Abstract
In recent years, growing awareness of the role of oxidative stress in brain health has prompted antioxidants, especially dietary antioxidants, to receive growing attention as possible treatments strategies for patients with neurodegenerative diseases (NDs). The most widely studied dietary antioxidants include active substances such as vitamins, carotenoids, flavonoids and polyphenols. Dietary antioxidants are found in usually consumed foods such as fresh fruits, vegetables, nuts and oils and are gaining popularity due to recently growing awareness of their potential for preventive and protective agents against NDs, as well as their abundant natural sources, generally non-toxic nature, and ease of long-term consumption. This review article examines the role of oxidative stress in the development of NDs, explores the ‘two-sidedness’ of the blood–brain barrier (BBB) as a protective barrier to the nervous system and an impeding barrier to the use of antioxidants as drug medicinal products and/or dietary antioxidants supplements for prevention and therapy and reviews the BBB permeability of common dietary antioxidant suplements and their potential efficacy in the prevention and treatment of NDs. Finally, current challenges and future directions for the prevention and treatment of NDs using dietary antioxidants are discussed, and useful information on the prevention and treatment of NDs is provided.
Key words: Dietary antioxidant supplements, Oxidative stress, Blood–brain barrier, Neurodegenerative diseases, Vitamins, Carotenoids, Flavonoids, Polyphenols
Graphical abstract
This review examines the role of oxidative stress in the development of neurodegenerative diseases (NDs), and summarizes the blood–brain barrier (BBB) permeability of common dietary antioxidant supplements and their potential efficacy in the prevention and treatment of NDs.
1. Introduction
Neurodegenerative diseases (NDs), including Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD) and amyotrophic lateral sclerosis (ALS), among others, are debilitating heterogeneous diseases that have been difficult to cure thus far. NDs seriously endanger people's health and quality of life. As the population ages, the incidence of NDs is increasing at an alarming rate worldwide1, 2, 3, 4 and placing a heavy burden on society and healthcare systems.
The etiology of NDs has not been fully elucidated, but numerous research studies have shown that the pathogenesis of NDs is closely related to oxidative stress5, 6, 7. Reactive oxygen species (ROS) are critical intermediates of cellular signaling pathways. Under normal conditions, cells have an antioxidant defense system that includes enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) and non-enzymatic antioxidants such as uric acid, glutathione and coenzyme Q10, among others. However, the brain is more vulnerable to oxidative stress than other organs due to the low activity of the antioxidant defense system in the brain8 for the following reasons. First of all, the oxygen demand of the brain is very high, accounting for 20% of the oxygen consumption of the human body. Second, oxidation-reduction active metals such as iron or copper are present in large quantities in the brain and they are actively involved in catalyzing the formation of ROS9. Third, although the production rate of ROS is very high, the brain's antioxidant defense system is relatively low due to the high rate of oxidative metabolism and the high content of polyunsaturated fatty acids (PUFA) in the cell membrane10. Furthermore, the level of glutathione (GSH) in the brain is relatively low, which plays the role of endogenous antioxidant in the removal of ROS11. Due to the important role of oxidative stress in the pathogenesis of NDs and the susceptibility of the central nervous system (CNS) to oxidative stress, delaying or preventing the degeneration of nerve cells by scavenging ROS or preventing their formation has become one of the promising strategies to prevent and treat NDs.
Due to their pathophysiology, NDs require long-term and sometimes lifelong drug treatment, which increases the risk of adverse effects of medicinal products on other clinical aspects of the patient. In fact, synthetic medicinal drugs are widely used to treat most NDs, but these medicinal drugs have adverse treatment effects12. Therefore, when prescribing exogenous dietary antioxidant supplements, special consideration should be given to those that can be used for a long time, are easily available, have few adverse effects and work best as part of the daily diet or as a dietary antioxidant supplement regardless of the age of the patient.
In addition, a major problem in the treatment of NDs is the limited delivery of many medicinal products to the brain due to the presence of the blood–brain barrier (BBB), which makes it difficult to reach sufficient concentrations of active substances in the brain and decreases their bioavailability13. This may be one of the main reasons why effective neurological treatments are difficult to develop. Although the BBB is an important factor to consider, few research studies have examined the BBB permeability of dietary antioxidant supplements.
In recent years, research studies have found that the risk of some NDs may be reduced by supplementing or ingesting various fruits, vegetables and other foods and consuming antioxidants in the diet14, 15, 16. The most widely studied dietary antioxidant supplements mainly include active substances such as vitamin C, vitamin E, carotenoids, polyphenols and flavonoids. Dietary antioxidant supplements are mainly found in foods such as fresh fruits, vegetables, nuts and oils. They are becoming increasingly popular in the prevention and treatment of NDs because of their abundant sources, their natural, non-toxic nature and the fact that they can be consumed in the usual human diet17. At the same time, because of their lipophilic properties, many dietary antioxidant supplements can pass through the BBB and play an important biological role in the CNS18. Numerous experimental and epidemiological studies have shown that dietary antioxidant suplements can effectively scavenge ROS, reduce lipid peroxidation, antagonize oxidative damage, protect neurons and improve and enhance cognitive function and memory19, 20, 21.
In recent years, much of the peer reviewed literature has described the impact of natural products on a certain ND or the impact of a certain natural product on NDs. Although there are some research studies on the effects of natural products on NDs, the antioxidant mechanism of dietary antioxidant supplements in this process has not been deeply explored, and there are few systematic summaries on preclinical and clinical data. In addition, the BBB permeability of dietary antioxidants is one of the obstacles to their use in ND treatment, but very scarce scientific literature has focused on this aspect. With the accumulation of basic research and the continuous expansion of dietary antioxidant functions, more and more people are exploring the beneficial effects of dietary components on diseases. Therefore, this manuscript review summarizes the underlying pathophysiological pathways of oxidative stress in the development of NDs and explores the physiological role of the BBB. This article reviews the BBB permeability of common dietary antioxidant supplements and the existing evidence for the prevention and treatment of NDs and summarizes the future direction of dietary antioxidant supplements in the prevention and treatment of NDs. It lays a theoretical foundation for the effective prevention, treatment and management of various NDs, thereby ensuring healthy aging of the global population.
2. Oxidative stress and neurodegenerative diseases
2.1. Oxidative stress plays an important role in the pathogenesis of neurodegenerative diseases
Oxidative stress is a potentially damaging imbalance of redox states in the body, involving excessive generation of toxic ROS and/or dysfunction of the protective antioxidant system22,23. When the production of ROS exceeds the counteracting mechanisms of the antioxidant system, oxidative stress will occur, resulting in oxidative damage to proteins, nucleic acids and lipids, affecting the normal functioning of the body and inducing the occurrence of various diseases such as NDs22,24.
NDs are a debilitating heterogenous group of diseases that have so far been difficult to cure. They are characterized by the slow and progressive loss of specific neuronal cell subsets, and/or the loss of their specific functions, which worsen over time, culminating in conditions such as memory impairment, movement disorders and other functional impairments25. NDs have become an important health and economic problem, and their aetiology has not been fully elucidated; however, increased oxidative stress has been recognized as one of the underlying common causes of various NDs26, 27, 28, 29.
As the main site of neurodegenerative immune responses, the brain is a highly metabolic organ with high concentrations of transition metals, which are capable of producing highly reactive hydroxyl radicals together with hydrogen peroxide30. At the same time, it has a relatively low antioxidant capacity and almost no regenerative function, factors which increase its susceptibility to damage from oxidative stress and neurodegeneration8,31,32.
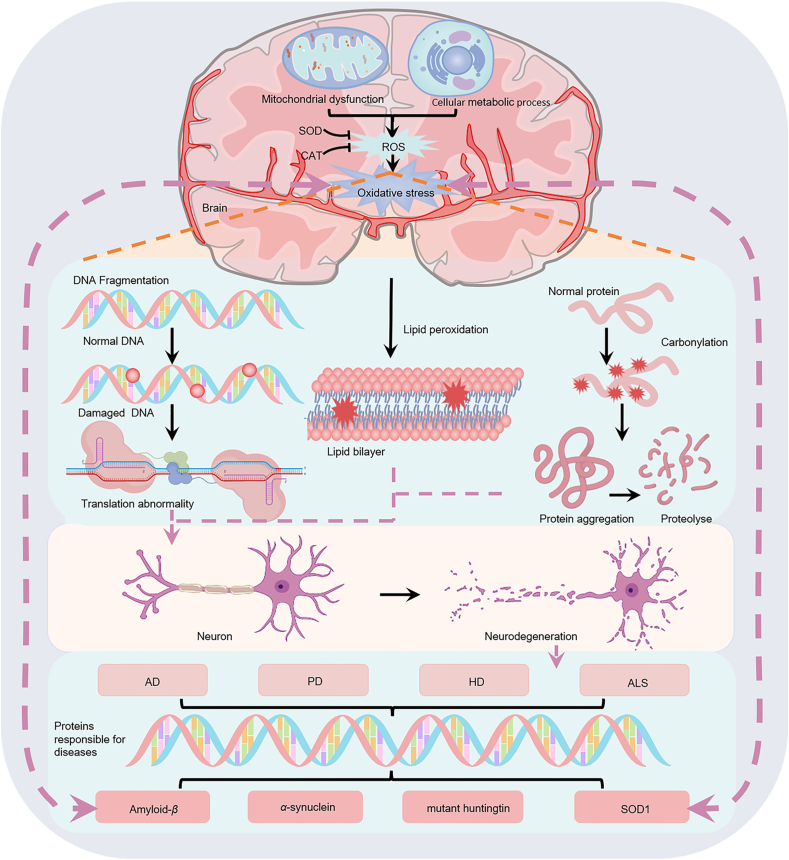
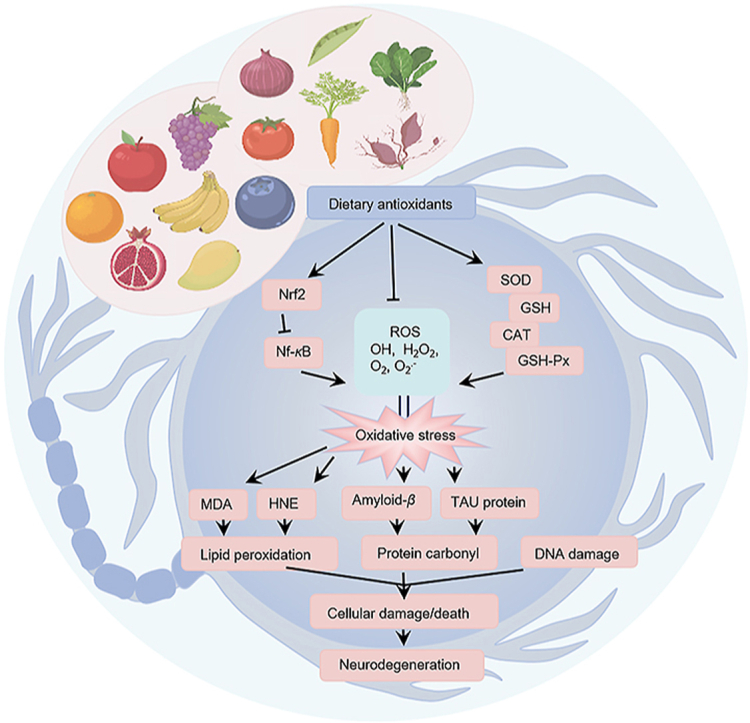
Accumulating data suggest that oxidative stress and the resulting neuronal damage may be closely related to the pathogenesis of a variety of NDs, including AD, HD, PD and ALS, among others5,6,33, 34, 35, as shown in Fig. 1. Large amounts of the lipid peroxides 4-hydroxynonenal and malondialdehyde (MDA), as well as protein carbonyl and 3-nitrotyrosine, associated by-products of protein oxidation, were found in the brains of AD patients36. Subsequent research studies have confirmed that the levels of ROS in AD patients are increased, and in severe clinical cases, the protein folding function of the endoplasmic reticulum is impaired, and the clearance of damaged proteins mediated by proteases and autophagy is reduced, which promotes the accumulation of amyloid-β (Aβ) and TAU proteins37. Oxidative stress may be the trigger or relay station of HD. Consistent with the immunohistochemical data, analysis of biochemical assays in HD patients show significant increases in MDA and 4-hydroxynonenal brain levels, almost 8-fold greater than in control subjects38. Several research studies have shown that oxidative stress is involved in the misfolding and accumulation of mutant huntingtin protein, which induces proteotoxicity and impairs oxidative metabolism, leading to neuronal damage and death39,40. Previous research studies have found that the activity of mitochondrial respiratory complex I in substantia nigra pars compacta of PD patients decreases and destroys the electron transport chain, which may lead to the excessive production of ROS and induce apoptosis41. Changes in antioxidant molecules have been reported even in the early stages of PD. Furthermore, oxidative stress induces the degeneration of motor neurons in the cerebral cortex of patients with ALS, damages mitochondria and leads to the apoptotic death of motor neuron cells42,43. Mutations in the superoxide dismutase 1 gene encoding Cu/Zn-SOD were found in patients with familial ALS, resulting in excessive production of hydroxyl radicals and massive oxidative stress, resulting in ALS disease occurrence44.
Figure 1.
Oxidative stress plays an important role in the pathogenesis of neurodegenerative diseases (NDs). The pathology of NDs [including Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), amyotrophic lateral sclerosis (ALS)] is closely related to the production of oxidative stress, which in turn promotes the further development of NDs. Excessive production of active substances will lead to oxidative damage of proteins, lipids and nucleic acids and induce the formation of misfolded amyloid-β (Aβ), α-synuclein (α-syn), mutant Huntington protein and superoxide dismutase 1, resulting in neurodegeneration.
In addition, the role of metabolic antioxidants, such as uric acid, in the treatment of stroke is a field of great concern45, 46, 47, 48. Animal model studies have shown that administering uric acid after stroke can prevent long-term cerebral arterial remodeling, alleviate brain damage, and protect endothelial cell function in the brain49. Systematic reviews and meta-analyses of animal studies have also found that uric acid therapy may have neuroprotective effects against ischemic stroke50. In a research study of emergency treatment for acute ischemic stroke, Llull et al.51 found that uric acid therapy significantly improved the clinical condition of patients and reduced neurological deficits. Li et al.52 conducted a literature review and found that uric acid has multiple protective effects on neurons, including antioxidant, anti-inflammatory, and anti-apoptotic effects. Uric acid can also inhibit endothelial cell adhesion and inflammatory reactions, thereby reducing brain damage. Overall, these research studies suggest that antioxidants may have beneficial effects in the treatment of neurological diseases, including stroke.
2.2. Do antioxidants play key roles in the treatment of neurodegenerative diseases?
Due to the susceptibility of the CNS to oxidative stress and the important role of oxidative stress in the pathogenesis of NDs53, delaying or preventing the degeneration of nerve cells by clearing ROS or preventing its formation has become one of the promising strategies for the prevention and treatment of NDs. In recent years, research studies have found that natural antioxidants in fruits, vegetables, edible flowers and tea have obvious antioxidant effects and low adverse effects, as well as preventive and protective effects on NDs and other diseases54. These dietary antioxidant suplements not only reduce the harmful activities of ROS and oxidative stress but also promote the regenerative capacity of the adult human brain55. Therefore, it is necessary to study and summarize the role of dietary antioxidant suplements in the prevention and treatment of NDs.
3. Bood–brain barrier permeability and drug delivery
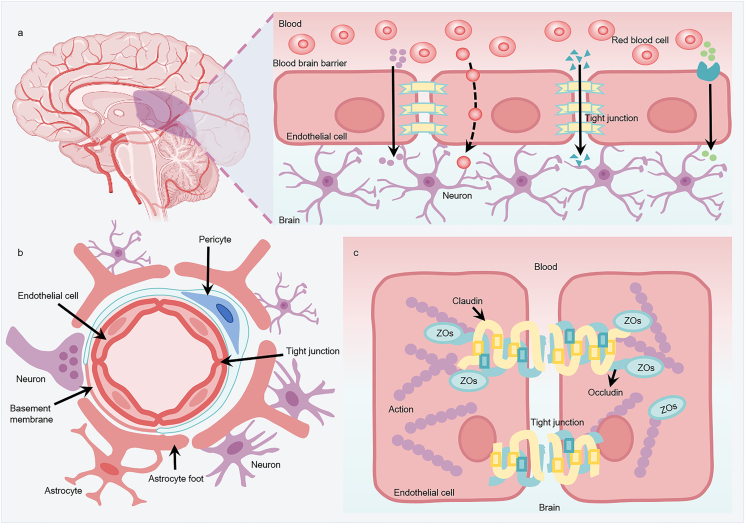
The blood–brain barrier (BBB) is a highly selective permeability barrier that regulates the passage of endogenous and exogenous compounds to facilitate the transport of specific nutrients, precisely regulates ion homeostasis, protects the brain against many pathogens and toxic compounds and is the structural basis for maintaining the homeostasis of the internal environment of the CNS56,57. The BBB is a dynamic structure composed of an assembly of brain endothelial cells (BECs), basement membrane and the pericytes embedded in it, astrocyte foot processes and intercellular tight junctions (TJs)58, as shown in Fig. 2. Among these, BECs are the main morphological structures of the BBB. In contrast to vascular endothelial cells in other parts of the body with high permeability, endothelial cells in the BBB lack fenestrations, and there are continuous and dense TJ proteins between cells59. These unique structural features limit the paracellular transport of active substances and can strictly regulate the transport of ions, molecules and cells between the blood and the brain.
Figure 2.
The structure and permeability of the blood–brain barrier (BBB). (a) Permeability of the BBB. The BBB separates the brain from the components of circulating blood and is formed by endothelial cells connected by tight junction proteins (TJs). TJs allow essential nutrients (such as oxygen, glucose, amino acids, among others) to enter the brain parenchyma through simple diffusion, passive diffusion between cells (paracellular) or through cells (transcellular) and via transporters that transport essential macromolecules. It limits the entry of potentially harmful molecules in the blood (about 98% of drugs) into the brain. (b) Structure of the neurovascular unit of the BBB. The capillary cavity is surrounded by endothelial cells, and the TJs are located between brain endothelial cells, preventing most substances from flowing into the brain from the blood. Endothelial cells and pericytes are surrounded by a common basement membrane. The ends of astrocytes surround endothelial cells and pericytes and provide connections between neurons and BBB. (c) The basic molecular structure of TJ protein complexes of BBB. Claudins and occludin compress two adjacent endothelial cells together. These proteins are linked to cytoskeletal proteins (actin) through helper proteins such as ZOs (zona occludens), which promote the formation of TJs.
Although the cerebrovascular system plays an important protective role in maintaining the internal environment balance necessary for neuronal function, the BBB also prevents the entry of drugs, making CNS diseases more difficult to treat than those that can be reached by the systemic circulation. Normally, the TJs of the BBB allow only H2O, some gases and lipid-soluble molecules to pass selectively via passive diffusion60,61, and most hydrophilic molecules and large hydrophobic substances cannot freely cross the BBB62,63. Molecules critical to neuronal function, such as glucose, purine bases, choline, nucleotides, amino acids, fatty acids and vitamins, are selectively transported by relatively high concentrations of specific membrane transporters in BECs64. These transporters mainly include ATP-binding cassette efflux transporters and solute carrier transporters. However, many potential neuroprotective substances may also be the substrates of these efflux transporters, resulting in reduced brain permeability65.
Many dietary antioxidants can reduce oxidative stress injury in vitro, whereas in vivo the non permeability of the BBB is considered one of the greatest challenges, which makes it particularly difficult for antioxidant compounds to enter the brain tissue. It is estimated that 98% of small molecules administered systemically cannot cross the BBB, which leads to the failure of almost all medicinal drugs discovery and development projects in NDs66. Therefore, the potential for dietary antioxidants to either protect or penetrate the BBB must be considered with respect to NDs.
4. The role of dietary antioxidant suplements in the prevention and treatment of neurodegenerative diseases
Dietary antioxidants are active substances that inhibit oxidation or repair oxidative damage to cellular components. They can effectively prevent damage to lipids, proteins and DNA67. In order to help clear excess ROS and maintain the balance between ROS production and antioxidant defense system, endogenous antioxidant system exists in all cells including neurons68, but the activity of antioxidant defense system in the brain is low. Antioxidant enzymes such as SOD, CAT, GSH-Px and GR (glutathione reductase) are able to participate in the regulation of ROS and RNS69, 70, 71, 72. Metabolic antioxidants are endogenous antioxidants that are produced by metabolic reactions in cells, such as uric acid, glutathione, coenzyme Q10, melatonin, transferrin, lipoic acid, and bilirubin, among others73. Nutritional dietary antioxidant suplements are exogenous antioxidants, which are active compounds that cannot be produced in the body and must be provided through food or dietary antioxidant supplements74 (Table 1).
Table 1.
Antioxidants in neurodegenerative diseases.
| Species | Source | Example |
|---|---|---|
| Endogenous enzymes | Endogenous | Sod, Cat, Gsh-Px, Gr |
| Metabolic antioxidants | Endogenous | Uric acid, GSH, coenzyme Q10, melatonin, transferrin, lipoic acid, bilirubin |
| Nutritional antioxidants | Exogenous | Vitamin C, vitamin E, carotenoids, polyphenols, flavonoids |
| Synthetic antioxidants | Exogenous | Bha, Bht, Tbhq |
BHA, butyl hydroxyanisole; BHT, butylated hydroxytoluene; CAT, catalase; GSH-Px, glutathione peroxidase; GR, glutathione reductase; GSH, glutathione; SOD, superoxide dismutase; TBHQ, tert-butylhydroquinone.
If the production of ROS increases too rapidly, the endogenous antioxidant defense system of the brain is not sufficient to prevent damage. Exogenous dietary oxidant suplement can help the body maintain homeostatic control of ROS to prevent oxidative stress. Particularly attractive agents are those that can be used in the long term with little to no adverse effects and are readily available.
