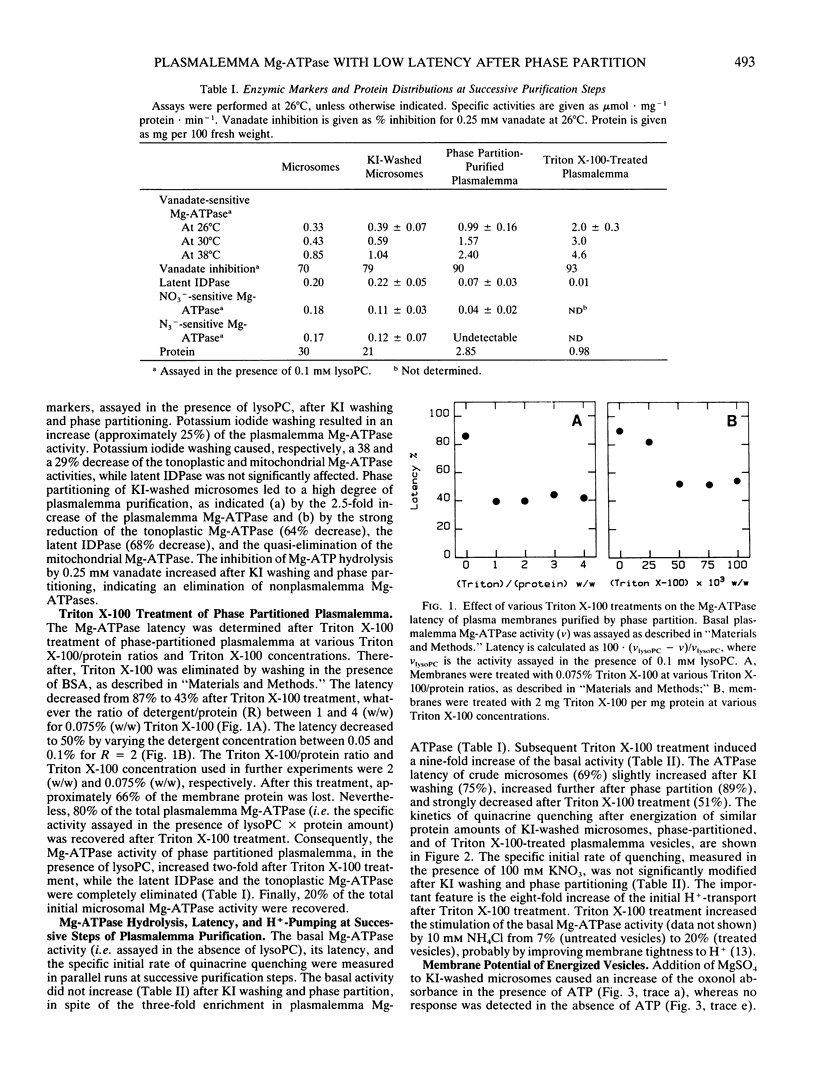
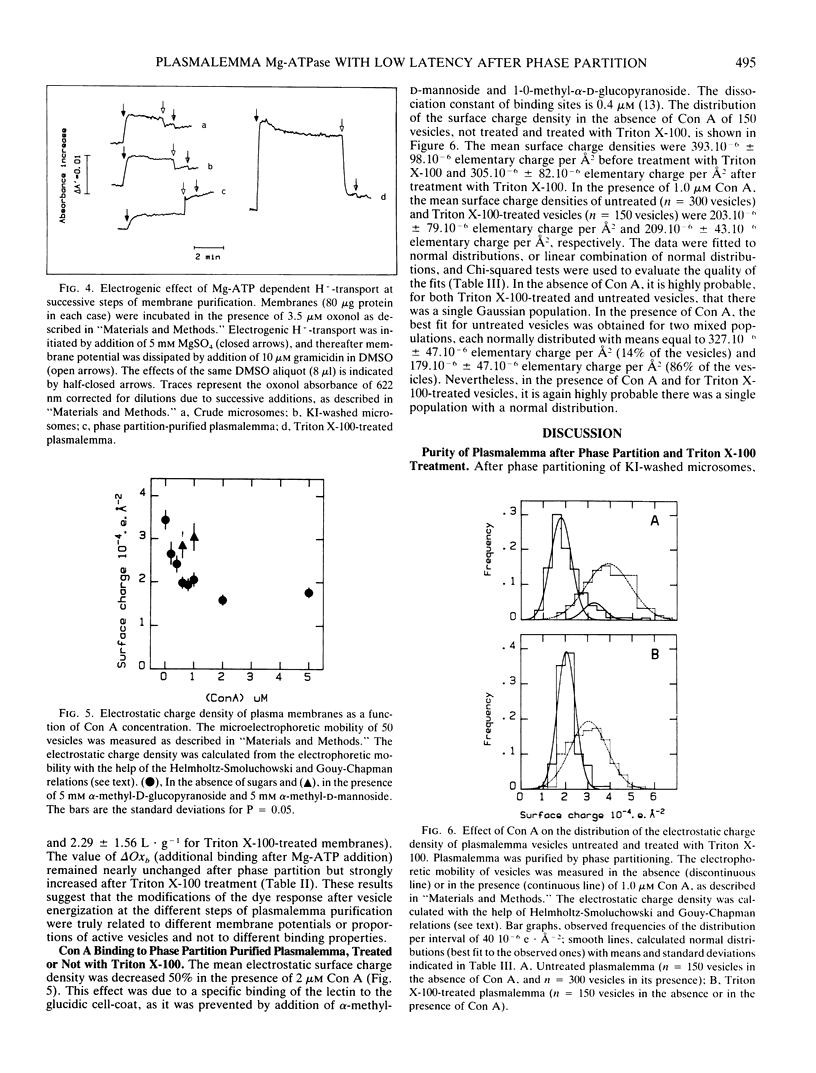
Crude plasma membranes of corn (Zea mays L.) roots were obtained according to MI De Michelis and RM Spanswick (1986 Plant Physiol 81: 542-547). This preparation, which contained tightly sealed vesicles displaying Mg-ATP dependent H+-transport, was purified by phase partitioning. The percentage of inside-out vesicles (10%) was determined from the Mg-ATPase latency, revealed with lysophosphatidylcholine. A Triton X-100 treatment described previously (JP Grouzis, R Gibrat, J Rigaud, C Grignon 1987 Biochim Biophys Acta 903: 449-464) was applied to phase-partitioned plasma membranes. The percentage of catalytic sites freely accessible to Mg-ATP increased to 50% after Triton X-100 treatment. Treated vesicles remained capable of electrogenic H+-pumping, as demonstrated by Mg:ATP-dependent quinacrine fluorescence quenching and oxonol absorbance shift. As expected from the large increase of the catalytic sites accessibility, increases of the dye responses were observed. Concanavalin A binding was estimated from microelectrophoretic measurements of individual vesicles. Statistical analysis of concanavalin A binding and Mg-ATPase latency suggest that treated membranes have lost their asymmetric structure.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANGHAM A. D., HEARD D. H., FLEMANS R., SEAMAN G. V. An apparatus for microelectrophoresis of small particles. Nature. 1958 Sep 6;182(4636):642–644. doi: 10.1038/182642a0. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Chance B., Prince R. C. Oxonol dyes as monitors of membrane potential. Their behavior in photosynthetic bacteria. Biochim Biophys Acta. 1979 Jan 11;545(1):46–57. doi: 10.1016/0005-2728(79)90112-9. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Chance B., Smith J. C., Yoshida T. The behavior of oxonol dyes in phospholipid dispersions. Biophys J. 1979 Jan;25(1):63–85. doi: 10.1016/S0006-3495(79)85278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Michelis M. I., Spanswick R. M. H-pumping driven by the vanadate-sensitive ATPase in membrane vesicles from corn roots. Plant Physiol. 1986 Jun;81(2):542–547. doi: 10.1104/pp.81.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlish S. J., Stein W. D. Passive rubidium fluxes mediated by Na-K-ATPase reconstituted into phospholipid vesicles when ATP- and phosphate-free. J Physiol. 1982 Jul;328:295–316. doi: 10.1113/jphysiol.1982.sp014265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. I. Action of detergents on membranes: differences between lipid extracted from red cell ghosts and from red cell lipid vesicles by Triton X-100. Biochemistry. 1980 Apr 29;19(9):1916–1922. doi: 10.1021/bi00550a029. [DOI] [PubMed] [Google Scholar]
- O'neill S. D., Bennett A. B., Spanswick R. M. Characterization of a NO(3)-Sensitive H-ATPase from Corn Roots. Plant Physiol. 1983 Jul;72(3):837–846. doi: 10.1104/pp.72.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado A., Arrondo J. L., Villena A., Goñi F. M., Macarulla J. M. Membrane-surfactant interactions. The effect of Triton X-100 on sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1983 Aug 24;733(1):163–171. doi: 10.1016/0005-2736(83)90102-5. [DOI] [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C., De Michelis M. I. Electrogenic transport of protons driven by the plasma membrane ATPase in membrane vesicles from radish : biochemical characterization. Plant Physiol. 1985 Jan;77(1):200–205. doi: 10.1104/pp.77.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Uemura M., Yoshida S. Isolation and Identification of Plasma Membrane from Light-Grown Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1983 Nov;73(3):586–597. doi: 10.1104/pp.73.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]