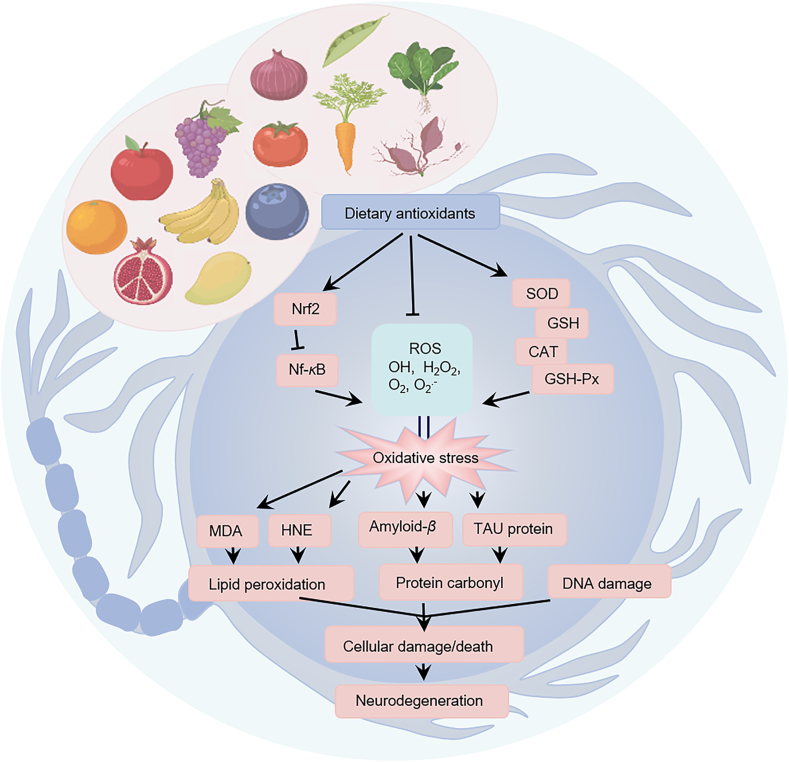
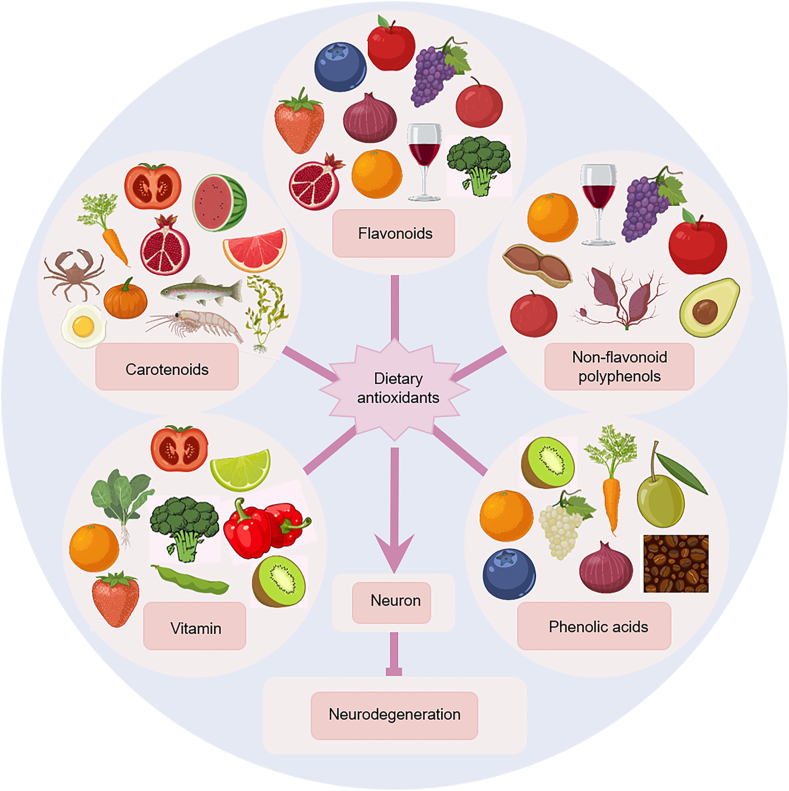
There is growing evidence that antioxidant intake from the diet, through supplementation or intake of various foods, may reduce the risk of certain NDs15,16,75,76 (Fig. 3). The most widely studied dietary antioxidant suplements are active substances such as vitamin C, vitamin E, carotenoids and polyphenols/flavonoids. Dietary antioxidant suplements, mainly found in fresh fruits, vegetables, nuts and oils (Fig. 4), are increasing in popularity for the prevention and treatment of NDs because they are easily sourced, natural and non-toxic and can be consumed in the normal human diet17,77. However, their uptake is limited by the BBB, making it difficult to reach active concentrations in the brain78. Although the BBB is an important factor to consider, few research studies have examined the BBB permeability of dietary antioxidant suplements. The list of dietary antioxidants and BBB permeability of dietary antioxidants is summarized in Table 2. The major dietary antioxidant suplements studied in preclinical and clinical trials in NDs are described in Table 3, Table 4.
Figure 3.
Antioxidant effects of dietary antioxidants in the prevention and treatment of neurodegenerative diseases (NDs). Dietary antioxidants reduce oxidative stress by inhibiting lipid peroxidation, scavenging reactive oxygen species (ROS), and increasing the activity of antioxidant enzymes [superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px)] and the level of antioxidant molecule glutathione (GSH). They significantly increase the expression of nuclear factor erythroid 2 like 2 (NRF2), inhibit the expression of nuclear transcription factor kappa B (NF-κB), and directly reduce the excessive production of ROS. By effectively reducing amyloid-β (Aβ) deposition and TAU protein phosphorylation, they can improve neurodegeneration.
Figure 4.
Main types and food sources of dietary antioxidants. The most widely studied dietary antioxidants include vitamins, carotenoids, flavonoids, non-flavonoid polyphenols, phenolic acids and other substances. They mainly exist in fresh fruits, vegetables, nuts and oils.
Table 2.
Dietary antioxidant supplements and blood–brain barrier permeability.
| Species | Studied material | Main source | Brain penetration | CAS number | Chemical formula | Molecular weight | Chemical structures | Ref. |
|---|---|---|---|---|---|---|---|---|
| Vitamins | Vitamin C | Oranges, strawberries, lemons, kiwifruit, spinach, bell peppers, kale, broccoli | + | 50-81-7 | C6H8O6 | 176.12 | 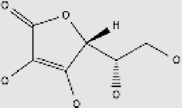 |
79 |
| Vitamin E | Wheat germ, soybeans, spinach, tomatoes, vegetable oil, cod liver oil | + | 2074-53-5 | C29H50O2 | 430.71 | 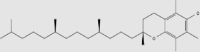 |
91,92 | |
| Carotenoids | Lycopene | Tomatoes, watermelons, grapefruit and pomegranates | + | 502-65-8 | C40H56 | 536.87 | 117,118 | |
| Astaxanthin | Shrimp, crab, salmon, trout, brown algae, yeast | + | 472-61-7 | C40H52O4 | 596.85 | 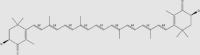 |
129–131 | |
| β-Carotene | Carrots, sweet potatoes, pumpkins | – | 7235-40-7 | C40H56 | 536.89 | 140 | ||
| Lutein | Kale, spinach, oranges, egg yolks, avocados | + | 127-40-2 | C40H56O2 | 568.87 | 144 | ||
| Fucoxanthin | Wakame (Undaria pinnatifida), Kombu (Laminaria japónica Aresch), Hijiki (Hijikia fusiformis) | Unknown | 3351-86-8 | C42H58O6 | 658.91 | 153 | ||
| Flavonoids | Epigallocatechin-3-gallate | Green tea, black tea, red wine | + | 989-51-5 | C22H18O11 | 458.37 | 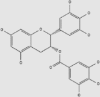 |
165 |
| Anthocyanidin | Blueberries, grapes, strawberries, cherries, pomegranates, cabbage | + | / | / | / | 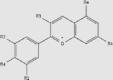 |
175 | |
| Quercetin | Onions, apples, broccoli, blueberries | + | 117-39-5 | C15H10O7 | 302.24 | 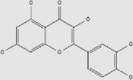 |
184–186 | |
| Rutin | Buckwheat, oranges, grapes, apples, tea | + | 153-18-4 | C27H30O16 | 610.52 | 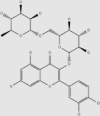 |
194,195 | |
| Silymarin | Milk thistle (Silybum marianum) | Unknown | 65,666-07-1 | C25H22O10 | 482.44 | 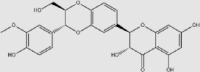 |
203 | |
| Genistein | Soybean | + | 446-72-0 | C15H10O5 | 270.24 | 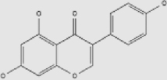 |
212 | |
| Hesperetin | Oranges, grapes, lemons | + | 520-33-2 | C16H14O6 | 302.28 | 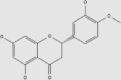 |
219 | |
| Non-flavonoid polyphenols | Resveratrol | Tea, red wine, grapes, berries, apples, plums, peanuts | + | 501-36-0 | C14H12O3 | 228.24 | 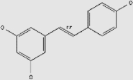 |
230–233 |
| Curcumin | Turmeric (Curcuma longa L.) | + | 458-37-7 | C21H20O6 | 368.38 | 240 | ||
| Phenolic acids | Gallic acid | Tea, blueberry, raspberries, grapes, bananas, wheat, barley, nuts | + | 149-91-7 | C7H6O5 | 170.12 | 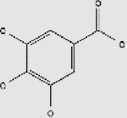 |
248–251 |
| Caffeic acid | + | 331-39-5 | C9H8O4 | 180.16 | 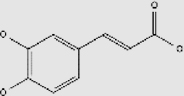 |
|||
| Protocatechuic acid | + | 99-50-3 | C7H6O4 | 154.12 |  |
|||
| Ferulic acid | + | 1135-24-6 | C10H10O4 | 194.18 | 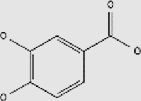 |
|||
| Others | Melatonin | Olives, tomatoes, grapes | + | 73-31-4 | C13H16N2O2 | 232.28 | 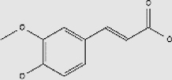 |
262,263 |
| Ergothioneine | Black fungus, king oyster mushroom, enoki, and shiitake mushrooms | + | 497-30-3 | C9H15N3O2S | 229.299 | 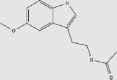 |
273–275 | |
| Sulforaphane | Broccoli, watercress, Brussels sprouts and cabbage | + | 4478-93-7 | C6H11NOS2 | 177.288 | 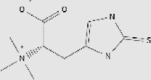 |
285 |
Table 3.
In vitro and in vivo studies of dietary antioxidant supplements with beneficial effects on neurodegenerative diseases.
| Species | Studied Material | In vitro/in vivo | Experimental model | Effective dose and duration | Mechanisms | Main results | Ref. |
|---|---|---|---|---|---|---|---|
| Vitamins | Vitamin C | In vitro | Neuro2a cells Hn33.11 cells |
200 μmol/L 36 h |
Increase of GSH concentration; Reduced ROS level; Regulate the expression of apoptosis genes (BCL-2, BAX, Caspase 8) |
Control oxidative stress in the brain; Prevent neuronal death |
83 |
| In vivo | Brains of 5 male pups | 100 mg/kg bw 60 days |
Decreased oxidative stress index and increased the activity of antioxidant enzymes; Reduced the number of apoptotic cells and dark neurons in sub-regions of hippocampus |
Against neuronal depletion in the hippocampus | 85 | ||
| In vivo | Charles-Foster rats Swiss albino mice |
200, 400 mg/kg bw 27 days |
Reduction of Aβ accumulation; Reduce ROS level |
Recovery of memory impairments; Prevention of neurodegeneration in the hippocampus |
88 | ||
| Vitamin E | In vivo | Sprague–Dawley rats | 1 μmol/L 72 h |
Inhibited the increase of intracellular Ca2+ induced by oxidative damage | Protect hippocampal neurons from oxidative damage | 94 | |
| In vivo | Tg2576 | 8 IU/day 8 months, 6 months |
Significantly reduce the level of lipid peroxidation; Significant reduction in Aβ levels and amyloid deposition |
Slow down the development of AD | 96 | ||
| In vitro | Primary cells of rat cortical neurons PSEN1dE9-85Dbo/J transgenic mice |
1 mmol/L 24 h 800 IU/kg bw 21 days |
Protects neurons from Aβ toxicity; Reduce GSH oxidation and lipid peroxidation; Reduce oxidative stress |
Prevent and improve AD | 97 | ||
| In vivo | Sprague–Dawley male rats | 100 IU/kg bw 5 weeks |
Significant increase in DA; Significantly increased SOD and GSH levels; Significantly reduced MDA levels; Significantly reduced lipid peroxidation |
Has a neuroprotective effect on PD | 295 | ||
| Carotenoids | Lycopene | In vivo | Adult male Wistar rats |
10 mg/kg bw 40 days |
Decreased the levels of Aβ1–42; Reduce Aβ-induced oxidative damage |
Prevention and treatment of AD | 118 |
| In vivo | Female Sprague–Dawley rats | 50, 100, 200 mg/kg bw 2 months |
Increased SOD activity in the hippocampus; Decrease ROS generation |
Alleviate the pathological characteristics of dementia | 123 | ||
| In vivo | CD-1 male mice | 50 mg/kg bw/day 8 weeks |
Increased the activities of antioxidant enzymes GSH-Px, GSH, and SOD | Alleviate oxidative stress induced cognitive impairments | 296 | ||
| In vitro | BV2 microglial cells Male C57BL/6 J mice |
0.03% (w/w) of standard chow 5 weeks 50 μmol/L 8 h |
Reduce LPS-induced amyloidogenesis, cognitive impairments, and oxidative stress; Reduce LPS-induced accumulation of Aβ |
Might be a promising drug candidate for the treatment of AD | 297 | ||
| In vitro | Hippocampal NSCs and cerebral cortical neurons | 0, 0.1, 1, 2, 4, 8 and 16 μmol/L 24 h |
Enhance neuronal survival and reduce oxidative damage; Reduced ROS generation significantly |
Prevention and treatment of oxidative stress-related AD lesions | 298 | ||
| In vivo | Adult male Wistar rats |
1–4 mg/kg bw 14 days |
Reduce learning and memory deficits by restoring the levels of CAT, GSH, SOD; Reduce Aβ1–42-induced mitochondrial dysfunction |
Remission and treatment of AD | 299 | ||
| In vivo | Young male Wistar rats |
2.5–5 mg/kg bw/day | Reduce Aβ1–42-induced memory loss, mitochondrial-oxidative damage; Reversed Aβ1–42-induced caspase-3 activities | Prevention and treatment of AD | 300 | ||
| In vitro | SH-SY5Y human neuroblastoma cells | 0.5–1 μmol/L 2 h, 24 h |
Reduce Aβ1–42 secretion in SH-SY5Y cells; Reduce H2O2-induced oxidative stress |
Attenuate onset and development of AD | 301 | ||
| In vivo | Mice model of MPTP-induced PD | 5–20 mg/kg bw/day 7 days |
Attenuates oxidative stress; Reduce destruction of DA neurons; Depletion in the levels of striatal DA and its metabolites |
Neuroprotective effect against MPTP induced experimental PD in mice | 302 | ||
| Astaxanthin | In vivo | Male C57BL/6 J mice | 30 mg/kg bw day 1 month |
Reduce oxidative stress; Enhanced synaptic plasticity |
Restore cognitive function; Improve performance in cognitive behavioral tasks | 131 | |
| In vivo | Male Wistar rats |
10, 20, 40 mg/kg bw 5 days |
Down regulate oxidative stress markers (MDA) in cerebral cortex and hippocampus, up regulate SOD and GSH | Have the protective effect on the brain cell of rat | 303 | ||
| In vitro | PC12 cells | 5, 10, 20 μmol/L 24–36 h |
Suppress MPP+-induced oxidative stress in PC12 cells | Strongly considered as a potential neuroprotectant and adjuvant therapy for patients with PD | 304 | ||
| In vitro and in vivo | SH-SY5Y cells C57BL/6 mice |
50 μmol/L, 25 h 30 mg/kg bw 28 days |
Inhibit MPP+-induced production of intracellular ROS; Up regulation of SOD and CAT |
Attenuate onset and development of PD | 305 | ||
| β-Carotene | In vivo | Male Wistar rats |
0.6, 3, 6 mg/kg bw/day 28 days |
Increase in antioxidant activity; Increased CAT activity in the cerebral cortex; Reduced the lipid peroxidation levels |
Protects the brain, and is a safer nutritional supplement | 140 | |
| In vivo | Male Albino mice |
1.02, 2.05 mg/kg bw/day 14 days |
Significantly increased levels of all antioxidant enzymes and decreased AChE activity; GSSG/GSH ratio was decreased significantly |
Improve cognitive ability and treat NDs such as AD | 141 | ||
| In vivo | Male C57BL/6 mice |
20, 30, 50 mg/kg bw/day 1 week |
Significantly reduce lipid peroxidation; Increase antioxidant enzyme activity; Increase SOD level and decrease MDA level; Activate the NRF2 signaling pathway, alleviate acute oxidative stress |
Confirm the direct protective effects of β-carotene supplementation on neuroprotection | 306 | ||
| Lutein | In vivo | Adult rhesus monkeys | 4.5 mg/kg bw/day 6–12 months |
Significantly reduce oxidative stress of polyunsaturated fatty acids in the brain | Protect the brain from oxidative stress | 144 | |
| In vivo | Male ICR mice |
30, 15, 7.5 mg/kg bw/day 7 days |
Elevated GSH/GSSG and SOD, GSH, and CAT activities; Decreased MDA contents, 8-OHdG expression |
Afford strong neuroprotective effect | 147 | ||
| In vivo | Male C57BL/6 mice |
5, 10, 20 mg/kg bw/day 7 days |
Reduce DA metabolism; Enhance the levels and activities of GSH and GSH-Px |
Improve the behavioral pattern and offer neuroprotection against PD | 150 | ||
| In vivo | The fruit flies (Drosophila melanogaster wild-type - Harwich strain) | Diet containing lutein-loaded nanoparticles, in water 2, 6 or 20 μmol/L 7 days |
Restore the DA levels, AChE activity and oxidative stress indicators | Protect the nerves of the brain, attenuate symptoms of PD | 152 | ||
| In vitro | Aβ1–42 fibrils | 1 μg/mL, 5 μg/mL 24 h |
Inhibition of Aβ fibril formation; Potent anti-amyloidogenic activity |
Maintain brain health and improve cognitive function | 307 | ||
| In vivo | Sprague–Dawley rats | 40, 80, 160 mg/kg bw/day 5 weeks |
Reduce serum ROS levels; Increase SOD and GSH activities; Up regulate NRF2 and exert antioxidant effect |
Prevent severe brain damage through antioxidation | 308 | ||
| Fucoxanthin | In vivo | Wild type mice and NRF2-deficient mice | 50, 100, 200 mg/kg bw/day 7 days | Reduce oxidative stress in injured brains; Reverse the up-regulation of MDA and increase the activity of GSH; Increase the neuron survival |
Alleviate neurological deficits, cerebral edema, brain lesion and neuronal apoptosis | 157 | |
| In vitro | Primary cortical neuron cultures | 5, 10, 20 μmol/L 24 h |
Significantly suppress ROS accumulation; Enhance NRF2 expression; Suppress apoptosis in cultured neurons |
Protect neurons from oxidative stress | 158 | ||
| In vivo | C57BL/6 mice | 10 mg/kg bw/day 14 days |
Repress α-synuclein abnormal accumulation, oxidative stress and motor impairment; Reverse the MPTP-mediated decline of DA neuron |
Exert the neural protective effect, might perform as a beneficial remedy toward PD amelioration | 159 | ||
| In vivo | Aβ oligomer-treated mice | 50, 100, 200 mg/kg bw/day 17 days, |
Significantly inhibited oxidative stress; Attenuate Aβ neurotoxicity |
Attenuate cognitive impairments in Aβ oligomer-injected mice, Prevention of AD | 309 | ||
| In vitro | SH-SY5Y cells | 0.3–3 μmol/L 2 h |
Reduce H2O2-induced intracellular ROS; Significantly decreased H2O2-induced neuronal apoptosis and neurotoxicity |
For the treatment of NDs caused by or characterized by oxidative stress | 310 | ||
| Flavonoids | Epigallo-catechin-3-gallate | In vivo | Male Sprague–Dawley rats | 100 mg/kg bw/day 4 weeks |
Significantly lower Aβ1–42 expression in the hippocampus and cortex; Significantly lower Aβ content; Reduce oxidative stress |
Ameliorate learning and memory impairment in aging rats, and is a potential substance for the treatment of AD | 165 |
| In vivo | Male Wistar rats |
40 mg/kg bw/day 4 weeks |
Inhibition of lipid peroxidation and protein oxidation; Significantly decreased the levels of oxidative stress markers; Significantly increased the levels of non-enzymatic antioxidants |
Improve the oxidative stress caused by sodium fluoride in rat hippocampus and weakened the neurotoxicity | 166 | ||
| In vivo | Male C57BL/6 mice | 40 mg/kg bw 2 h |
Decrease hippocampal Aβ plaque deposit number; Reduce oxidative stress |
Alleviate AD memory deficits, prevention and treatment of AD and other similar NDs | 168 | ||
| In vitro and in vivo | Primary brain microvascular endothelial cells Male C57BL/6 mice |
1.5, 5, 15, 50, 150, 500 μg/mL, 2 h 40 mg/kg bw 24 h |
Significantly decline in the accumulation of Aβ plaques; Reduce Aβ42 peptide levels; Reduce oxidative stress |
As a novel, safe and suitable therapeutic alternative for the treatment of AD | 169 | ||
| In vitro | PC12 cells | 1, 2, 5, 10, 20 μmol/L 24 h, 48 h |
Inhibit α-Syn fibrillation and aggregation, disaggregate α-Syn mature fibrils, as well as protect α-Syn overexpressed-PC12 cells against damage; Reduce ROS production |
Protect the nerves in the brain and have the potential to treat PD | 170 | ||
| In vivo | Male Wistar rats |
10 mg/kg bw/day 15 days |
Significantly reduce the level of lipid peroxidation; DA contents decreased in a dose-dependent manner |
Prevention and treatment of PD | 171 | ||
| In vivo | CD-1 male mice | 2 mg/kg bw 8 h, 24 h, 3 days |
Increase GSH-Px activity in striatum; Attenuate the METH-induced increase of striatal CAT and SOD protein levels |
Mitigate the METH-induced striatal toxicity in the mouse brain | 173 | ||
| In vitro | Murine neuroblastoma N2a cells |
1 μmol/L 48 h |
Reduce toxic levels of brain Aβ; Reduce ROS generation |
Hold the potential to protect neuronal function in AD | 174 | ||
| In vitro | PC12 cells | 2.5, 5, 10, 20, 40 μmol/L 24 h |
Suppressed intracellular ROS production; Reduce damage by oxidative stress; Increase the expression of antioxidant enzymes, remove free radicals |
Play an effective protection role in the pathogenesis of PD, reduce the risk of PD | 311 | ||
| Anthocyanidin | In vitro | SH-SY5Y cells | 25–500 μg/mL 24 h |
Significantly decreased intracellular ROS levels; Reduced cellular lipid peroxidation; Increased CAT activity |
Protect neurons from oxidative stress | 177 | |
| In vivo | Male C57BL/6 N mice | 12 mg/kg bw/day 30 days |
Significantly increased expression of NRF2, mitigate oxidative stress; Reduced MDA levels; Increased GSH levels |
Improved memory functions in AD mice | 178 | ||
| In vitro | SH-SY5Y cells | 100 μmol/L 24 h |
Significantly inhibited Aβ1–40-induced oxidative stress; Increase the level of SOD; Protect SH-SY5Y cells against oxidative stress-induced |
Provide a new treatment strategy for AD | 179 | ||
| In vitro | PC12 cells | 5–80 μmol/L 24 h |
Significantly attenuated Aβ; Protected Aβ-induced DNA damage by blocking ROS and superoxide accumulation |
Prevention of oxidative stress-mediated Aβ neurotoxicity | 312 | ||
| In vivo | Wistar rats | 200 mg/kg bw/day 25 days |
Induced an decrease in lipid peroxidation; Increased antioxidant enzymes levels; Reduced ROS generation |
Attenuate memory deficits, protects against oxidative damage in the brain | 313 | ||
| In vivo | C57BL/6 N mice | 24 mg/kg bw/day 14 days |
Prevented ROS production | Improve spatial memory | 314 | ||
| In vivo | Kunming mice | 30 mg/kg bw/day 8 weeks |
Adjust the balance of redox system; Significantly increase SOD level and decrease MDA level |
Maintain thinking and memory in aging mice, improve spatial memory ability | 315 | ||
| In vivo | Sprague–Dawley rats | 100 mg/kg bw/day 7 weeks |
Reduced ROS level and lipid peroxidation | Agent for age-related NDs such as AD | 316 | ||
| Quercetin | In vivo | Male Sprague–Dawley rats | 20 mg/kg bw/day 10 days |
Significantly reduce level of MDA and increased level of SOD; Scaveng free radicals and inhibits oxidative enzymes |
Protect the brain from oxidative stress | 187 | |
| In vitro | Mouse Mixed cortical neuronal cell | 1–10 μmol/L 30 min |
Significantly reduced ROS level; Reduce neuronal cell death and intracellular ROS accumulation |
Suggest their potential therapeutic effects on various NDs | 189 | ||
| In vivo | Adult Sprague–Dawley rats | 25–75 mg/kg bw/day 4 days |
Attenuation of rotenone- induced loss in striatal DA, and nigral oxidized and increased GSH; Increase in endogenous antioxidant enzymes (CAT and SOD) activities |
Potential properties for prevention and treatment of PD | 190 | ||
| In vitro | Immortalized murine microglial cells (BV-2 cell line) | 1–100 μg/mL 24 h |
Reduce tert-butyl hydroperoxide-induced oxidative stress | Protect the brain from oxidative stress | 193 | ||
| In vitro and in vivo | Dopaminergic MN9D cell line MitoPark transgenic mice |
10, 30 μmol/L, 24 h 25 mg/kg bw/day 8 weeks |
Protect DA cells from oxidative stress-induced cell death; Slow down the progressive degeneration of DA neurons |
Prevention and treatment of NDs, including PD | 317 | ||
| In vivo | APPswe/PS1dE9 mice (C57/BL) | 20, 40 mg/kg bw/day 16 weeks |
Inhibit radical induced stress; Reduce the production of ROS; Promote the clearance of intracellular Aβ; Attenuate Aβ-induced neurotoxicity |
Lessening learning and memory deficits, prevention of memory loss and Aβ-induced neurotoxicity | 318 | ||
| In vivo | Male Wistar rats |
0.3 mmol/L/day 3 months |
Reduced oxidative stress; Significantly restored GSH; Prevente changes in the brain ROS |
Protects the brain from sodium tungstate-induced oxidative stress of the nervous system | 319 | ||
| In vivo | Male Wistar rats |
10 mg/kg bw/day 6 days | Protect from oxidative stress and lipid peroxidation; Restore antioxidant enzymes activities and reduce MDA levels |
Protect the brain from oxidative stress | 320 | ||
| In vivo | Male C57BL/6 mice |
50, 100, 200 mg/kg bw/day 14 days |
Diminished reduction of DA levels; Increased SOD and GSH-Px |
Showing anti PD's properties | 321 | ||
| Rutin | In vitro | PC12 cells | 10, 50, 100 mol/L 8 h |
Significantly increased SOD and GSH; Increase CAT activity; Reduce MDA |
Protect the brain from oxidative stress | 191 | |
| In vitro | SH-SY5Y cells | 0.8, 8 μmol/L 30 min |
Decrease the production of ROS, NO and MDA; Enhance the antioxidant enzyme activity of SOD, CAT and GSH-Px |
Prevent the development of AD, protect the aging brain or slow down the neurodegenerative process | 196 | ||
| In vitro | SH-SY5Y cells | 25–100 nmol/L 24 h |
Decrease ROS generation; Increased intracellular GSH content; Reduce lipid peroxidation level |
Mitigation Aβ Induced neurotoxicity with neuroprotective effect | 197 | ||
| In vivo | APPswe/PSEN1dE9 double-transgenic mice | 18–25 mg/kg bw/day 7 months |
Significantly reduced Aβ deposits, and oxidative stress | Ameliorate synaptic plasticity impairment and reverse spatial learning and memory deficits | 198 | ||
| In vitro | SH-SY5Y cells | 0.8, 8 μmol/L 8 h |
Decrease ROS, NO and MDA; Enhance the activities of SOD, CAT and GSH-Px |
Prevention and treatment of AD | 199 | ||
| In vitro | SH-SY5Y cells | 0.1, 1 mg/mL 12 h, 60 h |
Prevent oxidative stress induced by Aβ; Interfere with Aβ aggregation and neurotoxicity; Reduce Aβ levels; Effectively reduced the generation of NO |
Rescue memory deficits in AD transgenic mice, prevention and treatment of AD | 200 | ||
| In vivo | Male Wistar rats | 25, 50 mg/kg bw/day 14 days |
Significantly decreased MDA level; Increased SOD, CAT and GSH-Px activities; Increased AChE activity |
Prevention and treatment of HD | 201 | ||
| In vivo | Male Wistar rats | 50, 100 mg/kg bw/day 31 days |
Significantly increased SOD, CAT and GSH-Px activities in the cerebrum and striatum; Decreased the MDA level; Increased AChE activity |
Reduce neurobehavioral deficits in rats and neurotoxicity | 202 | ||
| Silymarin | In vitro | SH-SY5Y cells | 50 μmol/L 72 h |
Reduce Aβ1-42 aggregation; Inhibit lipid peroxidation; Alleviate oxidative stress |
Might be a novel therapeutic agent for the treatment of AD | 204 | |
| In vivo | Caenorhabditis elegans | 25, 50 μmol/L, 24 h | Alleviate oxidative stress; Reduce Aβ1-42 aggregation |
Prevention and the treatment of AD | 206 | ||
| In vivo | Male Wistar rats |
100, 200, 300 mg/kg bw/day 15 days |
Significantly increased SOD and GSH activities; Suppress ROS production; Restore the brain's antioxidant capacity |
Prevention and treatment of PD | 207 | ||
| In vivo | Male albino Wistar rats |
200, 400, 800 mg/kg bw/day 14 days |
Significantly reduced MDA activity; Increased GSH activity; Decreased AchE; Reduce cortical and hippocampal lipid peroxides formation |
Improve cognitive impairment and enhance memory ability | 208 | ||
| In vivo | Adult male Wistar rats |
50 mg/kg bw/day 15 days | Suppress the production of ROS; Significantly increased SOD and GSH; Significantly reduce MDA |
Maintaine cognitive and behavioral functions, alleviate brain antioxidant status, and prevent and treat nervous system disease | 209 | ||
| In vivo | Male Wistar rats | 160 mg/kg bw/day 11 days |
Significantly increased in the activities of CAT, SOD and GSH-Px; MDA diminution; Suppress ROS production |
Alleviate neurotoxicity, potential useful candidate in the protection from nervous system | 210 | ||
| Genistein | In vivo | Male albino Wistar rats |
10, 50, 100 mg/kg bw/day 1 week |
Reduce hippocampal level of MDA; Increase activity of SOD, CAT and GSH; Ameliorat hippocampal AChE activity; Alleviated oxidative stress |
Prevention of cognitive dysfunction, attenuate spatial recognition, discrimination, and memory deficits | 214 | |
| In vitro | Hippocampal neurons | 0.1, 0.2, 0.4, 0.8, 1 μg/mL 24 h |
Reduce excessive production and deposition of Aβ peptides; Increased cell viability; Decrease ROS and MDA |
Prevention and the treatment of early-stage AD | 216 | ||
| In vivo | Male Swiss albino mice | 10, 20, 30 mg/kg bw/day 28 days |
Suppress oxidative stress in hippocampus; Reduce lipid peroxidation; Reduce ROS; Increase GSH, increase total antioxidant capacity |
Effectively protect cortical neurons against oxidative stress, ameliorate the cognitive defects | 217 | ||
| In vivo | C57BL/6 mice | 10 mg/kg bw/day 3 days |
Suppressed superoxide production; Increased GSH content and decreased MDA; Inhibit oxidative stress |
Effectively reduced cerebral infarction, attenuated neuronal injury and apoptosis | 218 | ||
| In vitro | PC12 cells | 25, 50, 100 μmol/L 2 h |
Alleviate oxidative damage induced by Aβ25-35; Increase GSH; Attenuate ROS levels |
Might possess neuroprotective role through its antioxidant activity | 322 | ||
| Hesperetin | In vivo | Male C57BL/6 N mice | 50 mg/kg bw/day 6 weeks |
Attenuate oxidative stress; Reduce the production of Aβ |
Restore memory impairment associated with neurodegeneration, could be a therapeutic agent to treat NDs | 220 | |
| In vivo | Male C57BL/6 N mice |
50 mg/kg bw/day 5 weeks |
Reduced ROS production and lipid peroxidation; Improve antioxidant protein level; Reduced ROS in the cortex and hippocampus regions |
Ameliorate cognition, spatial learning, and memory processing | 221 | ||
| In vivo | Male Wistar rats |
10, 20 mg/kg bw/day 3 weeks |
Decreased lipid peroxidation of hippocampal area; Increased GSH; Reduced oxidative stress and increased antioxidant enzymes |
Enhance learning and memory, potential properties for prevention and treatment of AD | 222 | ||
| In vivo | Male albino mice | 1, 5, 50 mg/kg bw/day 3 days |
Increased SOD and GSH in the hippocampus and prefrontal cortex | Prevented non-spatial/spatial learning and memory decline, enhanced antioxidant defense | 223 | ||
| In vivo | Male Sprague Dawley rats |
0, 50, 150 mg/kg bw/day 10 weeks |
Elevated GSH; Activated NRF2 pathway, decreased oxidative stress |
Ameliorate anxiety and depression-like behaviors and protect the brain | 225 | ||
| In vitro | SH-SY5Y cells | 10–40 μmol/L 6–48 h |
Ameliorate ROS; Increase SOD, GSH-Px, CAT; Reduce the production of Aβ |
Might be a potential agent for treating Aβ neurotoxicity | 226 | ||
| Non-flavonoid polyphenols | Resveratrol | In vitro | Immortalized lymphocytes from AD patients | 10, 50 μmol/L 18 h |
Increase the expression of antioxidants (CAT, SOD); Reduce oxidative stress |
Reinforce the protective mechanisms against memory loss in AD | 239 |
| In vivo | Adult male Wistar albino rats |
20 mg/kg bw/day 3 weeks |
Amelioration of oxidative stress; Restored redox balance; NRF2 and GSH-Px activation |
Maintaining intracellular antioxidant status is a promising way to prevent and treat PD | 323 | ||
| In vitro | Neuronal stem cells | 1–20 μmol/L 24 h |
Decrease apoptosis and the levels of MDA; Increase the activity of SOD and content of GSH; Activation of NRF2 |
Improved neuronal injury and enhanced neuroprotective effect | 324 | ||
| In vivo | Adult male Sprague–Dawley rats | 15, 30 mg/kg bw/day 7 days |
Activation of NRF2; Reduction of oxidation biomarkers; Reestablished SOD activity; Decreased MDA levels |
Improved neuronal injury and enhanced neuroprotective effect | 325 | ||
| Curcumin | In vitro | SH-SY5Y cells | 5 μmol/L 24 h |
Increase GSH; Reduce oxidative stress; Inhibit ROS accumulation |
Play a potential role in the treatment neurological diseases | 243 | |
| In vitro | Mouse neuroblastoma cells | 0.1, 1, 10 μmol/L 24 h |
Reduce ROS and oxidative stress | Prevention and treatment of AD | 244 | ||
| In vivo | Male Lewis rats |
100 mg/kg bw/day 50 days |
Increase GSH; Decrease accumulation of ROS and MDA; Ameliorate dopaminergic neuronal damage and oxidative injury |
Partly alleviate clinical symptoms of PD and exert potential neuroprotective therapeutic effects | 246 | ||
| In vitro | PC12 cells | 0.1, 1, 5, 10, 20 μmol/L 24 h |
Efficiently attenuated Aβ25-35-induced oxidative damage; Inhibit ROS; Activate NRF2 expression |
Prevention and treatment of NDs | 247 | ||
| Phenolic acids | Phenolic acids | In vivo | Caenorhabditis elegans | 25 mmol/L 30 min |
Reduce lipid peroxidation; Activate NRF2 pathway and increase antioxidant activity |
Exert antioxidant and neuroprotective effects | 253 |
| In vitro | Cerebellar granule neurons | 1 μg/mL 24 h |
Reduce oxidative stress caused by H2O2 and ROS production | Protect brain nerves, effectively alleviate NDs | 254 | ||
| In vitro | Neural stem and progenitor cells | 0.06 mmol/L 7 days |
Significantly augmented the activities of CAT in the cells; Significantly reduced the levels of endogenous of ROS |
Promote brain recovery and repair in NDs | 255 | ||
| In vivo | Adult male Wistar rats |
50, 100 mg/kg bw/day 14 days |
Reduce oxidative stress and lipid peroxidation; Increase CAT, SOD activities and GSH-Px in brain |
Prevent hyperlocomotion and brain oxidative damage | 256 | ||
| In vivo | Male Wistar rats | 50 mg/kg bw/day 4 weeks |
Restore antioxidant enzymes; Prevent glutathione depletion; Inhibit lipid peroxidation |
Might be used as potent neuroprotective substance in the prevention of PD | 258 | ||
| In vivo | Adult male Wistar rats |
60, 120 mg/kg bw/day 10 days |
Reduce oxidative stress and increase antioxidant defense system; Restoration of normal levels of cerebellar and cerebral CAT, SOD, MDA |
Ameliorate the neurotoxicity via oxidative stress reduction and increase antioxidant defense system | 326 | ||
| In vivo | Male Swiss mice |
0.01, 0.1, 1, 10 mg/kg bw/day 21 days |
Significantly increased SOD, CAT and GSH-Px activities; Significantly decreased on lipid peroxidation |
Prevention and treatment of nervous system disease | 327 | ||
| In vivo | Male C57BL/6 mice |
20, 40, 80 mg/kg bw/day 7 days |
Reduce oxidative stress; Reduce the production of by-products that interfere with antioxidant activity |
A potent neuroprotective substance in PD patients | 328 | ||
| In vitro | PC12 cells | 1 mmol/L 12 h |
Restore the loss of antioxidant enzyme activities and markedly ameliorate lipid peroxidation | Enhance neuroprotective effect, prevention and treatment of PD | 329 | ||
| In vivo | Male BALB/cA mice |
0.5%, 1%, or 2% in diet 8 weeks |
Decrease ROS and protein carbonyl content; Retain GSH content |
Might be helpful for the prevention or alleviation of aging | 330 | ||
| In vitro | PC12 cells | 50, 100, 150, 200 μmol/L 2 h |
Reduce the content of lipid peroxide and increase the activities of GSH-Px and SOD | Improve the cognition of aged rats, protect the nervous system | 331 | ||
| In vitro | PC12 cells | 1.2 mmol/L 24 h |
Reduce oxidative stress; Increase GSH level |
Might be a candidate chemical for the treatment of oxidative stress-induced NDs | 332 | ||
| Others | Melatonin | In vivo | C57BL/6 mice | 0.5 mg/kg bw/day 4 months |
Reduce Aβ deposition; Reduce oxidative stress |
Improve the spatial learning, alleviate the memory impairment | 269 |
| In vivo | C57BL/6 mice | 5 mg/kg bw/day 18 weeks |
Reduce oxidative stress; Preserve the nigrostriatal DA function |
Slow down idiopathic PD progression, ameliorate locomotor deficit in the chronic model of PD | 270 | ||
| In vitro | SH-SY5Y cells | 10 μmol/L 24 h |
Attenuate MPP+-induced apoptosis and oxidative stress; Increase GSH-Px and SOD |
Prevent and decelerate PD-like neurodegeneration | 271 | ||
| In vitro | Hippocampal slices of Wistar rats | 5, 15, 45, 135 nmol/L 2 h |
Scavenge free radicals to mitigate oxidative stress; Protected against oxidative stress and cell apoptosis |
Against neurodegenerative events in hippocampal neurons | 333 | ||
| In vivo | Wistar rats | 5 mg/kg bw/day 7 weeks |
Reduce oxidative stress; Efficiently attenuated Aβ-induced oxidative damage; | Might be an alternative way to alleviate the development of AD | 334 |
5XFAD, 5 familial Alzheimer's disease mutation; 8-OHdG, 8-hydroxy-2-deoxyguanosine; α-Syn, α-synuclein; Aβ, amyloid-β; AchE, acetylcholinesterase; AD, Alzheimer's Disease; CAT, catalase; DA, Dopamine; GSH, glutathione; GSH-Px, glutathione peroxidase; GSSG, Oxidized glutathione; H2O2, hydrogen peroxide; HD, Huntington Disease; LPS, lipopolysaccharide; MDA, malondialdehyde; METH, Methamphetamine; MPP+, 1-methyl-4-phenylpyridinium; NDs, neurodegenerative diseases; Nrf2, nuclear factor erythroid 2 like 2; NSCs, Neural stem cells; PD, Parkinson's disease; ROS, reactive oxygen species; SOD, superoxide dismutase; Tg2576, transgenic mouse model.
Table 4.
Application of dietary antioxidant supplements in clinical studies of neurodegenerative diseases.
| Studied material | Participant | Effective dose and duration | Outcome measure | Main result | Ref. |
|---|---|---|---|---|---|
| Vitamins | 7540 men | Vitamin E 400 IU/day 5.4 ± 1.2 years |
Screening for dementia and cognitive impairment | No significant preventive effect on the incidence of AD and dementia | 101 |
| 57 AD patients | Vitamin C 800 IU/day 6 months |
Measured blood oxidized GSSG, Mini-Mental State, Blessed-Dementia Scale, and Clock Drawing Test | Reduce oxidative stress in some AD patients and maintains cognitive status | 102 | |
| 214 young adults aged 20–39 | Vitamin C 500 mg/day 4 weeks |
Stroop color-word test | Significantly increased attention and cognitive functions | 335 | |
| 47,335 participants | Vitamin C <400 mg/day, 400 < 700 mg/day, and ≥700 mg/day |
Risk indicators for ALS | No association between supplemental use of vitamin C and risk of ALS | 336 | |
| 45,837 men and 38,937 women aged 74–76 years | Vitamin E 14.9 years |
Total antioxidant capacity; Risk of PD | Intake of dietary vitamin E was associated with a lower risk of PD | 337 | |
| 1036 PD cases | Vitamin E 6.0, 7.6, 9.3, 14.6, 176.8 IU/day, 4 years |
PD clinical symptoms | Do not substantially affect the risk of PD | 338 | |
| Carotenoids | 62 older adults | 12 mg/day 1 year |
The memory, executive function and cognitive flexibility | Improve cognitive function in community-dwelling | 145 |
| 60 adult participants 25–45 years old |
Diet | Assess attentional inhibition; Assess response inhibition | Slow cognitive decline. Protective role of carotenoids in CNS may be evident during early and middle adulthood |
149 | |
| 682 participants without a clinical diagnosis of PD | Dietary intakes of total carotenoids, alpha-carotene, beta-carotene, lutein-zeaxanthin, lycopene, and beta-cryptoxanthin. 5.7 ± 3.0 years |
Assesses the severity of four parkinsonian signs (bradykinesia, gait, tremors, and rigidity) | A higher level of dietary antioxidant nutrients may slow the rate of parkinsonian clinical sign progression in older adults | 339 | |
| 193 healthy community dwellers 45–102 years old |
Daily intake of fruits and vegetables | Mini-mental state examination; clock drawing test; dem-tect scale | Reduce the prevalence of cognitive impairment in later life | 340 | |
| 1092 nondemented older participants | Daily intake fruits and vegetables 10 years |
Mini-mental state examination; Isaac's set test; Benton visual retention test | Moderately decrease the risk of dementia and AD | 341 | |
| 6958 participants aged older than 50 years | Diet 12 years |
AD-associated mortality | Reduce the AD mortality risk | 342 | |
| 2983 middle-aged adults | Diet 13 years |
The cued recall task; Backward digit span task; Trail making test and semantic fluency task |
Contribute to the preservation of cognitive function during ageing | 343 | |
| 49 healthy women | 12 mg/day 1 year |
Verbal fluency, memory, processing speed and accuracy, and self-reports of mood | Improve cognitive function | 344 | |
| 295 adult participants 65–84 years of age, overweight, at risk for AD and eating a suboptimal diet in the Boston and Chicago city areas |
Diet 3 years |
The global measure of cognitive function included a neuropsychological test battery of twelve performance-based tests. | Prevention of cognitive decline | 345 | |
| 63,257 men and women 45–74 years old |
Diet Average 19.4 years |
Incident cases were identified through follow-up interviews, hospital records, or PD registries | Not associated with the risk of developing PD in Singaporean Chinese | 346 | |
| Flavonoids | 25 working mothers 40–50 years old |
12 ounce (355 mL)/day 12 weeks |
Visual verbal learning test; Immediate recall (verbal memory); visual spatial learning test; rapid visual information processing | Improve performance on everyday tasks and cognitive ability | 183 |
| 28 participants 55+ years old |
200 mL/day 8 weeks |
Rey auditory; verbal learning test; verbal fluency task; digit-span backwards task; stroop task; counting span | Low-dose anthocyanin did not have any significant effect on cognition, nerve growth factor | 347 | |
| 40 men and women | Diet 6 weeks |
Levels of physical activity; Fatigue levels; Fatigability; Health descriptives | Improve the fatigue experienced early on in those with the nervous system disease and improved mobility and physical activity | 348 | |
| 92 patients fulfilling clinical criteria for PD or multiple system atrophy | 400 mg/day 48 weeks |
Clinical scales; lab-tests | Delay PD or multiple system atrophy and other related diseases | 349 | |
| Healthy 50–69 years old subjects | 450, 900 mg/day 3 months |
The modified Rey auditory learning task; The ModBent task | Improve a cognitive phenotype that characterizes the aging hippocampal circuit | 350 | |
| 96 subjects | 6, 12 mg/day 12 weeks |
Somatometry; haematology; urine screens; CogHealth and Groton maze learning test | Improve cognitive function in the healthy aged individuals | 351 | |
| 27 healthy adults | 135, 270 mg 45 min |
Near-infrared spectroscopy; Oddball reaction time task; rapid visual information processing task; stroop task | No significant differences were observed for the level of the cognitive performance/mood measures | 352 | |
| Non-flavonoid polyphenols | Forty subjects 51–84 years old |
180 mg/day 18 months |
Buschke selective reminding test; verbal memory outcome measure; consistent long term recall; brief visual memory test | Improved memory and attention in non-demented adults | 353 |
| 60 adults 18 and 30 years old |
500 mg/day 28 days |
Rapid visual information processing; serial subtractions; measures of cerebral blood flow | Subjective ratings of ‘fatigue’ were significantly lower; Significantly increased diastolic blood pressure; Levels of resveratrol metabolites were significantly higher | 354 | |
| 120 AD patients | 500, 1000, 1500, 2000 mg/day 52 weeks |
Magnetic resonance imaging acquisition and analyses | Resveratrol and its major metabolites penetrated the blood–brain barrier to have central nervous system effects | 355 | |
| 23 healthy adults | 250 mg 45 min |
Near-infrared spectroscopy; serial subtractions; rapid visual information processing; mood visual analogue scales | Cognitive function, mood and blood pressure were not affected | 356 | |
| Healthy older adults 50–80 years old |
200 mg/day 26 weeks |
Neuropsychological testing; magnetic resonance imaging acquisition and analyses | Improve memory performance in association and increase hippocampal functional connectivity in older adults | 357 | |
| 36 persons with mild-to-moderate AD | 2, 4 g/day 48 weeks |
AD assessment scale-cognitive subscale; AD cooperative study-activities of daily living | Anti-oxidant; Anti-amyloid effects; The efficacy of AD is unknown | 358 | |
| Phenolic acids | 56 participants 65–85 years old with mild cognitive impairment |
200 mg/day 48 weeks |
Magnetic resonance imaging; ADAS-Jcog score | Reduce AD pathological mechanisms; Improve cognitive functioning | 359 |
| 38 healthy participants | 300 mg/day 16 weeks |
Verbal and visual memory test; finger tapping test; symbol digit cording; stroop test; shifting attention test; continuous performance test | Improvement of cognitive functions including motor speed, psychomotor speed, and executive functions | 360 | |
| 8 healthy elderly men and women | 330 mg/day 6 months |
Verbal and visual memory test; finger tapping test; symbol digit cording; stroop test; shifting attention test; continuous performance test | Improvement of attentional, executive, and memory functions | 361 | |
| 411 non-demented older adults | 2 cups/day 3 years |
Measurement of cerebral Aβ deposition; measurement of cognitive activity; vascular risk score | Reduce the risk of AD or related cognitive decline by reducing pathological cerebral Aβ deposition | 362 | |
| 38 men and 37 women 38.5 ± 9 years old |
400 mg/day 8 weeks |
Plasma antioxidant capacity; lipid profile; vascular function | The antioxidant will be quickly absorbed; It has a neutral effect on blood lipids and blood vessel function | 363 | |
| 5632 subjects 65–84 years old |
1–2 cups 3.5 years |
Mini-mental state; Babcock story recall test; activities of daily living scale | Reduction of mild cognitive impairment and AD risk | 364 | |
| 60 healthy older adults 50 years old or older |
540 mg 40, 120 min |
Rapid visual information processing reaction time; inspection time; Jensen box decision/reaction times; serial subtraction N-Back working memory |
Significantly improve symptoms of headache; Did not significantly improve cognitive function | 365 | |
| Others | 85 patients diagnosed as mild cognitive impairment | 0.15 mg/day 6 months |
Magnetic resonance imaging examination; cerebrospinal fluid protein analysis | Reduced cerebrospinal fluid total TAU level; Improve the learning and memory function of patients | 366 |
| 8 patients with mild-to-moderate AD | 5 mg/day 3 days |
Electroencephalographic recordings; relative power, inter/intrahemispheric, Fronto-Posterior correlations | Significantly reduces nocturnal sleep onset in patients with mild-to-moderate AD | 367 | |
| 80 patients diagnosed with mild to moderate AD | 2 mg/day 24 weeks |
AD assessment scale-cognition; instrumental activities of daily living; mini-mental state examination; sleep quality index; a daily sleep diary; safety parameters | Positive effects on cognitive functioning and sleep maintenance in AD patients | 368 | |
| 25 elderly subjects 86 ± 6 years old with mild cognitive impairment |
Melatonin-containing supplement of docosahexaenoic acid with tryptophan 12 weeks |
Mini-mental state examination; digit, verbal, and spatial span; Rey's auditory-verbal learning test; Rey–Osterrieth complex figure | Improve cognitive function and attentional abilities | 369 |
Aβ, amyloid-β; AD, Alzheimer's disease; ALS, amyotrophic lateral sclerosis; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; GSSG, oxidized glutathione; PD, Parkinson's disease.
4.1. Vitamins
4.1.1. Vitamin C
Vitamin C (ascorbate) is mainly obtained from fruits and vegetables such as oranges (Citrus sinensis L. Osbeck), strawberries (Fragaria × ananassa Duch.), lemons (Citrus limon), kiwifruit (Actinidia chinensis Planch), spinach (Spinacia oleracea L.), bell peppers (Capsicum annuum L.), kale (Brassica oleracea L. var. acephala DC.) and broccoli (Brassica olearecea var. italica) (Table 2)79. It is one of the most important water-soluble antioxidants. Vitamin C has been reported to cross the BBB via glucose transporter 1 (Table 2). Glucose transporter 1 is a facilitative glucose transporter imports oxidized vitamin C (dehydroascorbic acid). Reduced vitamin C is taken up by another set of reduced vitamin C transporters, which are sodium-dependent vitamin C transporters (i.e., SVCT1 and SVCT2)62,80. Vitamin C has been linked to uptake by its transporters and a dysregulation of this system contributes to neurodegeneration in HD81,82.
Recent research studies also seem to confirm the role of vitamin C in preventing oxidative stress injury in the brain83. In vivo studies have shown that ascorbic acid can inactivate the main by-products of neuronal metabolism, superoxide radical and hydroxyl radical84. The fact that vitamin C neutralizes the oxygen free radicals that are abundantly produced during brain neurodegeneration seems to support its role in counteracting neurodegeneration. Ascorbic acid has also been shown to prevent lipid peroxidation induced by various oxidants in brain microsomes and sections, as well as cultured cells85. However, it is also noteworthy that randomized clinical trials have still failed to demonstrate any association between vitamin C and pathophysiological remission of NDs86, suggesting that prevention of deficiency appears to be more beneficial than vitamin C supplemen-tation.
It is worth noting that ascorbic acid has a dual role as an antioxidant and a pro-oxidant87. One research study showed that low doses of vitamin C (200 and 400 mg/kg bw) protected neurons by scavenging free radicals; however, higher doses (600 mg/kg bw) resulted in oxidative stress and cognitive impairment88 (Table 3)83,85,88. Therefore, much basic research and many clinical experiments are still needed to explore the specific mechanism of vitamin C in NDs.
4.1.2. Vitamin E
Vitamin E is a class of fat-soluble vitamins, including four tocopherols (designated as α, β, γ, and δ) and four tocotrienols (designated as α, β, γ, and δ)89,90, of which the most biologically active isomer is α-tocopherol91 that can be obtained from wheat germ (Triticum vulgare), soybeans (Glycine max (Linn.) Merr.), spinach (Spinacia oleracea), tomatoes (Solanum lycopersicum L.), vegetable oil, cod (Gadus morhua) liver oil and other foods92. Vitamin E is able to cross the BBB by passive diffusion and accumulate at therapeutic levels in the CNS (Table 2)91,92.
The ability of vitamin E to reduce oxidative damage to the brain has been demonstrated in preclinical and clinical human studies. In brain tissue, vitamin E can increase the level of GSH and the activities of various endogenous antioxidant enzymes93. Using neuronal cells, Crouzin et al.94 demonstrated that vitamin E can provide protection against antioxidant damage through genomic effects. In a human clinical study, de Wilde et al.95 showed that dietary antioxidant supplementation with vitamin E slowed the onset of dementia in patients with AD. In an AD mouse model, increasing dietary intake of vitamin E inhibited lipid peroxidation and effectively reduced the risk of AD prior to the occurrence of pathophysiological changes such as Aβ deposition96. Vitamin E inhibits p38 mitogen-activated protein kinase (p38) activation by preventing oxidative stress, thereby preventing TAU protein phosphorylation97 (Table 3)94,96,97. Increasing dietary intake of vitamin E can slow down the development of PD in humans. A meta-analysis showed a protective effect against PD in people with moderate and high dietary vitamin E intake, which protected the cell membrane from ROS damage by blocking peroxidation of cell membrane lipids98. Studies in animal models of PD have shown that vitamin E is neuroprotective against 6-hydroxydopamine (6-OHDA)-induced ROS and can significantly increase GSH levels in most brain regions and reduce the adverse effects of 6-OHDA on the brain99. In addition, vitamin E may reduce the progression of ALS and neuronal damage by reducing lipid peroxidation100.
However, while there is evidence to support the role of vitamin E supplementation in preventing neurodegeneration by scavenging excess free radicals, many research studies have yet to confirm these findings, some with contradictory results, and the exact role of vitamin E in the remains hotly debated. A clinical investigation study published in 2017 evaluated 7540 cognitively intact elderly men and found that taking a low dose (400 IU/day) of vitamin E did not delay the onset of AD101. Lloret et al.102 showed that vitamin E did not reduce plasma oxidative stress in AD patients (Table 4)101,102. And it has recently been shown that high-dose vitamin E supplementation is not as safe as previously thought. Taking too much vitamin E can lead to a variety of risks, including hemorrhagic stroke, retinopathy, impaired immune function, impaired clotting, and neoplastic diseases103, 104, 105. A randomized controlled trial showed that postmenopausal women who received high doses of vitamin E increased cardiovascular mortality within 2 years106. Vitamin E also has anticoagulant activity, and some clinical studies have shown that excess vitamin E can affect blood clotting in the fetus107,108. A clinical trial showed that vitamin E can significantly increase the risk of prostate cancer in men109. In addition, dietary studies have shown that the intake of large amounts of vitamin E from food alone or from food supplements is related to the increase in the prevalence of retinopathy in Caucasian patients110. In the future, more neurological research is needed to determine its efficacy and safe therapeutic doses.
4.2. Carotenoids
Carotenoids, a natural pigment found in fruits, vegetables and seaweed, have a variety of biological activities, including antioxidant properties, and play an important role in warding off brain disease111,112. Most carotenoids are essentially lipophilic and have the ability to penetrate the BBB (Table 2). A growing number of neurological studies have shown that various dietary antioxidant carotenoids, including lycopene, astaxanthin, β-carotene, lutein and fucoxanthin, have protective effects on people with NDs113, 114, 115, 116.
4.2.1. Lycopene
Lycopene is a natural carotenoid pigment with a broad presence in fruits and vegetables, such as tomatoes (Solanum lycopersicum), watermelons (Citrullus lanatus), grapefruit (Citrus paradisi Macf.) and pomegranates (Punica granatum L.)117. Due to its lipophilicity, lycopene can adequately reach the brain by crossing the BBB and play an important biological role in the CNS118 (Table 2 and 3)117,118.
Lycopene has been shown to antagonize oxidative stress damage and protect neurons, and long-term intake of lycopene-rich foods can effectively prevent the occurrence or development of NDs119,120. The antioxidant potential of lycopene is further reflected in its ability to inhibit membrane lipid peroxidation and the accumulation of hydrogen peroxide and superoxide, upregulating the intracellular antioxidant defense system121, 122, 123 (Table 3)123. Hwang et al.121 have confirmed that lycopene can inhibit Aβ-induced SH-SY5Y cell apoptosis by reducing intracellular ROS levels and inhibiting NF-κB activation, suggesting that lycopene can effectively inhibit Aβ-mediated oxidative stress and cellular apoptosis. Kaur et al.124 demonstrated that lycopene reduced oxidative damage, inhibited liposomal superoxide production, and increased the level of GSH and the activity of SOD in a rotenone-induced PD model. In mouse models of PD, lycopene exhibits antioxidant properties. Administration of enriched lycopene (10 mg/kg bw) significantly avoided the degeneration of substantia nigra dopaminergic neurons and the decrease of striatal dopamine (DA) levels in a PD model125. In addition, Huang et al.126 showed that lycopene can effectively resist synaptic damage induced by oxidative stress induced by tert-butyl hydroperoxide in vitro, and the possible mechanism of its protective effect is related to activation of the PI3K/AKT pathway.
The antioxidant properties of lycopene are particularly important in the protection of mitochondria. Lycopene treatment prevents loss of mitochondrial inner membrane potential, restores mitochondrial redox homeostasis and reduces ROS production127. Qu et al.128 demonstrated that lycopene improves energy metabolism in primary cortical neurons by preventing the loss of mitochondrial complex I, II, III and IV activity during Aβ treatment.
4.2.2. Astaxanthin
Astaxanthin (Table 2 and 3)129, 130, 131 is a carotenoid with antioxidant activity that is often found in shrimp (Caridea), crab (Brachyura), salmon (Oncorhynchus keta), trout (Salmo trutta L.), brown algae (Phaeophyceae), and yeast (Saccharomyces cerevisiae)129. Astaxanthin can be carried directly by fat molecules and cross the BBB to exert a neuroprotective effect on the brain130,131. Astaxanthin could be detected in the hippocampus of rats following oral administration at 100 mg/kg bw130. A randomized clinical trial found that consumption of astaxanthin at 10 mg per day is beneficial to the human body and has no adverse effects on a healthy adult132. Due to its powerful antioxidant properties and lack of adverse effects, astaxanthin was approved by the U.S. Food and Drug Administration as a dietary antioxidant supplement in 1999.
Astaxanthin exhibits strong antioxidant properties, and it has been demonstrated to exert antioxidant protection in people with neurological disorders133. Administration of astaxanthin reduces Aβ-mediated damage to cultured cells through multiple mechanisms, key among them being the reduction of ROS. Research studies have shown that astaxanthin can reduce lipid peroxidation and the level of ROS-generating enzymes and increase the activity of antioxidant enzymes134. Astaxanthin has also been shown to increase the expression of nuclear factor erythroid 2 like 2 (NRF2), thereby preventing oxidative stress135. Astaxanthin can restore the activity of antioxidant enzymes, reduce the production of ROS, and reduce the damage caused by 1-methyl-4-phenylpyridinium (MPP+)-induced oxidative stress on PC12 cells136. In addition, astaxanthin was able to scavenge H2O2-induced ROS in PC12 cells and inhibit Ca2+ influx137. Astaxanthin can also regulate the ratio of BCL-2/BAX, down-regulate the expression of α-synuclein (α-syn) and inhibit MPP+-induced SH-SY5Y cell damage138. The results of a research study on a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mouse model showed that astaxanthin can alleviate the clinical signs associated with PD in mice, slow down the metabolic rate of DA and promote the release of DA, all of which are related to the ability of astaxanthin to increase the level of antioxidants in the brain139.
4.2.3. β-Carotene
β-Carotene is a carotenoid found mainly in fruits and vegetables such as carrots (Daucus carota var. sativa Hoffm.), sweet potatoes (Ipomoea batatas L. Lam.) and pumpkins (Cucurbita moschata Duch.) and is considered a potent active biological antioxidant, especially in lipid-rich tissues such as neurons. However, the lack of research studies thus far demonstrating the ability of β-carotene to cross the BBB into the brain limits its use in the treatment of NDs.
β-Carotene can reduce the accumulation of ROS, including hydrogen peroxide and lipid peroxide free radicals (Tables 2 and 3)140,141. In addition, β-carotene has been shown to protect neurons from hydrogen peroxide damage and to improve the retention of intracellular antioxidants such as GSH and SOD141,142. The ability of β-carotene to combat lipid peroxidation and ROS is a key factor in it suitability as a treatment for brain-related diseases. A previous meta-analysis showed that dietary intake containing sufficient β-carotene reduced the incidence of AD and associated dementia143. β-Carotene increases protein expression of nuclear NRF2 and upregulates downstream targets containing antioxidant response elements (ARE) such as NADPH quinone oxidoreductase 1 (NQO1) and haeme oxygenase-1 (HO-1), thereby reducing oxidative damage to neurons142. Hira et al.141 showed that β-carotene was able to attenuate streptozotocin-induced cognitive deficits by inhibiting acetylcholinesterase (AChE) and reducing Aβ accumulation through its antioxidant effects. These results suggest that β-carotene could be used to treat NDs such as AD. β-Carotene has no prooxidative activity in the brain and is therefore considered to be a safe antioxidant140.
4.2.4. Lutein
Lutein is a carotenoid sourced from green leafy vegetables, particularly kale (Brassica oleracea var. sabellica L.) and spinach (Spinacia oleracea), in addition to orange and yellow fruits and vegetables, egg yolks, avocados (Persea americana Mill.) and other foods. Lutein is thought to pass through the BBB and play an important active biological role in the nervous system (Table 2 and 3)144. Research studies have shown that lutein can be detected in the brains of rhesus monkeys (Macaca mulatta) after oral administration of 0.25–0.5 μmol/kg bw of lutein144.
Known for its vision-enhancing properties, lutein has also been shown in research studies to play an important role in brain health and may even help prevent NDs145,146. Lutein reduces lipid peroxide accumulation and MDA formation, restores antioxidant levels in the brain, and reduces oxidative stress in a concentration-dependent manner (Table 3)147. In addition, research studies have shown that lutein can increase the expression of NRF2 and participate in the transcriptional regulation of antioxidant enzymes148. A clinical study conducted in 2017 showed that human beings with high concentrations of lutein in their brains had younger neural responses than those with low concentrations149 (Table 4)145,149. Nataraj et al.150 demonstrated the potential benefits lutein in the treatment of PD. Specifically, lutein can improve MPTP-induced mitochondrial dysfunction, oxidative stress and dyskinesia and protect dopaminergic neurons by increasing antioxidant defence and reducing mitochondrial dysfunction. Badgujar et al.151 showed that the long-term administration of lutein at a dose of 40 mg/kg bw in rats could regulate ROS in the brain and protect the brain from oxidative damage. A recent research study showed that in Drosophila exposed to rotenone, lutein-loaded nanoparticles restored DA levels, tyrosine hydroxylase and AChE activity and oxidative stress indicators and improved the survival rate of Drosophila152 (Table 3)150,152. Furthermore, dietary lutein has not been shown to have any adverse effects in humans.
4.2.5. Fucoxanthin
Fucoxanthin is a carotenoid that occurs naturally in edible brown seaweeds such as Undaria pinnatifida, Laminaria japonica Aresch and Hijikia fusiformis, among others, and has antioxidant properties (Table 2)153. However, it is unclear whether fucoxanthin can cross the BBB.
Although there are few research studies on the function of fucoxanthin in comparison with other carotenoids, a growing body of research suggests that fucoxanthin has a potential protective role in NDs154,155. Fucoxanthin and its metabolites have strong free-radical-scavenging activity. Fucoxanthin pre-treatment can reduce the content of MDA, increase the activity of SOD, increase the antioxidant capacity of neurons and reduce the level of lipid peroxidation. Fucoxanthin can prevent Aβ-induced neurotoxicity by reducing oxidative stress156. Research studies have shown that fucoxanthin can protect SH-SY5Y cells from oxidative damage caused by ROS and Aβ154. Fucoxanthin activates NRF2 and key redox targets downstream of NRF2, such as the expression of HO-1, SOD and BCL-2, protecting rodent brains from oxidative damage157,158. Sun et al.159 showed that fucoxanthin exerted neuroprotective effects on MPTP-mediated PD mice by inhibiting α-syn expression and oxidative stress and could be used as a potential therapeutic drug to improve PD (Table 3)157, 158, 159.
4.3. Flavonoids
Flavonoids are polyphenolic substances isolated from higher plants, mainly obtained from fruits, vegetables, grains and other foods, and are the most ingested polyphenolic compounds. A diet rich in flavonoids helps fight oxidative stress160,161. Thus far, a variety of flavonoids have been determined to have high antioxidant activity, including gren and black tea (Camellia sinensis) polyphenols, anthocyanins, quercetin and others. Many research studies have also confirmed that most flavonoids can cross the BBB and prevent neuronal degeneration162, 163, 164. Epidemiological studies support the idea that daily intake of dietary flavonoids can be beneficial in many NDs. Therefore, the following passages summarize research studies conducted on some flavonoids against NDs to enhance the understanding of flavonoids.
4.3.1. Epigallocatechin-3-gallate
Epigallocatechin-3-gallate (EGCG) is a flavonoid mainly found in green and black tea (Camellia sinensis), and red wine (Vitis vinifera L.). It has powerful antioxidant activity and can cross the BBB (Tables 2 and 3)165 where it has a positive impact on brain function.
EGCG may exert neuroprotective effects by scavenging free radicals and regulating the balance of oxidation and antioxidation. One research study showed that enzymatic and non-enzymatic antioxidant properties were significantly increased and markers of oxidative stress decreased in the brains of sodium fluoride-intoxicated rats after 4 weeks of EGCG administration (Table 3)166. In rat neural cell lines, EGCG significantly increased the expression of NRF2 and HO-1, inhibited nuclear transcription factor kappa B (NF-κB), directly reduced the overproduction of ROS and protected cultured neurons from oxidative stress-induced cell death167. In a mouse model of familial AD, EGCG significantly reduced Aβ production, attenuated Aβ-induced ROS production and induced a significant increase in brain synapses, improving spatial learning and memory (Table 3)168,169. The researchers demonstrated that EGCG inhibits Cu(II)-induced production of ROS, thereby reducing α-syn overexpression and primary systemic fibrosis in cells170. In vivo research studies in a rat model of PD induced by 6-OHDA showed that EGCG at 10 mg/kg bw by gavage reversed oxidative stress and immunohistochemical changes in the striatum and improved cognitive dysfunction (Table 3)170,171.
In addition to the direct antioxidant capacity, the neuroprotective effects of EGCG are also shown indirectly by modulating antioxidant enzymes172. Pan et al.173 showed that pre-treatment with EGCG for 30 min prevented methamphetamine-induced reduction in striatal GSH-Px activity. Furthermore, EGCG pre-treatment was observed to regulate methamphetamine-induced changes in striatal CAT and Cu/Zn SOD protein levels. Supplementation of EGCG upregulates the antioxidant system and enhances the activity of key enzymes of the tricarboxylic acid cycle (TCA cycle) and electron transport chain complexes in the mitochondria of aging brains, thereby demonstrating its antioxidant potential at the mitochondrial level (Table 3)173,174.
Nevertheless, there are still some obstacles to translating these preclinical data into human clinical trials, mainly related to the dosage regime (dose, interval and duration), and pharmacokinetics, particularly bioavailability issues, and these aspects should be the main focus of future research.
4.3.2. Anthocyanidins
Anthocyanins are flavonoids found widely in dark fruits, vegetables and grains. Common dietary sources include blueberries (Vaccinium spp.), grapes (Vitis vinifera L.), strawberries, cherries (Prunus spp.), pomegranates (Punica granatum), cabbage (Brassica oleracea var. capitata L.) and other foods that contain high amounts of natural pigments. Anthocyanins have strong antioxidant properties and can cross the BBB to reach brain tissue, where they exert neuroprotective effects (Table 2)175.
Research studies have shown that anthocyanins can neutralize ROS in cells, inhibit the generation of oxidative stress in nerve cells and simultaneously reduce the level of intracellular oxidative stress by effectively activating the production of endogenous antioxidant enzymes176. Anthocyanins can prevent oxidative damage to nerve cells by inhibiting both Aβ-induced oxidative damage and the activation of the BAX pro-apoptotic protein in mitochondria and by regulating the mitochondrial membrane potential, thus protecting the CNS from various NDs (Table 3)177, 178, 179. Ali et al.178 showed that anthocyanins can activate the NRF2/HO-1 pathway and its target genes, thereby reducing ROS production and neurodegeneration. Nutritional epidemiological studies have shown that long-term intake of anthocyanin-rich foods can significantly improve memory and cognition in the elderly and slow the progression of neurological diseases180,181.
Research studies in young, healthy adults have produced controversial results: some believe anthocyanins to have no effect on cognition182, while others find them to have cognitive benefits183 (Table 4)183. In conclusion, animal studies and randomized human clinical trials suggest that anthocyanins can reduce oxidative stress in the brain and improve cognition and neuroprotection. Further research studies must focus on finding the correct dosing regime (dose, interval and duration) for anthocyanin therapy in humans to achieve optimal neuroprotective benefits.
4.3.3. Quercetin
Quercetin is a strong antioxidant and a major dietary flavonoid widely found in vegetables and fruits such as onions (Allium cepa L.), apples (Malus domestica), broccoli (Brassica oleracea var. italica) and blueberries (Vaccinium caesariense)184,185.
Research studies have shown that quercetin is able to penetrate the BBB; however, the concentration of quercetin is much lower in the brain than in the plasma, suggesting that mice have low BBB permeability. A possible reason for this is that quercetin is a substrate for BBB efflux transporters such as P-glycoprotein (Table 2)184, 185, 186.
Interestingly, quercetin has properties that protect the rat brain from oxidative stress damage. It has been proven that quercetin can improve neuronal resistance to oxidative stress and excitotoxicity by regulating the mechanism of cell death. Firgany et al. (Table 3)187 showed that quercetin may inhibit oxidative stress and motor neuron excitotoxicity by inhibiting the p38/MAPK pathway. Moujahed et al.188 reported that quercetin reduces intracellular ROS production and restores mitochondrial membrane depolarization, thereby alleviating the neurotoxic effects caused by styrene 7,8-oxide. Lee and Jung (Table 3)189 showed for the first time that quercetin directly interferes with the activation of protein kinase C-ε and p38 MAPK and ameliorates the deleterious effects of R-sulfoximine (BSO)-induced oxidative stress by activating extracellular signal-regulated kinase 1/2 in neuronal cells. Furthermore, in a rotenone-induced PD mouse model, quercetin has been shown to upregulate the activity of mitochondrial complex I and increase the activity of CAT and SOD (Table 3)190. Another research study in a 6-OHDA-induced rat PD model showed that quercetin treatment increased antioxidant and striatal DA levels, decreased dopaminergic neuron loss, and significantly increased neuronal survival191. In fact, quercetin and its structurally related flavonoids have been identified as potential lead compounds for the development of treatments for neurodegeneration in the human brain.
However, a major therapeutic limitation stems from the fact that quercetin has poor solubility and absorption capacity, as well as low brain bioavailability192, and does not cross the normal BBB with high efficiency (Table 3)193. Further research is needed to expand on the above aspects of quercetin.
4.3.4. Rutin
Rutin is a flavonoid found in a variety of plants, including buckwheat (Fagopyrum esculentum Moench), oranges (Citrus sinensis), grapes (Vitis vinifera), apples (Malus domestica) and tea (Camellia sinensis (L.) O. Ktze.)194. Rutin has been shown to cross the BBB and act as an antioxidant in the brain (Table 2)194,195.
Several research studies have shown that rutin, as a potent antioxidant, has potential protective effects against NDs196. Rutin was able to dose-dependently reduce the formation of Aβ fibres in vitro, suggesting that its effect may be related to its free-radical scavenging activity and that it may inhibit neurotoxicity197. Pan et al.198 found that rutin can specifically target microglia in the brain, improve energy metabolism in microglia, enhance the clearance of Aβ by microglia and delay the pathological process of AD. Wang et al.199 found that rutin dose-dependently attenuated Aβ42-induced neurotoxicity and enhanced antioxidant enzyme activity in human neuroblastoma SH-SY5Y cells. Hu et al.200 demonstrated that treatment of SH-SY5Y cells with rutin-loaded nanoparticles was protective against Aβ-induced cytotoxicity and reduced NO and ROS levels. Rutin protects dopaminergic neurons from 6-OHDA-induced neurotoxicity by upregulating the activities of antioxidant enzymes, including SOD, CAT, GSH-Px and total GSH, and inhibiting lipid peroxidation activity191. Suganya and Sumathi reported that rutin ameliorated striatal damage in a 3-NP-induced HD model by reducing the activity of lipid peroxides201. Furthermore, rutin inhibited lipid peroxidation, enhanced the activity of AChE and attenuated sodium fluoride-induced neurotoxicity in the brain and striatum of rats202 (Table 3)191,196, 197, 198, 199, 200, 201, 202.
Although these basic research studies have presented concrete evidence of its biological activity, there is an urgent need for human clinical trials to provide additional data and further clarify the therapeutic potential of rutin.
4.3.5. Silymarin
Silymarin, a polyphenolic compound extracted from Silybum marianum (a species of thistle) is one of the most widely used flavonoids and is well known for its hepatoprotective activity (Table 2)203. However, its ability to cross the BBB has not yet been reported.
The neuroprotective effect of silymarin has been demonstrated in models of NDs204,205. Silymarin has strong free-radical scavenging activity and can protect the CNS from damage. Research studies have shown that silymarin significantly reduces the expression of Aβ1–42 in the muscle tissue of Caenorhabditis elegans by enhancing resistance to oxidative stress206. In addition, silymarin can inhibit the formation of Aβ fibres in PC12 cells and improve behavioural abnormalities in a mouse model of AD by ameliorating oxidative stress and inflammatory responses204. A recent research study in a 6-OHDA-induced PD model showed that intraperitoneal injection of different doses of silymarin (100, 200 or 300 mg/kg bw) for 15 days could increase the activity of antioxidant enzymes in the brain, reduce the level of lipid peroxidation, repair 6-OHDA-induced sports injury and dose-dependently increase the total number of surviving neurons in the dense part of the substantia nigra207. Another research study reported that silymarin pre-treatment attenuated scopolamine-induced oxidative stress by increasing ROS-scavenging activity in cortical and hippocampal regions, increasing GSH content and reducing MDA levels208. Silymarin (50 mg/kg bw) significantly maintained cognitive and behavioural functions, reduced the antioxidant state of the brain, and inhibited microglia activation209. Silymarin can also provide neuroprotection against acrylamide-induced neurotoxicity by reducing oxidative stress and inhibiting cathepsin D activity in the cerebellum of rats210 (Table 3)204,206, 207, 208, 209, 210. Meanwhile, silymarin has poor water solubility and low bioavailability, and only 23%–47% can reach the systemic circulation after oral administration which limits its activity, a silymarin-loaded liquid nanoemulsion could be useful to deliver poorly water-soluble silymarin with excellent hepatic protection by enhanced oral bioavailability via nanosized particles211.
4.3.6. Genistein
Genistein is the most active isoflavone in soybean (Glycine max (Linn.) Merr.) and a phytoestrogen that can cross the BBB (Table 2)212. Genistein has low water solubility and bioavailability, which limits its absorption.
Genistein is an effective free-radical scavenger with high antioxidant capacity in vitro213. Many recent research studies have shown that genistein has certain preventive and therapeutic effects on NDs such as AD. Mirahmadi et al.214 showed that in an animal model of lipopolysaccharide-induced cognitive dysfunction, oral administration of genistein flavonoids (10 mg/kg bw) for one week reduced lipid peroxidation and increased antioxidant defences (SOD, CAT and GSH) in the hippocampus. More importantly, high concentrations of genistein exhibited stronger antioxidant activity in preventing Aβ25–35-induced neuronal cell death compared to low concentrations of genistein, and at the micromolar level, the neuroprotective effect of genistein is mainly mediated by its antioxidant activity215,216. Rumman et al.217 showed that genistein ameliorated hypoxia-induced cognitive impairment in mice by reducing lipid peroxidation, reducing nitrite and ROS levels and increasing GSH and total antioxidant activity. Li et al.218 showed that genistein may increase the antioxidant level and reduce the level of lipid peroxide by regulating the NRF2/HO-1 signaling pathway, so as to reduce the oxidative stress induced by hypoxic–ischemic brain damage in neonatal mice (Table 3)214,216, 217, 218.
However, because of the unpredictability of genistein and the uncertainty of the results obtained through various biopharmacokinetic studies, the consistency of genistein in terms of pharmacokinetics remains unclear, limiting its further development.
4.3.7. Hesperetin
Hesperetin (4′-methoxy derivative of eriodictyol) is a citrus flavonoid found in fruits such as oranges (Citrus sinensis), grapes (Vitis vinifera L.) and lemons (Citrus limon). Hesperetin has been shown to cross the BBB (Table 2)219, and it has been suggested that the permeability of these active compounds depends on their lipophilicity.
It has been widely reported that in different models of neurodegeneration, hesperetin can exert neuroprotective effects by counteracting free radicals generated during cellular metabolism and by enhancing endogenous antioxidant defence mechanisms220,221. A research study evaluating the effect of hesperetin on Aβ-induced AD found that hesperetin significantly reduced oxidative stress-mediated neuroinflammation, apoptosis and neurodegeneration162. According to Kheradmand et al.222, that the most important protective mechanism provided by hesperetin and nano-hesperetin was a significant reduction in oxidative stress in a rat model of AD through its reduction of hippocampal lipid peroxidation and increased GSH levels as well as increased antioxidant enzyme activity. In addition, hesperetin treatment attenuated scopolamine-induced changes in oxidative-antioxidant balance, AChE activity, and neurogenesis in the hippocampus and the prefrontal cortex to ameliorate scopolamine-induced nonspatial/spatial learning and memory impairments223. To analyse the effect of hesperetin on PD, hesperetin was administered at a dose of 50 mg/kg bw for one week, and the results showed that hesperetin reduced oxidative stress by regulating NRF2, NF-κB and mitochondrial apoptosis224. Likewise, hesperetin attenuated high-glucose-induced neuronal oxidative damage by activating the canonical NRF2/ARE pathway in SH-SY5Y cells225. A recent research study showed that hesperetin protects SH-SY5Y cells from advanced glycation end-product-induced ROS and neuronal cell damage by downregulating Aβ production and enhancing Aβ degradation, leading to reduced Aβ accumulation226, which may be an excellent option for the treatment of NDs under enhanced glycation conditions (Table 3)220, 221, 222, 223,225,226.
Future research should investigate the use of hesperetin as a potential treatment for the prevention or management of NDs.
4.4. Non-flavonoid polyphenols
Polyphenols are chemicals that are widely found in plants and foods. Due to their lipophilicity, they can cross the BBB and exert powerful antioxidant and free-radical-scavenging activities within the brain tissue227, 228, 229.
4.4.1. Resveratrol
Resveratrol is widely found in vegetables, fruits, and other plants and at particularly high levels in wine (Vitis vinifera L.) and is one of the most predominant natural polyphenolic compounds with high biological activity230,231. In vitro and in vivo studies have shown that resveratrol can cross the BBB and exert a positive effect on NDs232,233 (Table 2)230, 231, 232, 233. However, its oral bioavailability is limited234, and the presence of specific transporters that allow it to cross the BBB, in addition to passive diffusion, remains to be conclusively demonstrated.
Resveratrol is a potent antioxidant both in vitro and in vivo, which exerts neuroprotective effects by scavenging free radicals and upregulating cellular antioxidants against oxidative stress233. Several research studies have shown that its ability to delay ND progression is mainly manifested as inhibition of Aβ aggregation and destabilization of fibrotic Aβ, activation of sirtuin protein and reduction of intracellular ROS accumulation, thus reducing neurodegenerative lesions in the hippocampus235,236. A research study conducted on mouse cortical neuronal cells showed that resveratrol selectively induced the expression of NRF2 and HO-1 in a dose- and time-dependent manner and provided protection against neurological damage caused by free radicals and excitotoxicity237. Resveratrol can also protect SK-N-BE cells (neuroblastoma model) from Aβ and α-syn-induced oxidative stress and toxicity238. Resveratrol can reduce oxidative stress by stimulating endogenous antioxidant enzymes. This effect was demonstrated by Cosin-Tomás et al. (Table 3)239 in a research study of a lymphoblastoid cell line from AD patients showing that 50 μmol/L resveratrol treatment was able to significantly upregulate gene expression in antioxidant systems, including CAT and SOD2, compared with AD controls.
Overall, basic research suggests that resveratrol has a powerful neuroprotective effect in humans. Although it penetrates the BBB, its low bioavailability minimizes its neuroprotective effects. Further research could focus on increasing its bioavailability to improve its therapeutic effect.
4.4.2. Curcumin
Curcumin is a polyphenol extracted from the rhizomes of Curcuma L. and has the ability to cross the BBB (Table 2)240. Recently, several research studies have demonstrated its potential in NDs.
The beneficial effects of curcumin on NDs may be related to its antioxidant properties. There is evidence to support the idea that curcumin can reduce the level of ROS, protect the brain from lipid peroxidation and reduce neuronal death caused by oxidative damage241. Fikry et al.242 showed that curcumin could alleviate degenerative histological changes and reduce oxidative stress in a rotenone-induced rat model of PD. Uğuz et al.243 showed that curcumin reduced lipid peroxidation in a H2O2-treated SH-SY5Y human neuroblastoma cell model, and thus protected neurons from oxidative damage. In a mouse model of AD, dietary antioxidant supplementation with curcumin reduced the accumulation of Aβ and oxidative stress in the cerebral cortex244 (Table 3)243,244.
Besides direct scavenging of ROS, curcumin also promotes antioxidant function by enhancing the activity of endogenous antioxidant molecules245. Cui et al.246 showed that curcumin treatment successfully reversed the decrease in HO-1, NQO1 and GSH activities in the substantia nigra pars compacta (SNc) in a rotenone-induced PD rat model. The research study found that curcumin treatment restored depleted GSH levels in neuronal cells, maintained mitochondrial complex I activity, activated the AKT/NRF2/HO-1 signaling pathway and prevented protein oxidation247 (Table 3)246,247.
Research also shows that curcumin is relatively non-toxic and has few adverse effects. Its inclusion in the diet is recommended for the elderly and those at risk for NDs. However, the poor bioavailability of curcumin limits its therapeutic application in brain diseases. Therefore, the improvement of curcumin bioavailability is an important pharmacological challenge.
4.5. Phenolic acids
Phenolic acids (such as gallic acid, caffeic acid, protocatechuic acid or ferulic acid) are an important class of polyphenols widely distributed in vegetables and fruits, and the phenolic hydroxyl and carboxyl groups in their structures can react with ROS to exert anti-free-radical effects248,249. Some research studies have shown that dietary plant phenolics, when absorbed into the bloodstream, can cross the body's BBB and directly affect the function of brain cells18,250. These results are consistent with previous experiments on experimental animals, suggesting that phenolic acids may penetrate the mouse brain through the BBB to exert neuroprotective activity (Table 2)18,248, 249, 250.
From various research studies conducted so far, phenolic acids have good nutritional function and antioxidant and other pharmacological activities and can potentially be applied to inhibit the progression of NDs. Caffeic acid has been shown to restore the levels of endogenous antioxidants in the brain, such as CAT, GSH and glutathione-S-transferase251, and prevent oxidative brain damage induced by different prooxidants, such as ferrous sulphate252. Protocatechin readily crosses the BBB, protects neurons from oxidative stress caused by glutamate excitotoxicity and nitrosative stress caused by nitric oxide (NO); it also works by retaining mitochondrial glutathione to inhibit mitochondrial oxidative stress and consequent apoptosis and to reduce NO production in microglia treated with lipopolysaccharide, showing antioxidant activity253. Guan et al.254 found that it can improve the survival rate of cultured neural stem cells and progenitor cells, upregulate the activity of intracellular antioxidant enzymes and further protect nerve cells. On the other hand, gallic acid can effectively reduce the generation of ROS, significantly increase the activity of antioxidant enzymes in animal models of ketamine-induced mania and reduce the activity of AChE and oxidative stress in the hippocampus and striatum255. Teixeira et al.256 demonstrated the ability of gallic acid to prevent mitochondrial lipid peroxidation in a cellular model using thiobarbituric acid reactive substances (TBARS) as the biological endpoint. In addition, ferulic acid can rescue DA neurons in the SNc region and nerve endings in the striatum from rotenone damage, restore antioxidant enzyme (SOD and CAT) activity, prevent glutathione overconsumption, and inhibit lipid peroxidation257(Table 3)252, 253, 254, 255,257. However, further human clinical trials are needed to fully define the beneficial activities and therapeutic effects of phenolic acids on neurons.
4.6. Others
Melatonin, specifically an indoleamine, is a tryptophan-derived hormone released by the pineal gland at night and has a role in regulating the circadian rhythm258, 259, 260. In addition to endogenous synthesis, some foods are rich sources of melatonin, especially olives (Canarium album (Lour.) Raeusch.), tomatoes (Solanum lycopersicum L.) and grapes (Vitis vinifera L.)261. The lipophilic and hydrophilic properties of melatonin allow it to easily cross the BBB and confer antioxidant protection on the nervous system262 (Table 2)261,262.
Melatonin functions as an antioxidant and may play an important role in the development of various NDs induced by oxidative stress263. Melatonin can increase the expression of antioxidant enzymes (such as SOD and CAT) and inhibit the excessive production of ROS264. It can also reduce oxidative damage to DNA, proteins and lipids265. Alghamdi et al.266 showed that melatonin can reduce Aβ production and neuronal oxidative damage in AD to promote the recovery of cognitive processes. Others research studies have reported that melatonin exerts neuroprotective effects in AD mouse model by reducing the production of Aβ and TAU proteins, oxidative stress and neuronal damage267. Sun et al.268 in a mouse model of AD showed that melatonin could reduce Aβ accumulation and ROS production, improve cognition and alleviate memory deficits. Patki et al.269 showed that melatonin attenuated oxidative stress and alleviated neurobehavioral deficits in a MPTP-induced PD mouse model. In addition, melatonin can attenuate MPP+-induced apoptosis and oxidative stress in SH-SY5Y human neuroblastoma cells and reduce neuronal toxicity270 (Table 3)268, 269, 270. Conversely, some research studies suggest that melatonin triggers PD271. Therefore, further research is needed to ensure better treatments for NDs.
Ergothioneine is an antioxidant of food origin and is known to be present in only a few foods and microorganisms. Some of the main sources include fungal foods such as black fungus (Auricularia auricula), king oyster mushroom (Pleurotus eryngii), enoki (Flammulina velutipes), and shiitake mushrooms (Lentinus edodes). Ergothioneine does cross the BBB and its concentration in vivo is controlled by the membrane transporter protein OCTN1272. Research studies have shown that it can be measured in human cerebrospinal fluid and post-mortem brain tissue samples273 and is readily accessible to the brains of mice274 (Table 2)272, 273, 274.
Ergothioneine, is a naturally occurring amino acid and is a thiourea derivative of histidine, containing a sulfur atom on the imidazole ring, that may protect the nervous system and reduce the risk of neurological disorders. A number of research studies have investigated the neuroprotective effects and therapeutic potential of ergothioneine275, 276, 277, 278, 279. Song et al.280 showed that ergothioneine attenuated oxidative stress and prevented cognitive deficits in a mouse model of D-galactose-induced dementia. Ergothioneine may prevent cisplatin-induced neuronal damage and enhance cognitive function in mice by inhibiting oxidative stress and restoring AChE activity in neuronal cells281. In addition, a protective effect of ergothioneine against the toxicity of Aβ was also observed. Mice injected with Aβ showed learning and memory deficits, whereas mice pretreated with ergothioneine did not show memory deficits282. A research study showed that when subjects ate mushrooms rich in ergothioneine, there was a negative correlation between their mild cognitive impairment and mushroom intake283. Overall, ergothioneine has potential therapeutic value as an antioxidant to protect the nervous system and reduce the risk of nervous system diseases. However, more research studies are needed to determine its efficacy and optimal dose.
Sulforaphane is an effective antioxidant, mainly found in cruciferous vegetables, such as broccoli (Brassica olearecea var. italica), watercress (Nasturtium officinale), Brussels sprouts (Brassica oleracea var. gemmifera) and cabbage (Brassica oleracea var. sabellica L.). Research studies have shown that sulforaphane has good oral bioavailability and can easily cross the BBB (Table 2)284.
Sulforaphane has been proved to play a neuroprotective role in vitro and in vivo model285,286. Sulforaphane can increase the level of NRF2 protein in various brain regions including the basal ganglia, leading to the up-regulation of antioxidant enzymes under different stress conditions287. Morroni et al.288 proved that in the 6-OHDA-PD mouse model treated with sulforaphane, the GSH level in the striatum was significantly increased. Lee et al.289 showed that sulforaphane significantly reduced AChE activity and reduced scopolamine-induced memory impairment. In addition, sulforaphane can improve the cognitive function of the Aβ-induced AD acute mouse model in the Y-maze and passive behavior avoidance tests290. Overall, these preclinical studies suggest that sulforaphane may have potential as a neuroprotective agent in the treatment of NDs, and more research studies are still needed in the future to determine its safety and efficacy in humans.
In conclusion, dietary antioxidant suplements are potent bioactive compounds with pleiotropic effects and good prospects as safe and effective treatments for NDs. However, some of the above-mentioned antioxidant compounds have poor bioavailability, which needs further improvement. At present, the research on nanotechnology is the most extensive. Zhang et al.291 have shown that a natural small molecule, betulinic acid, isolated from medicinal plants, can be assembled into nanoparticles, which can be used as both an effective therapeutic agent for stroke treatment and an effective carrier for drug delivery to the brain. The polylactic-glycolic acid copolymer formed by curcumin has 40-fold increased bioavailability compared to curcumin alone in rats229,292. With the continuous development of research, polymer nanoparticles or liposome nanocapsules have been used to increase permeability through BBB and improve bioavailability. Vanaja et al.293 showed that liposomal encapsulation of resveratrol increased its bioavailability. In addition, focused ultrasound is an emerging non-invasive technology for targeted drug delivery, which can safely and reversibly disrupt the BBB and enhance drug delivery57. In the future, extensive research and human clinical trials are still required to realize the therapeutic potential of these medicinal products. Other preclinical and clinical studies of dietary antioxidant suplements with beneficial effects on NDs are described in Table 3136,142,154,294, 295, 296, 297, 298, 299, 300, 301, 302, 303, 304, 305, 306, 307, 308, 309, 310, 311, 312, 313, 314, 315, 316, 317, 318, 319, 320, 321, 322, 323, 324, 325, 326, 327, 328, 329, 330 and Table 4331, 332, 333, 334, 335, 336, 337, 338, 339, 340, 341, 342, 343, 344, 345, 346, 347, 348, 349, 350, 351, 352, 353, 354, 355, 356, 357, 358, 359, 360, 361, 362, 363, 364, 365.
5. Conclusions and perspectives
The treatment of NDs is a global health challenge, and their etiopathology has not been fully elucidated. Without understanding the pathophysiologic basis for these diseases, developing effective treatments will remain challenging.
The role of oxidative stress in the pathogenesis of NDs has been well established in numerous preclinical and clinical human studies. Thus, blocking the production of ROS or promoting antioxidant defense systems in the brain may help treat NDs. Most of the findings reviewed here examine the efficacy of dietary antioxidant supplements in the treatment of NDs. Overall, these dietary antioxidants supplements have shown great promise in preclinical studies for NDs (AD, PD, HD and ALS). The reduction of ROS-induced neurogenesis limits the ability of the adult brain to regenerate. Dietary antioxidant supplements not only directly and indirectly reduce the harmful activities of ROS and oxidative stress but also promote the regenerative capacity of the adult human brain. In this regard, it is important to point out that a possible advantage of using dietary antioxidant supplements from fruits, vegetables, nuts and oils in the treatment of NDs is that they usually contain multiple antioxidant active compounds that can enhance each other. Low cytotoxicity, broad population availability and the ability to cross the BBB make antioxidant therapy a suitable candidate for combating NDs.
In order to optimize the intake of potentially beneficial oral antioxidants, it is important to have a diversified and balanced diet. People can incorporate a variety of antioxidant-rich fruits and vegetables into their diet, including berries, citrus fruits, leafy greens, sweet potatoes, nuts, and seeds. In addition to fruits and vegetables, antioxidant-rich beverages such as green tea or coffee can also be considered for daily consumption. It is equally important to note that, in general, obtaining antioxidants through whole foods rather than supplements is best. This is because whole foods contain a variety of other beneficial nutrients that work together to provide health benefits, while antioxidant supplements may provide isolated nutrients in high doses that can be harmful if taken in excess.
However, although preclinical animal studies have shown promising results, success in clinical trials has been limited, and the benefits of dietary antioxidant therapy in human NDs remain controversial. Moreover, epidemiological data remain sparse and controversial, perhaps due in part to the inherent difficulties in conducting epidemiological surveys of the dietary habits of large populations. Potential reasons for the conflicting results from these experiments may include the use of inappropriate doses for NDs or their different stages of development. Moreover, their effectiveness in the brain may be limited by their bioavailability, including an insufficient ability to cross the BBB and poor distribution in the brain regions, making it difficult for the antioxidant compound to reach target sites. It is also possible that treatment is initiated when the human patient is already in an advanced state, where a certain number of neurons have already died, at which point dietary antioxidant supplements may only save the surviving neurons and may not be sufficient to alleviate neurological symptoms. Therefore, it is suggested that dietary antioxidant supplementation can be started at a young age, when it can not only prevent but also delay the progression of diseases. In addition, for a medicinal product to be effective, it must be administered at its therapeutic concentration for the correct time frame and for a sufficiently long period of time from the point of injury. Therefore, any candidate medicinal product must be carefully evaluated to determine the appropriate therapeutic dosing regime. An important criterion that must be considered in the treatment of NDs is the ability to cross the BBB. The BBB is the main barrier between the CNS environment and peripheral blood flow, and if the medicinal product does not pass through the BBB, it will prevent the drug from entering the damaged site in the brain, in which case the effectiveness of neuroprotective medications may be limited. And studies in human beings must focus on assessing the average dose associated with a beneficial outcome without adverse effects, avoiding safety concerns whenever possible. Therefore, there is an urgent need for larger and more thorough human clinical trials to better understand the mechanisms of dietary antioxidant supplements and reveal their therapeutic potential, especially in various segments of the population (i.e. infants, children, adults and the elderly). It is expected that a medication with good effectiveness and few adverse effects will be developed, with almost no biophar-maceutical adverse effects, which will be good news for patients with NDs.
Acknowledgments
This work was supported by National Natural Science Foundation of China (NSFC) (32072925), Fundamental Research Funds for the Central Universities (2662020DKPY020, China) and Project Ref. PID 2020-115979RR-C33 from the Ministerio de Ciencia e Innovación, Spain.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Xu Wang, Email: wangxu@mail.hzau.edu.cn.
Arturo Anadón, Email: aanadon@ucm.es.
Author contributions
Jin Feng, Youle Zheng, Mingyue Guo, Irma Ares, Marta Martínez, Bernardo Lopez-Torres and María-Rosa Martínez-Larrañaga: Conceptualization, Methodology, Investigation, Data Curation, Writing—Original Draft, Visualization. Xu Wang, Arturo Anadón and María-Aránzazu Martinez: Supervision, Visualization, Writing—Review & Editing, Project administration, Funding acquisition. All authors have approved the final version of this manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Alladi S., Hachinski V. World dementia: one approach does not fit all. Neurology. 2018;91:264–270. doi: 10.1212/WNL.0000000000005941. [DOI] [PubMed] [Google Scholar]
- 2.Behl T., Makkar R., Sehgal A., Singh S., Sharma N., Zengin G., et al. Current trends in neurodegeneration: cross talks between oxidative stress, cell death, and inflammation. Int J Mol Sci. 2021;22:7432. doi: 10.3390/ijms22147432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feigin V.L., Vos T., Nichols E., Owolabi M.O., Carroll W.M., Dichgans M., et al. The global burden of neurological disorders: translating evidence into policy. Lancet Neurol. 2020;19:255–265. doi: 10.1016/S1474-4422(19)30411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Schependom J., D’haeseleer M. Advances in neurodegenerative diseases. J Clin Med. 2023;12:1709. doi: 10.3390/jcm12051709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uddin M.S., Al Mamun A., Rahman M.A., Behl T., Perveen A., Hafeez A., et al. Emerging proof of protein misfolding and interactions in multifactorial Alzheimer's disease. Curr Top Med Chem. 2020;20:2380–2390. doi: 10.2174/1568026620666200601161703. [DOI] [PubMed] [Google Scholar]
- 6.Singh E., Devasahayam G. Neurodegeneration by oxidative stress: a review on prospective use of small molecules for neuroprotection. Mol Biol Rep. 2020;47:3133–3140. doi: 10.1007/s11033-020-05354-1. [DOI] [PubMed] [Google Scholar]
- 7.Hacioglu G., Senturk A., Ince I., Alver A. Assessment of oxidative stress parameters of brain-derived neurotrophic factor heterozygous mice in acute stress model. Iran J Basic Med Sci. 2016;19:388–393. [PMC free article] [PubMed] [Google Scholar]
- 8.Cobley J.N., Fiorello M.L., Bailey D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503. doi: 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valko M., Jomova K., Rhodes C.J., Kuča K., Musílek K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol. 2016;90:1–37. doi: 10.1007/s00204-015-1579-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Michaelis E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira M.E., de Vasconcelos A.S., da Costa Vilhena T., da Silva T.L., da Silva Barbosa A., Gomes A.R., et al. Oxidative stress in Alzheimer's disease: should we keep trying antioxidant therapies?. Cell Mol Neurobiol. 2015;35:595–614. doi: 10.1007/s10571-015-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uddin M.S., Al Mamun A., Kabir M.T., Ahmad J., Jeandet P., Sarwar M.S., et al. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. Eur J Pharmacol. 2020;886 doi: 10.1016/j.ejphar.2020.173412. [DOI] [PubMed] [Google Scholar]
- 13.Sweeney M.D., Zhao Z., Montagne A., Nelson A.R., Zlokovic B.V. Blood–brain barrier: from physiology to disease and back. Physiol Rev. 2019;99:21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oppedisano F., Maiuolo J., Gliozzi M., Musolino V., Carresi C., Nucera S., et al. The potential for natural antioxidant supplementation in the early stages of neurodegenerative disorders. Int J Mol Sci. 2020;21:2618. doi: 10.3390/ijms21072618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianchi V.E., Herrera P.F., Laura R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr Neurosci. 2021;24:810–834. doi: 10.1080/1028415X.2019.1681088. [DOI] [PubMed] [Google Scholar]
- 16.Barbaresko J., Lellmann A.W., Schmidt A., Lehmann A., Amini A.M., Egert S., et al. Dietary factors and neurodegenerative disorders: an umbrella review of meta-analyses of prospective studies. Adv Nutr. 2020;11:1161–1173. doi: 10.1093/advances/nmaa053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winiarska-Mieczan A., Baranowska-Wójcik E., Kwiecień M., Grela E.R., Szwajgier D., Kwiatkowska K., et al. The role of dietary antioxidants in the pathogenesis of neurodegenerative diseases and their impact on cerebral oxidoreductive balance. Nutrients. 2020;12:435. doi: 10.3390/nu12020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velásquez-Jiménez D., Corella-Salazar D.A., Zuñiga-Martínez B.S., Domínguez-Avila J.A., Montiel-Herrera M., Salazar-López N.J., et al. Phenolic compounds that cross the blood–brain barrier exert positive health effects as central nervous system antioxidants. Food Funct. 2021;12:10356–10369. doi: 10.1039/d1fo02017j. [DOI] [PubMed] [Google Scholar]
- 19.Park H.A., Ellis A.C. Dietary antioxidants and Parkinson's disease. Antioxidants. 2020;9:570. doi: 10.3390/antiox9070570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh N., Ghosh K.K. In: Pathology, prevention and therapeutics of neurodegenerative disease. Singh S., Joshi N., editors. Springer; Singapore: 2019. Recent advances in the antioxidant therapies for Alzheimer's disease: emphasis on natural antioxidants; pp. 253–263. [Google Scholar]
- 21.Liaquat L., Haider S., Batool Z., Suleman A., Gul N., Jabbar S. Memory enhancement and reduction in lipid peroxidation by dietary antioxidants in brain. Pakistan J Biochem Mol Biol. 2016;49:53–59. [Google Scholar]
- 22.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules. 2019;24:1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng J., Wang X., Ye X., Ares I., Lopez-Torres B., Martínez M., et al. Mitochondria as an important target of metformin: the mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol Res. 2022;177 doi: 10.1016/j.phrs.2022.106114. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z., Zhou T., Ziegler A.C., Dimitrion P., Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dugger B.N., Dickson D.W. Pathology of neurodegenerative diseases. Cold Spring Harbor Perspect Biol. 2017;9:a028035. doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarafdar A., Pula G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int J Mol Sci. 2018;19:3824. doi: 10.3390/ijms19123824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rekatsina M., Paladini A., Piroli A., Zis P., Pergolizzi J.V., Varrassi G. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: a narrative review. Adv Ther. 2020;37:113–139. doi: 10.1007/s12325-019-01148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abramov A.Y. Redox biology in neurodegenerative disorders. Free Radic Biol Med. 2022;188:24–25. doi: 10.1016/j.freeradbiomed.2022.06.229. [DOI] [PubMed] [Google Scholar]
- 29.Barnham K.J., Masters C.L., Bush A.I. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 30.Collin F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int J Mol Sci. 2019;20:2407. doi: 10.3390/ijms20102407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheignon C., Tomas M., Bonnefont-Rousselot D., Faller P., Hureau C., Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol. 2018;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaganjac M., Milkovic L., Zarkovic N., Zarkovic K. Oxidative stress and regeneration. Free Radic Biol Med. 2022;181:154–165. doi: 10.1016/j.freeradbiomed.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Cassina P., Miquel E., Martínez-Palma L., Cassina A. Glial metabolic reprogramming in amyotrophic lateral sclerosis. Neuroimmunomodulation. 2021;28:204–212. doi: 10.1159/000516926. [DOI] [PubMed] [Google Scholar]
- 34.Goshtasbi H., Pakchin P.S., Movafeghi A., Barar J., Castejon A.M., Omidian H., et al. Impacts of oxidants and antioxidants on the emergence and progression of Alzheimer's disease. Neurochem Int. 2022;153 doi: 10.1016/j.neuint.2021.105268. [DOI] [PubMed] [Google Scholar]
- 35.Jurcau A. Insights into the pathogenesis of neurodegenerative diseases: focus on mitochondrial dysfunction and oxidative stress. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222111847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cioffi F., Adam R.H.I., Bansal R., Broersen K. A review of oxidative stress products and related genes in early Alzheimer's disease. J Alzheimers Dis. 2021;83:977–1001. doi: 10.3233/JAD-210497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang F., Zhong R.J., Cheng C., Li S., Le W.D. New therapeutics beyond amyloid-β and tau for the treatment of Alzheimer's disease. Acta Pharmacol Sin. 2021;42:1382–1389. doi: 10.1038/s41401-020-00565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoy N., Mackay G.M., Forrest C.M., Christofides J., Egerton M., Stone T.W., et al. Tryptophan metabolism and oxidative stress in patients with Huntington's disease. J Neurochem. 2005;93:611–623. doi: 10.1111/j.1471-4159.2005.03070.x. [DOI] [PubMed] [Google Scholar]
- 39.Zheng J., Winderickx J., Franssens V., Liu B. A mitochondria-associated oxidative stress perspective on Huntington's disease. Front Mol Neurosci. 2018;11:329. doi: 10.3389/fnmol.2018.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Essa M.M., Moghadas M., Ba-Omar T., Walid Qoronfleh M., Guillemin G.J., Manivasagam T., et al. Protective effects of antioxidants in Huntington's disease: an extensive review. Neurotox Res. 2019;35:739–774. doi: 10.1007/s12640-018-9989-9. [DOI] [PubMed] [Google Scholar]
- 41.Jin H., Kanthasamy A., Ghosh A., Anantharam V., Kalyanaraman B., Kanthasamy A.G. Mitochondria-targeted antioxidants for treatment of Parkinson's disease: preclinical and clinical outcomes. Biochim Biophys Acta. 2014;1842:1282–1294. doi: 10.1016/j.bbadis.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obrador E., Salvador R., López-Blanch R., Jihad-Jebbar A., Vallés S.L., Estrela J.M. Oxidative stress, neuroinflammation and mitochondria in the pathophysiology of amyotrophic lateral sclerosis. Antioxidants. 2020;9:901. doi: 10.3390/antiox9090901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obrador E., Salvador-Palmer R., López-Blanch R., Jihad-Jebbar A., Vallés S.L., Estrela J.M. The link between oxidative stress, redox status, bioenergetics and mitochondria in the pathophysiology of ALS. Int J Mol Sci. 2021;22:6352. doi: 10.3390/ijms22126352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peggion C., Scalcon V., Massimino M.L., Nies K., Lopreiato R., Rigobello M.P., et al. SOD1 in ALS: taking stock in pathogenic mechanisms and the role of glial and muscle cells. Antioxidants. 2022;11:614. doi: 10.3390/antiox11040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiménez-Xarrié E., Pérez B., Dantas A.P., Puertas-Umbert L., Martí-Fabregas J., Chamorro Á., et al. Uric acid treatment after stroke prevents long-term middle cerebral artery remodelling and attenuates brain damage in spontaneously hypertensive rats. Transl Stroke Res. 2020;11:1332–1347. doi: 10.1007/s12975-018-0661-8. [DOI] [PubMed] [Google Scholar]
- 46.Chamorro Á., Amaro S., Castellanos M., Gomis M., Urra X., Blasco J., et al. Uric acid therapy improves the outcomes of stroke patients treated with intravenous tissue plasminogen activator and mechanical thrombectomy. Int J Stroke. 2017;12:377–382. doi: 10.1177/1747493016684354. [DOI] [PubMed] [Google Scholar]
- 47.Dhanesha N., Vázquez-Rosa E., Cintrón-Pérez C.J., Thedens D., Kort A.J., Chuong V., et al. Treatment with uric acid reduces infarct and improves neurologic function in female mice after transient cerebral ischemia. J Stroke Cerebrovasc Dis. 2018;27:1412–1416. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.043. [DOI] [PubMed] [Google Scholar]
- 48.Aliena-Valero A., López-Morales M.A., Burguete M.C., Castelló-Ruiz M., Jover-Mengual T., Hervás D., et al. Emergent uric acid treatment is synergistic with mechanical recanalization in improving stroke outcomes in male and female rats. Neuroscience. 2018;388:263–273. doi: 10.1016/j.neuroscience.2018.07.045. [DOI] [PubMed] [Google Scholar]
- 49.Vila E., Solé M., Masip N., Puertas-Umbert L., Amaro S., Dantas A.P., et al. Uric acid treatment after stroke modulates the Krüppel-like factor 2-VEGF-A axis to protect brain endothelial cell functions: impact of hypertension. Biochem Pharmacol. 2019;164:115–128. doi: 10.1016/j.bcp.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Aliena-Valero A., Baixauli-Martín J., Castelló-Ruiz M., Torregrosa G., Hervás D., Salom J.B. Effect of uric acid in animal models of ischemic stroke: a systematic review and meta-analysis. J Cerebr Blood Flow Metabol. 2021;41:707–722. doi: 10.1177/0271678X20967459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Llull L., Amaro S., Chamorro Á. Administration of uric acid in the emergency treatment of acute ischemic stroke. Curr Neurol Neurosci Rep. 2016;16:4. doi: 10.1007/s11910-015-0604-7. [DOI] [PubMed] [Google Scholar]
- 52.Li R., Huang C., Chen J., Guo Y., Tan S. The role of uric acid as a potential neuroprotectant in acute ischemic stroke: a review of literature. Neurol Sci. 2015;36:1097–1103. doi: 10.1007/s10072-015-2151-z. [DOI] [PubMed] [Google Scholar]
- 53.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cichon N., Dziedzic A., Gorniak L., Miller E., Bijak M., Starosta M., et al. Unusual bioactive compounds with antioxidant properties in adjuvant therapy supporting cognition impairment in age-related neurodegenerative disorders. Int J Mol Sci. 2021;22 doi: 10.3390/ijms221910707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramli N.Z., Yahaya M.F., Tooyama I., Damanhuri H.A. A mechanistic evaluation of antioxidant nutraceuticals on their potential against age-associated neurodegenerative diseases. Antioxidants. 2020;9:1019. doi: 10.3390/antiox9101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Langen U.H., Ayloo S., Gu C. Development and cell biology of the blood–brain barrier. Annu Rev Cell Dev Biol. 2019;35:591–613. doi: 10.1146/annurev-cellbio-100617-062608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pandit R., Chen L., Götz J. The blood–brain barrier: physiology and strategies for drug delivery. Adv Drug Deliv Rev. 2020;165–166:1–14. doi: 10.1016/j.addr.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 58.Kadry H., Noorani B., Cucullo L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17:69. doi: 10.1186/s12987-020-00230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coelho-Santos V., Shih A.Y. Postnatal development of cerebrovascular structure and the neurogliovascular unit. Wiley Interdiscip Rev Dev Biol. 2020;9:e363. doi: 10.1002/wdev.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serlin Y., Shelef I., Knyazer B., Friedman A. Anatomy and physiology of the blood–brain barrier. Semin Cell Dev Biol. 2015;38:2–6. doi: 10.1016/j.semcdb.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seo S., Kim H., Sung J.H., Choi N., Lee K., Kim H.N. Microphysiological systems for recapitulating physiology and function of blood–brain barrier. Biomaterials. 2020;232 doi: 10.1016/j.biomaterials.2019.119732. [DOI] [PubMed] [Google Scholar]
- 62.Patching S.G. Glucose transporters at the blood–brain barrier: function, regulation and gateways for drug delivery. Mol Neurobiol. 2017;54:1046–1077. doi: 10.1007/s12035-015-9672-6. [DOI] [PubMed] [Google Scholar]
- 63.Zhao Y., Gan L., Ren L., Lin Y., Ma C., Lin X. Factors influencing the blood–brain barrier permeability. Brain Res. 2022;1788 doi: 10.1016/j.brainres.2022.147937. [DOI] [PubMed] [Google Scholar]
- 64.Gil-Martins E., Barbosa D.J., Silva V., Remião F., Silva R. Dysfunction of ABC transporters at the blood–brain barrier: role in neurological disorders. Pharmacol Ther. 2020;213 doi: 10.1016/j.pharmthera.2020.107554. [DOI] [PubMed] [Google Scholar]
- 65.Strazielle N., Ghersi-Egea J.F. Efflux transporters in blood–brain interfaces of the developing brain. Front Neurosci. 2015;9:21. doi: 10.3389/fnins.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J., Zheng M., Shimoni O., Banks W.A., Bush A.I., Gamble J.R., et al. Development of novel therapeutics targeting the blood–brain barrier: from barrier to carrier. Adv Sci. 2021;8 doi: 10.1002/advs.202101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petrovic S., Arsic A., Ristic-Medic D., Cvetkovic Z., Vucic V. Lipid peroxidation and antioxidant supplementation in neurodegenerative diseases: a review of human studies. Antioxidants. 2020;9:1128. doi: 10.3390/antiox9111128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee K.H., Cha M., Lee B.H. Neuroprotective effect of antioxidants in the brain. Int J Mol Sci. 2020;21:7152. doi: 10.3390/ijms21197152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guggilla S., Karthik M., Shylendra B. Regulation of antioxidant enzyme levels in rat brain. Adv Exp Med Biol. 2021;1339:21–26. doi: 10.1007/978-3-030-78787-5_3. [DOI] [PubMed] [Google Scholar]
- 70.Aranda-Rivera A.K., Cruz-Gregorio A., Arancibia-Hernández Y.L., Hernández-Cruz E.Y., Pedraza-Chaverri J. RONS and oxidative stress: an overview of basic concepts. Oxygen. 2022;2:437–478. [Google Scholar]
- 71.Simonian N.A., Coyle J.T. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- 72.Akanji M.A., Rotimi D.E., Elebiyo T.C., Awakan O.J., Adeyemi O.S. Redox homeostasis and prospects for therapeutic targeting in neurodegenerative disorders. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/9971885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mirończuk-Chodakowska I., Witkowska A.M., Zujko M.E. Endogenous non-enzymatic antioxidants in the human body. Adv Med Sci. 2018;63:68–78. doi: 10.1016/j.advms.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 74.Pisoschi A.M., Pop A., Iordache F., Stanca L., Predoi G., Serban A.I. Oxidative stress mitigation by antioxidants—an overview on their chemistry and influences on health status. Eur J Med Chem. 2021;209 doi: 10.1016/j.ejmech.2020.112891. [DOI] [PubMed] [Google Scholar]
- 75.Maccioni R.B., Calfío C., González A., Lüttges V. Novel nutraceutical compounds in Alzheimer prevention. Biomolecules. 2022;12:249. doi: 10.3390/biom12020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh P., Mishra G., Molla M., Shumet Yimer Y., Sisay W., Andargie Y., et al. Dietary and nutraceutical-based therapeutic approaches to combat the pathogenesis of Huntington's disease. J Funct Foods. 2022;92 [Google Scholar]
- 77.Ramírez-Salazar S.A., Herren C., McCartney J., Ortiz García J.G. Dietary insights in neurological diseases. Curr Neurol Neurosci Rep. 2021;21:55. doi: 10.1007/s11910-021-01143-w. [DOI] [PubMed] [Google Scholar]
- 78.Fernandes C., Pinto M., Martins C., Gomes M.J., Sarmento B., Oliveira P.J., et al. Development of a PEGylated-based platform for efficient delivery of dietary antioxidants across the blood–brain barrier. Bioconjugate Chem. 2018;29:1677–1689. doi: 10.1021/acs.bioconjchem.8b00151. [DOI] [PubMed] [Google Scholar]
- 79.Mieszczakowska-Frąc M., Celejewska K., Płocharski W. Impact of innovative technologies on the content of vitamin C and its bioavailability from processed fruit and vegetable products. Antioxidants. 2021;10:54. doi: 10.3390/antiox10010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bürzle M., Hediger M.A. Functional and physiological role of vitamin C transporters. Curr Top Membr. 2012;70:357–375. doi: 10.1016/B978-0-12-394316-3.00011-9. [DOI] [PubMed] [Google Scholar]
- 81.Acuña A.I., Esparza M., Kramm C., Beltrán F.A., Parra A.V., Cepeda C., et al. A failure in energy metabolism and antioxidant uptake precede symptoms of Huntington's disease in mice. Nat Commun. 2013;4:2917. doi: 10.1038/ncomms3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Covarrubias-Pinto A., Parra A.V., Mayorga-Weber G., Papic E., Vicencio I., Ehrenfeld P., et al. Impaired intracellular trafficking of sodium-dependent vitamin C transporter 2 contributes to the redox imbalance in Huntington's disease. J Neurosci Res. 2021;99:223–235. doi: 10.1002/jnr.24693. [DOI] [PubMed] [Google Scholar]
- 83.Ferrada L., Barahona M.J., Salazar K., Vandenabeele P., Nualart F. Vitamin C controls neuronal necroptosis under oxidative stress. Redox Biol. 2020;29 doi: 10.1016/j.redox.2019.101408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kontoghiorghes G.J., Kolnagou A., Kontoghiorghe C.N., Mourouzidis L., Timoshnikov V.A., Polyakov N.E. Trying to solve the puzzle of the interaction of ascorbic acid and iron: redox, chelation and therapeutic implications. Medicines. 2020;7:45. doi: 10.3390/medicines7080045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khordad E., Alipour F., Beheshti F., Hosseini M., Rajabzadeh A.A., Asiaei F., et al. Vitamin C prevents hypothyroidism associated neuronal damage in the hippocampus of neonatal and juvenile rats: a stereological study. J Chem Neuroanat. 2018;93:48–56. doi: 10.1016/j.jchemneu.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 86.Monacelli F., Acquarone E., Giannotti C., Borghi R., Nencioni A. Vitamin C, aging and Alzheimer's disease. Nutrients. 2017;9:670. doi: 10.3390/nu9070670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen G., Chang T.M.S. Dual effects include antioxidant and pro-oxidation of ascorbic acid on the redox properties of bovine hemoglobin. Artif Cells, Nanomed Biotechnol. 2018;46:983–992. doi: 10.1080/21691401.2018.1476374. [DOI] [PubMed] [Google Scholar]
- 88.Sil S., Ghosh T., Gupta P., Ghosh R., Kabir S.N., Roy A. Dual role of vitamin C on the neuroinflammation mediated neurodegeneration and memory impairments in colchicine induced rat model of Alzheimer disease. J Mol Neurosci. 2016;60:421–435. doi: 10.1007/s12031-016-0817-5. [DOI] [PubMed] [Google Scholar]
- 89.Galli F., Azzi A., Birringer M., Cook-Mills J.M., Eggersdorfer M., Frank J., et al. Vitamin E: emerging aspects and new directions. Free Radic Biol Med. 2017;102:16–36. doi: 10.1016/j.freeradbiomed.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 90.Müller L., Theile K., Böhm V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol Nutr Food Res. 2010;54:731–742. doi: 10.1002/mnfr.200900399. [DOI] [PubMed] [Google Scholar]
- 91.Lee P., Ulatowski L.M. Vitamin E: mechanism of transport and regulation in the CNS. IUBMB Life. 2019;71:424–429. doi: 10.1002/iub.1993. [DOI] [PubMed] [Google Scholar]
- 92.Zaaboul F., Liu Y. Vitamin E in foodstuff: nutritional, analytical, and food technology aspects. Compr Rev Food Sci Food Saf. 2022;21:964–998. doi: 10.1111/1541-4337.12924. [DOI] [PubMed] [Google Scholar]
- 93.Badgujar P.C., Chandratre G.A., Pawar N.N., Telang A.G., Kurade N.P. Fipronil induced oxidative stress involves alterations in SOD1 and catalase gene expression in male mice liver: protection by vitamins E and C. Environ Toxicol. 2016;31:1147–1158. doi: 10.1002/tox.22125. [DOI] [PubMed] [Google Scholar]
- 94.Crouzin N., Ferreira M.C., Cohen-Solal C., Barbanel G., Guiramand J., Vignes M. Neuroprotection induced by vitamin E against oxidative stress in hippocampal neurons: involvement of TRPV1 channels. Mol Nutr Food Res. 2010;54:496–505. doi: 10.1002/mnfr.200900188. [DOI] [PubMed] [Google Scholar]
- 95.de Wilde M.C., Vellas B., Girault E., Yavuz A.C., Sijben J.W. Lower brain and blood nutrient status in Alzheimer's disease: results from meta-analyses. Alzheimers Dement. 2017;3:416–431. doi: 10.1016/j.trci.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sung S., Yao Y., Uryu K., Yang H., Lee V.M., Trojanowski J.Q., et al. Early vitamin E supplementation in young but not aged mice reduces Aβ levels and amyloid deposition in a transgenic model of Alzheimer's disease. Faseb J. 2004;18:323–325. doi: 10.1096/fj.03-0961fje. [DOI] [PubMed] [Google Scholar]
- 97.Giraldo E., Lloret A., Fuchsberger T., Viña J. Aβ and tau toxicities in Alzheimer's are linked via oxidative stress-induced p38 activation: protective role of vitamin E. Redox Biol. 2014;2:873–877. doi: 10.1016/j.redox.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Etminan M., Gill S.S., Samii A. Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson's disease: a meta-analysis. Lancet Neurol. 2005;4:362–365. doi: 10.1016/S1474-4422(05)70097-1. [DOI] [PubMed] [Google Scholar]
- 99.Gaba B., Khan T., Haider M.F., Alam T., Baboota S., Parvez S., et al. Vitamin E loaded naringenin nanoemulsion via intranasal delivery for the management of oxidative stress in a 6-OHDA Parkinson's disease model. BioMed Res Int. 2019;2019 doi: 10.1155/2019/2382563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Galbussera A., Tremolizzo L., Brighina L., Testa D., Lovati R., Ferrarese C., et al. Vitamin E intake and quality of life in amyotrophic lateral sclerosis patients: a follow-up case series study. Neurol Sci. 2006;27:190–193. doi: 10.1007/s10072-006-0668-x. [DOI] [PubMed] [Google Scholar]
- 101.Kryscio R.J., Abner E.L., Caban-Holt A., Lovell M., Goodman P., Darke A.K., et al. Association of antioxidant supplement use and dementia in the prevention of Alzheimer's disease by vitamin E and selenium trial (PREADViSE) JAMA Neurol. 2017;74:567–573. doi: 10.1001/jamaneurol.2016.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lloret A., Badía M.C., Mora N.J., Pallardó F.V., Alonso M.D., Viña J. Vitamin E paradox in Alzheimer's disease: it does not prevent loss of cognition and may even be detrimental. J Alzheimers Dis. 2009;17:143–149. doi: 10.3233/JAD-2009-1033. [DOI] [PubMed] [Google Scholar]
- 103.Schürks M., Glynn R.J., Rist P.M., Tzourio C., Kurth T. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702. doi: 10.1136/bmj.c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garg A., Lee J.C. Vitamin E: where are we now in vascular diseases?. Life. 2022;12:310. doi: 10.3390/life12020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dow C., Mancini F., Rajaobelina K., Boutron-Ruault M.C., Balkau B., Bonnet F., et al. Diet and risk of diabetic retinopathy: a systematic review. Eur J Epidemiol. 2018;33:141–156. doi: 10.1007/s10654-017-0338-8. [DOI] [PubMed] [Google Scholar]
- 106.Waters D.D., Alderman E.L., Hsia J., Howard B.V., Cobb F.R., Rogers W.J., et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002;288:2432–2440. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 107.Allan K.M., Prabhu N., Craig L.C., McNeill G., Kirby B., McLay J., et al. Maternal vitamin D and E intakes during pregnancy are associated with asthma in children. Eur Respir J. 2015;45:1027–1036. doi: 10.1183/09031936.00102214. [DOI] [PubMed] [Google Scholar]
- 108.Chen H., Qian N., Yan L., Jiang H. Role of serum vitamin A and E in pregnancy. Exp Ther Med. 2018;16:5185–5189. doi: 10.3892/etm.2018.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klein E.A., Thompson I.M., Jr., Tangen C.M., Crowley J.J., Lucia M.S., Goodman P.J., et al. Vitamin E and the risk of prostate cancer: the selenium and vitamin E cancer prevention trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Millen A.E., Klein R., Folsom A.R., Stevens J., Palta M., Mares J.A. Relation between intake of vitamins C and E and risk of diabetic retinopathy in the atherosclerosis risk in communities study. Am J Clin Nutr. 2004;79:865–873. doi: 10.1093/ajcn/79.5.865. [DOI] [PubMed] [Google Scholar]
- 111.Pérez-Gálvez A., Viera I., Roca M. Carotenoids and chlorophylls as antioxidants. Antioxidants. 2020;9:505. doi: 10.3390/antiox9060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kabir M.T., Rahman M.H., Shah M., Jamiruddin M.R., Basak D., Al-Harrasi A., et al. Therapeutic promise of carotenoids as antioxidants and anti-inflammatory agents in neurodegenerative disorders. Biomed Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112610. [DOI] [PubMed] [Google Scholar]
- 113.Moratilla-Rivera I., Sánchez M., Valdés-González J.A., Gómez-Serranillos M.P. Natural products as modulators of Nrf2 signaling pathway in neuroprotection. Int J Mol Sci. 2023;24:3748. doi: 10.3390/ijms24043748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beydoun M.A., Canas J.A., Fanelli-Kuczmarski M.T., Maldonado A.I., Shaked D., Kivimaki M., et al. Association of antioxidant vitamins A, C, E and carotenoids with cognitive performance over time: a cohort study of middle-aged adults. Nutrients. 2020;12:3558. doi: 10.3390/nu12113558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rakowski M., Porębski S., Grzelak A. Nutraceuticals as modulators of autophagy: relevance in Parkinson's disease. Int J Mol Sci. 2022;23:3625. doi: 10.3390/ijms23073625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fu C., Zheng Y., Zhu J., Chen B., Lin W., Lin K., et al. Lycopene exerts neuroprotective effects after hypoxic–ischemic brain injury in neonatal rats via the nuclear factor erythroid-2 related factor 2/nuclear factor-κ-gene binding pathway. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.585898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Poojary M.M., Passamonti P. Extraction of lycopene from tomato processing waste: kinetics and modelling. Food Chem. 2015;173:943–950. doi: 10.1016/j.foodchem.2014.10.127. [DOI] [PubMed] [Google Scholar]
- 118.Wang R., Xu Z., Li Y., Li W., Gao X., Liu C., et al. Lycopene can modulate the LRP1 and RAGE transporters expression at the choroid plexus in Alzheimer's disease rat. J Funct Foods. 2021;85 [Google Scholar]
- 119.Crowe-White K.M., Phillips T.A., Ellis A.C. Lycopene and cognitive function. J Nutr Sci. 2019;8 doi: 10.1017/jns.2019.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saini R.K., Rengasamy K.R.R., Mahomoodally F.M., Keum Y.S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: an update on epidemiological and mechanistic perspectives. Pharmacol Res. 2020;155 doi: 10.1016/j.phrs.2020.104730. [DOI] [PubMed] [Google Scholar]
- 121.Hwang S., Lim J.W., Kim H. Inhibitory effect of lycopene on amyloid-β-induced apoptosis in neuronal cells. Nutrients. 2017;9:883. doi: 10.3390/nu9080883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bin-Jumah M.N., Nadeem M.S., Gilani S.J., Mubeen B., Ullah I., Alzarea S.I., et al. Lycopene: a natural arsenal in the war against oxidative stress and cardiovascular diseases. Antioxidants. 2022;11:232. doi: 10.3390/antiox11020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu N.W., Yin X.L., Lin R., Fan X.L., Chen S.J., Zhu Y.M., et al. Possible mechanisms of lycopene amelioration of learning and memory impairment in rats with vascular dementia. Neural Regen Res. 2020;15:332–341. doi: 10.4103/1673-5374.265565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaur H., Chauhan S., Sandhir R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson's disease. Neurochem Res. 2011;36:1435–1443. doi: 10.1007/s11064-011-0469-3. [DOI] [PubMed] [Google Scholar]
- 125.Liu C.B., Wang R., Pan H.B., Ding Q.F., Lu F.B. Effect of lycopene on oxidative stress and behavioral deficits in rotenone induced model of Parkinson's disease. Chin J Appl Physiol. 2013;29:380–384. [PubMed] [Google Scholar]
- 126.Huang C., Wen C., Yang M., Gan D., Fan C., Li A., et al. Lycopene protects against t-BHP-induced neuronal oxidative damage and apoptosis via activation of the PI3K/Akt pathway. Mol Biol Rep. 2019;46:3387–3397. doi: 10.1007/s11033-019-04801-y. [DOI] [PubMed] [Google Scholar]
- 127.Chen D., Huang C., Chen Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed Pharmacother. 2019;111:791–801. doi: 10.1016/j.biopha.2018.12.151. [DOI] [PubMed] [Google Scholar]
- 128.Qu M., Jiang Z., Liao Y., Song Z., Nan X. Lycopene prevents amyloid [beta]-induced mitochondrial oxidative stress and dysfunctions in cultured rat cortical neurons. Neurochem Res. 2016;41:1354–1364. doi: 10.1007/s11064-016-1837-9. [DOI] [PubMed] [Google Scholar]
- 129.Ambati R.R., Phang S.M., Ravi S., Aswathanarayana R.G. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review. Mar Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Manabe Y., Komatsu T., Seki S., Sugawara T. Dietary astaxanthin can accumulate in the brain of rats. Biosci Biotechnol Biochem. 2018;82:1433–1436. doi: 10.1080/09168451.2018.1459467. [DOI] [PubMed] [Google Scholar]
- 131.Grimmig B., Hudson C., Moss L., Peters M., Subbarayan M., Weeber E.J., et al. Astaxanthin supplementation modulates cognitive function and synaptic plasticity in young and aged mice. GeroScience. 2019;41:77–87. doi: 10.1007/s11357-019-00051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ono A., Sekita K., Saitoh M., Umemura T., Ogawa Y., Furuya T., et al. A 13-week subchronic oral toxicity study of Haematococcus color in F344 rats. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyujo Hokoku. 1999;117:91–98. [PubMed] [Google Scholar]
- 133.Kim S.H., Kim H. Inhibitory effect of astaxanthin on oxidative stress-induced mitochondrial dysfunction—a mini-review. Nutrients. 2018;10:1137. doi: 10.3390/nu10091137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Deng X., Wang M., Hu S., Feng Y., Shao Y., Xie Y., et al. The neuroprotective effect of astaxanthin on pilocarpine-induced status epilepticus in rats. Front Cell Neurosci. 2019;13:123. doi: 10.3389/fncel.2019.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin W.N., Kapupara K., Wen Y.T., Chen Y.H., Pan I.H., Tsai R.K. Haematococcus pluvialis-derived astaxanthin is a potential neuroprotective agent against optic nerve ischemia. Mar Drugs. 2020;18:85. doi: 10.3390/md18020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ye Q., Huang B., Zhang X., Zhu Y., Chen X. Astaxanthin protects against MPP+-induced oxidative stress in PC12 cells via the HO-1/NOX2 axis. BMC Neurosci. 2012;13:156. doi: 10.1186/1471-2202-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chang C.S., Chang C.L., Lai G.H. Reactive oxygen species scavenging activities in a chemiluminescence model and neuroprotection in rat pheochromocytoma cells by astaxanthin, beta-carotene, and canthaxanthin. Kaohsiung J Med Sci. 2013;29:412–421. doi: 10.1016/j.kjms.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 138.Shen D.F., Qi H.P., Ma C., Chang M.X., Zhang W.N., Song R.R. Astaxanthin suppresses endoplasmic reticulum stress and protects against neuron damage in Parkinson's disease by regulating miR-7/SNCA axis. Neurosci Res. 2021;165:51–60. doi: 10.1016/j.neures.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 139.Grimmig B., Daly L., Subbarayan M., Hudson C., Williamson R., Nash K., et al. Astaxanthin is neuroprotective in an aged mouse model of Parkinson's disease. Oncotarget. 2017;9:10388–10401. doi: 10.18632/oncotarget.23737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schnorr C.E., Morrone Mda S., Simões-Pires A., Bittencourt Lda S., Zeidán-Chuliá F., Moreira J.C. Supplementation of adult rats with moderate amounts of β-carotene modulates the redox status in plasma without exerting pro-oxidant effects in the brain: a safer alternative to food fortification with vitamin A?. Nutrients. 2014;6:5572–5582. doi: 10.3390/nu6125572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hira S., Saleem U., Anwar F., Sohail M.F., Raza Z., Ahmad B. β-Carotene: a natural compound improves cognitive impairment and oxidative stress in a mouse model of streptozotocin-induced Alzheimer's disease. Biomolecules. 2019;9:441. doi: 10.3390/biom9090441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen P., Li L., Gao Y., Xie Z., Zhang Y., Pan Z., et al. β-Carotene provides neuroprotection after experimental traumatic brain injury via the Nrf2–ARE pathway. J Integr Neurosci. 2019;18:153–161. doi: 10.31083/j.jin.2019.02.120. [DOI] [PubMed] [Google Scholar]
- 143.Li F.J., Shen L., Ji H.F. Dietary intakes of vitamin E, vitamin C, and β-carotene and risk of Alzheimer's disease: a meta-analysis. J Alzheimers Dis. 2012;31:253–258. doi: 10.3233/JAD-2012-120349. [DOI] [PubMed] [Google Scholar]
- 144.Mohn E.S., Erdman J.W., Jr., Kuchan M.J., Neuringer M., Johnson E.J. Lutein accumulates in subcellular membranes of brain regions in adult rhesus macaques: relationship to DHA oxidation products. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hammond B.R., Jr., Miller L.S., Bello M.O., Lindbergh C.A., Mewborn C., Renzi-Hammond L.M. Effects of lutein/zeaxanthin supplementation on the cognitive function of community dwelling older adults: a randomized, double-masked, placebo-controlled trial. Front Aging Neurosci. 2017;9:254. doi: 10.3389/fnagi.2017.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stringham J.M., Johnson E.J., Hammond B.R. Lutein across the lifespan: from childhood cognitive performance to the aging eye and brain. Curr Dev Nutr. 2019;3:nzz066. doi: 10.1093/cdn/nzz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sun Y.X., Liu T., Dai X.L., Zheng Q.S., Hui B.D., Jiang Z.F. Treatment with lutein provides neuroprotection in mice subjected to transient cerebral ischemia. J Asian Nat Prod Res. 2014;16:1084–1093. doi: 10.1080/10286020.2014.939584. [DOI] [PubMed] [Google Scholar]
- 148.Li H., Huang C., Zhu J., Gao K., Fang J., Li H. Lutein suppresses oxidative stress and inflammation by Nrf2 activation in an osteoporosis rat model. Med Sci Mon Int Med J Exp Clin Res. 2018;24:5071–5075. doi: 10.12659/MSM.908699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Walk A.M., Edwards C.G., Baumgartner N.W., Chojnacki M.R., Covello A.R., Reeser G.E., et al. The role of retinal carotenoids and age on neuroelectric indices of attentional control among early to middle-aged adults. Front Aging Neurosci. 2017;9:183. doi: 10.3389/fnagi.2017.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nataraj J., Manivasagam T., Thenmozhi A.J., Essa M.M. Lutein protects dopaminergic neurons against MPTP-induced apoptotic death and motor dysfunction by ameliorating mitochondrial disruption and oxidative stress. Nutr Neurosci. 2016;19:237–246. doi: 10.1179/1476830515Y.0000000010. [DOI] [PubMed] [Google Scholar]
- 151.Badgujar L., Saraf M. Behavioral, biochemical and molecular modulation in brain and spinal cord: a potential antinociceptive-antidepressant mechanism of Xanthophylls [abstract] FASEB J. 2015;29:LB502. [Google Scholar]
- 152.Fernandes E.J., Poetini M.R., Barrientos M.S., Bortolotto V.C., Araujo S.M., Santos Musachio E.A., et al. Exposure to lutein-loaded nanoparticles attenuates Parkinson's model-induced damage in Drosophila melanogaster: restoration of dopaminergic and cholinergic system and oxidative stress indicators. Chem Biol Interact. 2021;340 doi: 10.1016/j.cbi.2021.109431. [DOI] [PubMed] [Google Scholar]
- 153.Kim S.M., Jung Y.J., Kwon O.N., Cha K.H., Um B.H., Chung D., et al. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl Biochem Biotechnol. 2012;166:1843–1855. doi: 10.1007/s12010-012-9602-2. [DOI] [PubMed] [Google Scholar]
- 154.Yu J., Lin J.J., Yu R., He S., Wang Q.W., Cui W., et al. Fucoxanthin prevents H2O2-induced neuronal apoptosis via concurrently activating the PI3-K/Akt cascade and inhibiting the ERK pathway. Food Nutr Res. 2017;61 doi: 10.1080/16546628.2017.1304678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li N., Gao X., Zheng L., Huang Q., Zeng F., Chen H., et al. Advances in fucoxanthin chemistry and management of neurodegenerative diseases. Phytomedicine. 2022;105 doi: 10.1016/j.phymed.2022.154352. [DOI] [PubMed] [Google Scholar]
- 156.Zhao X., Zhang S., An C., Zhang H., Sun Y., Li Y., et al. Neuroprotective effect of fucoxanthin on β-amyloid-induced cell death. J Chin Pharmaceut Sci. 2015;24:467–474. [Google Scholar]
- 157.Zhang L., Wang H., Fan Y., Gao Y., Li X., Hu Z., et al. Fucoxanthin provides neuroprotection in models of traumatic brain injury via the Nrf2–ARE and Nrf2–autophagy pathways. Sci Rep. 2017;7 doi: 10.1038/srep46763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hu L., Chen W., Tian F., Yuan C., Wang H., Yue H. Neuroprotective role of fucoxanthin against cerebral ischemic/reperfusion injury through activation of Nrf2/HO-1 signaling. Biomed Pharmacother. 2018;106:1484–1489. doi: 10.1016/j.biopha.2018.07.088. [DOI] [PubMed] [Google Scholar]
- 159.Sun G., Xin T., Zhang R., Liu C., Pang Q. Fucoxanthin attenuates behavior deficits and neuroinflammatory response in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease in mice. Phcog Mag. 2020;16:51. [Google Scholar]
- 160.Treml J., Šmejkal K. Flavonoids as potent scavengers of hydroxyl radicals. Compr Rev Food Sci Food Saf. 2016;15:720–738. doi: 10.1111/1541-4337.12204. [DOI] [PubMed] [Google Scholar]
- 161.Sharma K., Verma R., Kumar D., Nepovimova E., Kuča K., Kumar A., et al. Ethnomedicinal plants used for the treatment of neurodegenerative diseases in Himachal Pradesh, India in Western Himalaya. J Ethnopharmacol. 2022;293 doi: 10.1016/j.jep.2022.115318. [DOI] [PubMed] [Google Scholar]
- 162.Khan A., Ikram M., Hahm J.R., Kim M.O. Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: special focus on neurological disorders. Antioxidants. 2020;9:609. doi: 10.3390/antiox9070609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hajialyani M., Hosein Farzaei M., Echeverría J., Nabavi S.M., Uriarte E., Sobarzo-Sánchez E. Hesperidin as a neuroprotective agent: a review of animal and clinical evidence. Molecules. 2019;24:648. doi: 10.3390/molecules24030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou D., Bai Z., Guo T., Li J., Li Y., Hou Y., et al. Dietary flavonoids and human top-ranked diseases: the perspective of in vivo bioactivity and bioavailability. Trends Food Sci Technol. 2022;120:374–386. [Google Scholar]
- 165.Wei B.B., Liu M.Y., Zhong X., Yao W.F., Wei M.J. Increased BBB permeability contributes to EGCG-caused cognitive function improvement in natural aging rats: pharmacokinetic and distribution analyses. Acta Pharmacol Sin. 2019;40:1490–1500. doi: 10.1038/s41401-019-0243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shanmugam T., Abdulla S., Yakulasamy V., Selvaraj M., Mathan R. A mechanism underlying the neurotoxicity induced by sodium fluoride and its reversal by epigallocatechin gallate in the rat hippocampus: involvement of NrF2/Keap-1 signaling pathway. J Basic Appl Zool. 2018;79:17. [Google Scholar]
- 167.Zhang Y., Xu Y.Y., Sun W.J., Zhang M.H., Zheng Y.F., Shen H.M., et al. FBS or BSA inhibits EGCG induced cell death through covalent binding and the reduction of intracellular ROS production. BioMed Res Int. 2016;2016 doi: 10.1155/2016/5013409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ettcheto M., Cano A., Manzine P.R., Busquets O., Verdaguer E., Castro-Torres R.D., et al. Epigallocatechin-3-gallate (EGCG) improves cognitive deficits aggravated by an obesogenic diet through modulation of unfolded protein response in APPswe/PS1dE9 mice. Mol Neurobiol. 2020;57:1814–1827. doi: 10.1007/s12035-019-01849-6. [DOI] [PubMed] [Google Scholar]
- 169.Cano A., Ettcheto M., Chang J.H., Barroso E., Espina M., Kühne B.A., et al. Dual-drug loaded nanoparticles of epigallocatechin-3-gallate (EGCG)/ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer's disease mice model. J Control Release. 2019;301:62–75. doi: 10.1016/j.jconrel.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Teng Y., Zhao J., Ding L., Ding Y., Zhou P. Complex of EGCG with Cu(II) suppresses amyloid aggregation and Cu(II)-induced cytotoxicity of α-synuclein. Molecules. 2019;24:2940. doi: 10.3390/molecules24162940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bitu Pinto N., da Silva Alexandre B., Neves K.R., Silva A.H., Leal L.K., Viana G.S. Neuroprotective properties of the standardized extract from Camellia sinensis (green tea) and its main bioactive components, epicatechin and epigallocatechin gallate, in the 6-OHDA model of Parkinson's disease. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/161092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bernatoniene J., Kopustinskiene D.M. The role of catechins in cellular responses to oxidative stress. Molecules. 2018;23:965. doi: 10.3390/molecules23040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pan A.L., Hasalliu E., Hasalliu M., Angulo J.A. Epigallocatechin gallate mitigates the methamphetamine-induced striatal dopamine terminal toxicity by preventing oxidative stress in the mouse brain. Neurotox Res. 2020;37:883–892. doi: 10.1007/s12640-020-00177-1. [DOI] [PubMed] [Google Scholar]
- 174.Dragicevic N., Smith A., Lin X., Yuan F., Copes N., Delic V., et al. Green tea epigallocatechin-3-gallate (EGCG) and other flavonoids reduce Alzheimer's amyloid-induced mitochondrial dysfunction. J Alzheimers Dis. 2011;26:507–521. doi: 10.3233/JAD-2011-101629. [DOI] [PubMed] [Google Scholar]
- 175.Figueira I., Tavares L., Jardim C., Costa I., Terrasso A.P., Almeida A.F., et al. Blood–brain barrier transport and neuroprotective potential of blackberry-digested polyphenols: an in vitro study. Eur J Nutr. 2019;58:113–130. doi: 10.1007/s00394-017-1576-y. [DOI] [PubMed] [Google Scholar]
- 176.Li P., Feng D., Yang D., Li X., Sun J., Wang G., et al. Protective effects of anthocyanins on neurodegenerative diseases. Trends Food Sci Technol. 2021;117:205–217. [Google Scholar]
- 177.Cásedas G., González-Burgos E., Smith C., López V., Gómez-Serranillos M.P. Sour cherry (Prunus cerasus L.) juice protects against hydrogen peroxide-induced neurotoxicity by modulating the antioxidant response. J Funct Foods. 2018;46:243–249. [Google Scholar]
- 178.Ali T., Kim T., Rehman S.U., Khan M.S., Amin F.U., Khan M., et al. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer's disease. Mol Neurobiol. 2018;55:6076–6093. doi: 10.1007/s12035-017-0798-6. [DOI] [PubMed] [Google Scholar]
- 179.Meng L.S., Li B., Li D.N., Wang Y.H., Lin Y., Meng X.J., et al. Cyanidin-3-O-glucoside attenuates amyloid-beta1–40-induced oxidative stress and apoptosis in SH-SY5Y cells through a Nrf2 mechanism. J Funct Foods. 2017;38:474–485. [Google Scholar]
- 180.Spagnuolo C., Moccia S., Russo G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur J Med Chem. 2018;153:105–115. doi: 10.1016/j.ejmech.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 181.dos Santos N.M., Batista P.B., Batista Â.G., Júnior M.R.M. Current evidence on cognitive improvement and neuroprotection promoted by anthocyanins. Curr Opin Food Sci. 2019;26:71–78. [Google Scholar]
- 182.Igwe E.O., Charlton K.E., Roodenrys S., Kent K., Fanning K., Netzel M.E. Anthocyanin-rich plum juice reduces ambulatory blood pressure but not acute cognitive function in younger and older adults: a pilot crossover dose-timing study. Nutr Res. 2017;47:28–43. doi: 10.1016/j.nutres.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 183.Lamport D.J., Lawton C.L., Merat N., Jamson H., Myrissa K., Hofman D., et al. Concord grape juice, cognitive function, and driving performance: a 12-wk, placebo-controlled, randomized crossover trial in mothers of preteen children. Am J Clin Nutr. 2016;103:775–783. doi: 10.3945/ajcn.115.114553. [DOI] [PubMed] [Google Scholar]
- 184.Anand David A.V., Arulmoli R., Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharm Rev. 2016;10:84–89. doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sharifi-Rad J., Rodrigues C.F., Sharopov F., Docea A.O. Can Karaca A, Sharifi-Rad M, et al. Diet, lifestyle and cardiovascular diseases: linking pathophysiology to cardioprotective effects of natural bioactive compounds. Int J Environ Res Publ Health. 2020;17:2326. doi: 10.3390/ijerph17072326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Singh A., Patel S.K., Kumar P., Das K.C., Verma D., Sharma R., et al. Quercetin acts as a P-gp modulator via impeding signal transduction from nucleotide-binding domain to transmembrane domain. J Biomol Struct Dyn. 2022;40:4507–4515. doi: 10.1080/07391102.2020.1858966. [DOI] [PubMed] [Google Scholar]
- 187.Firgany A.E.L., Sarhan N.R. Quercetin mitigates monosodium glutamate-induced excitotoxicity of the spinal cord motoneurons in aged rats via p38 MAPK inhibition. Acta Histochem. 2020;122 doi: 10.1016/j.acthis.2020.151554. [DOI] [PubMed] [Google Scholar]
- 188.Moujahed S., Ruiz A., Hallegue D., Sakly M. Quercetin alleviates styrene oxide-induced cytotoxicity in cortical neurons in vitro via modulation of oxidative stress and apoptosis. Drug Chem Toxicol. 2022;45:1634–1643. doi: 10.1080/01480545.2020.1851706. [DOI] [PubMed] [Google Scholar]
- 189.Lee B.K., Jung Y.S. Allium cepa extract and quercetin protect neuronal cells from oxidative stress via PKC-ε inactivation/ERK1/2 activation. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/2495624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Karuppagounder S.S., Madathil S.K., Pandey M., Haobam R., Rajamma U., Mohanakumar K.P. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson's disease in rats. Neuroscience. 2013;236:136–148. doi: 10.1016/j.neuroscience.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 191.Magalingam K.B., Radhakrishnan A., Haleagrahara N. Protective effects of quercetin glycosides, rutin, and isoquercetrin against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity in rat pheochromocytoma (PC-12) cells. Int J Immunopathol Pharmacol. 2016;29:30–39. doi: 10.1177/0394632015613039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Khan H., Ullah H., Aschner M., Cheang W.S., Akkol E.K. Neuroprotective effects of quercetin in Alzheimer's disease. Biomolecules. 2020;10:59. doi: 10.3390/biom10010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Oliveira A.I., Pinho C., Sarmento B., Dias A.C.P. Quercetin-biapigenin nanoparticles are effective to penetrate the blood–brain barrier. Drug Deliv Transl Res. 2022;12:267–281. doi: 10.1007/s13346-021-00917-6. [DOI] [PubMed] [Google Scholar]
- 194.Huang W.Y., Zhang H.C., Liu W.X., Li C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J Zhejiang Univ Sci B. 2012;13:94–102. doi: 10.1631/jzus.B1100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Habtemariam S. Rutin as a natural therapy for Alzheimer's disease: insights into its mechanisms of action. Curr Med Chem. 2016;23:860–873. doi: 10.2174/0929867323666160217124333. [DOI] [PubMed] [Google Scholar]
- 196.Yu X.L., Li Y.N., Zhang H., Su Y.J., Zhou W.W., Zhang Z.P., et al. Rutin inhibits amylin-induced neurocytotoxicity and oxidative stress. Food Funct. 2015;6:3296–3306. doi: 10.1039/c5fo00500k. [DOI] [PubMed] [Google Scholar]
- 197.Jiménez-Aliaga K., Bermejo-Bescós P., Benedí J., Martín-Aragón S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011;89:939–945. doi: 10.1016/j.lfs.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 198.Pan R.Y., Ma J., Kong X.X., Wang X.F., Li S.S., Qi X.L., et al. Sodium rutin ameliorates Alzheimer's disease-like pathology by enhancing microglial amyloid-β clearance. Sci Adv. 2019;5 doi: 10.1126/sciadv.aau6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang S.W., Wang Y.J., Su Y.J., Zhou W.W., Yang S.G., Zhang R., et al. Rutin inhibits β-amyloid aggregation and cytotoxicity, attenuates oxidative stress, and decreases the production of nitric oxide and proinflammatory cytokines. Neurotoxicology. 2012;33:482–490. doi: 10.1016/j.neuro.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 200.Hu B., Dai F., Fan Z., Ma G., Tang Q., Zhang X. Nanotheranostics: Congo red/rutin-MNPs with enhanced magnetic resonance imaging and H2O2-responsive therapy of Alzheimer's disease in APPswe/PS1dE9 transgenic mice. Adv Mater. 2015;27:5499–5505. doi: 10.1002/adma.201502227. [DOI] [PubMed] [Google Scholar]
- 201.Suganya S.N., Sumathi T. Effect of rutin against a mitochondrial toxin, 3-nitropropionicacid induced biochemical, behavioral and histological alterations-a pilot study on Huntington's disease model in rats. Metab Brain Dis. 2017;32:471–481. doi: 10.1007/s11011-016-9929-4. [DOI] [PubMed] [Google Scholar]
- 202.Nkpaa K.W., Onyeso G.I. Rutin attenuates neurobehavioral deficits, oxidative stress, neuro-inflammation and apoptosis in fluoride treated rats. Neurosci Lett. 2018;682:92–99. doi: 10.1016/j.neulet.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 203.AbouZid S.F., Chen S.N., McAlpine J.B., Friesen J.B., Pauli G.F. Silybum marianum pericarp yields enhanced silymarin products. Fitoterapia. 2016;112:136–143. doi: 10.1016/j.fitote.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yin F., Liu J., Ji X., Wang Y., Zidichouski J., Zhang J. Silibinin: a novel inhibitor of Aβ aggregation. Neurochem Int. 2011;58:399–403. doi: 10.1016/j.neuint.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 205.Devi K.P., Malar D.S., Braidy N., Nabavi S.M., Nabavi S.F. A mini review on the chemistry and neuroprotective effects of silymarin. Curr Drug Targets. 2017;18:1529–1536. doi: 10.2174/1389450117666161227125121. [DOI] [PubMed] [Google Scholar]
- 206.Kumar J., Park K.C., Awasthi A., Prasad B. Silymarin extends lifespan and reduces proteotoxicity in C. elegans Alzheimer's model. CNS Neurol Disord: Drug Targets. 2015;14:295–302. doi: 10.2174/1871527314666150116110212. [DOI] [PubMed] [Google Scholar]
- 207.Haddadi R., Eyvari-Brooshghalan S., Nayebi A.M., Sabahi M., Ahmadi S.A. Neuronal degeneration and oxidative stress in the SNc of 6-OHDA intoxicated rats; improving role of silymarin long-term treatment. Naunyn-Schmiedeberg’s Arch Pharmacol. 2020;393:2427–2437. doi: 10.1007/s00210-020-01954-7. [DOI] [PubMed] [Google Scholar]
- 208.El-Marasy S.A., Abd-Elsalam R.M., Ahmed-Farid O.A. Ameliorative effect of silymarin on scopolamine-induced dementia in rats. Open Access Maced J Med Sci. 2018;6:1215–1224. doi: 10.3889/oamjms.2018.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tabaa M.M.E., Aboalazm H.M., Shaalan M., Khedr N.F. Silymarin constrains diacetyl-prompted oxidative stress and neuroinflammation in rats: involvements of Dyn/GDNF and MAPK signaling pathway. Inflammopharmacology. 2022;30:961–980. doi: 10.1007/s10787-022-00961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Elsawy H., Alzahrani A.M., Alfwuaires M., Sedky A., El-Trass E.E., Mahmoud O., et al. Analysis of silymarin-modulating effects against acrylamide-induced cerebellar damage in male rats: biochemical and pathological markers. J Chem Neuroanat. 2021;115 doi: 10.1016/j.jchemneu.2021.101964. [DOI] [PubMed] [Google Scholar]
- 211.Yang K.Y., Hwang du H., Yousaf A.M., Kim D.W., Shin Y.J., Bae O.N., et al. Silymarin-loaded solid nanoparticles provide excellent hepatic protection: physicochemical characterization and in vivo evaluation. Int J Nanomed. 2013;8:3333–3343. doi: 10.2147/IJN.S50683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chang H.C., Churchwell M.I., Delclos K.B., Newbold R.R., Doerge D.R. Mass spectrometric determination of genistein tissue distribution in diet-exposed Sprague–Dawley rats. J Nutr. 2000;130:1963–1970. doi: 10.1093/jn/130.8.1963. [DOI] [PubMed] [Google Scholar]
- 213.Kładna A., Berczyński P., Kruk I., Piechowska T., Aboul-Enein H.Y. Studies on the antioxidant properties of some phytoestrogens. Luminescence. 2016;31:1201–1206. doi: 10.1002/bio.3091. [DOI] [PubMed] [Google Scholar]
- 214.Mirahmadi S.M., Shahmohammadi A., Rousta A.M., Azadi M.R., Fahanik-Babaei J., Baluchnejadmojarad T., et al. Soy isoflavone genistein attenuates lipopolysaccharide-induced cognitive impairments in the rat via exerting anti-oxidative and anti-inflammatory effects. Cytokine. 2018;104:151–159. doi: 10.1016/j.cyto.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 215.Zhao B. Natural antioxidants for neurodegenerative diseases. Mol Neurobiol. 2005;31:283–293. doi: 10.1385/MN:31:1-3:283. [DOI] [PubMed] [Google Scholar]
- 216.Guo J., Yang G., He Y., Xu H., Fan H., An J., et al. Involvement of α7nAChR in the protective effects of genistein against β-amyloid-induced oxidative stress in neurons via a PI3K/Akt/Nrf2 pathway-related mechanism. Cell Mol Neurobiol. 2021;41:377–393. doi: 10.1007/s10571-020-01009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rumman M., Pandey S., Singh B., Gupta M., Ubaid S., Mahdi A.A. Genistein prevents hypoxia-induced cognitive dysfunctions by ameliorating oxidative stress and inflammation in the hippocampus. Neurotox Res. 2021;39:1123–1133. doi: 10.1007/s12640-021-00353-x. [DOI] [PubMed] [Google Scholar]
- 218.Li Y., Zhang J.J., Chen R.J., Chen L., Chen S., Yang X.F., et al. Genistein mitigates oxidative stress and inflammation by regulating Nrf2/HO-1 and NF-κB signaling pathways in hypoxic-ischemic brain damage in neonatal mice. Ann Transl Med. 2022;10:32. doi: 10.21037/atm-21-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Parhiz H., Roohbakhsh A., Soltani F., Rezaee R., Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 2015;29:323–331. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 220.Ikram M., Muhammad T., Rehman S.U., Khan A., Jo M.G., Ali T., et al. Hesperetin confers neuroprotection by regulating Nrf2/TLR4/NF-κB signaling in an Aβ mouse model. Mol Neurobiol. 2019;56:6293–6309. doi: 10.1007/s12035-019-1512-7. [DOI] [PubMed] [Google Scholar]
- 221.Muhammad T., Ikram M., Ullah R., Rehman S.U., Kim M.O. Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients. 2019;11:648. doi: 10.3390/nu11030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kheradmand E., Hajizadeh Moghaddam A., Zare M. Neuroprotective effect of hesperetin and nano-hesperetin on recognition memory impairment and the elevated oxygen stress in rat model of Alzheimer's disease. Biomed Pharmacother. 2018;97:1096–1101. doi: 10.1016/j.biopha.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 223.Ishola I.O., Jacinta A.A., Adeyemi O.O. Cortico-hippocampal memory enhancing activity of hesperetin on scopolamine-induced amnesia in mice: role of antioxidant defense system, cholinergic neurotransmission and expression of BDNF. Metab Brain Dis. 2019;34:979–989. doi: 10.1007/s11011-019-00409-0. [DOI] [PubMed] [Google Scholar]
- 224.Kiasalari Z., Khalili M., Baluchnejadmojarad T., Roghani M. Protective effect of oral hesperetin against unilateral striatal 6-hydroxydopamine damage in the rat. Neurochem Res. 2016;41:1065–1072. doi: 10.1007/s11064-015-1796-6. [DOI] [PubMed] [Google Scholar]
- 225.Zhu X., Zhang Y.M., Zhang M.Y., Chen Y.J., Liu Y.W. Hesperetin ameliorates diabetes-associated anxiety and depression-like behaviors in rats via activating Nrf2/ARE pathway. Metab Brain Dis. 2021;36:1969–1983. doi: 10.1007/s11011-021-00785-6. [DOI] [PubMed] [Google Scholar]
- 226.Lai M.C., Liu W.Y., Liou S.S., Liu I.M. The citrus flavonoid hesperetin encounters diabetes-mediated Alzheimer-type neuropathologic changes through relieving advanced glycation end-products inducing endoplasmic reticulum stress. Nutrients. 2022;14:745. doi: 10.3390/nu14040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Vučić V., Grabež M., Trchounian A., Arsić A. Composition and potential health benefits of pomegranate: a review. Curr Pharmaceut Des. 2019;25:1817–1827. doi: 10.2174/1381612825666190708183941. [DOI] [PubMed] [Google Scholar]
- 228.Rudrapal M., Khairnar S.J., Khan J., Dukhyil A.B., Ansari M.A., Alomary M.N., et al. Dietary polyphenols and their role in oxidative stress-induced human diseases: insights into protective effects, antioxidant potentials and mechanism(s) of action. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.806470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yan L., Guo M.S., Zhang Y., Yu L., Wu J.M., Tang Y., et al. Dietary plant polyphenols as the potential drugs in neurodegenerative diseases: current evidence, advances, and opportunities. Oxid Med Cell Longev. 2022;2022 doi: 10.1155/2022/5288698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tian B., Liu J. Resveratrol: a review of plant sources, synthesis, stability, modification and food application. J Sci Food Agric. 2020;100:1392–1404. doi: 10.1002/jsfa.10152. [DOI] [PubMed] [Google Scholar]
- 231.Ji H.F., Zhang H.Y. Multipotent natural agents to combat Alzheimer's disease. Functional spectrum and structural features. Acta Pharmacol Sin. 2008;29:143–151. doi: 10.1111/j.1745-7254.2008.00752.x. [DOI] [PubMed] [Google Scholar]
- 232.Renaud J., Martinoli M.G. Considerations for the use of polyphenols as therapies in neurodegenerative diseases. Int J Mol Sci. 2019;20:1883. doi: 10.3390/ijms20081883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Griñán-Ferré C., Bellver-Sanchis A., Izquierdo V., Corpas R., Roig-Soriano J., Chillón M., et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer's disease pathology: from antioxidant to epigenetic therapy. Ageing Res Rev. 2021;67 doi: 10.1016/j.arr.2021.101271. [DOI] [PubMed] [Google Scholar]
- 234.Xiong S., Liu W., Zhou Y., Mo Y., Liu Y., Chen X., et al. Enhancement of oral bioavailability and anti-Parkinsonian efficacy of resveratrol through a nanocrystal formulation. Asian J Pharm Sci. 2020;15:518–528. doi: 10.1016/j.ajps.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Huang J., Huang N., Xu S., Luo Y., Li Y., Jin H., et al. Signaling mechanisms underlying inhibition of neuroinflammation by resveratrol in neurodegenerative diseases. J Nutr Biochem. 2021;88 doi: 10.1016/j.jnutbio.2020.108552. [DOI] [PubMed] [Google Scholar]
- 236.Rahman M.H., Akter R., Bhattacharya T., Abdel-Daim M.M., Alkahtani S., Arafah M.W., et al. Resveratrol and neuroprotection: impact and its therapeutic potential in Alzheimer's disease. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.619024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Farkhondeh T., Folgado S.L., Pourbagher-Shahri A.M., Ashrafizadeh M., Samarghandian S. The therapeutic effect of resveratrol: focusing on the Nrf2 signaling pathway. Biomed Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110234. [DOI] [PubMed] [Google Scholar]
- 238.Freyssin A., Page G., Fauconneau B., Rioux Bilan A. Natural polyphenols effects on protein aggregates in Alzheimer's and Parkinson's prion-like diseases. Neural Regen Res. 2018;13:955–961. doi: 10.4103/1673-5374.233432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cosín-Tomàs M., Senserrich J., Arumí-Planas M., Alquézar C., Pallàs M., Martín-Requero Á, et al. Role of resveratrol and selenium on oxidative stress and expression of antioxidant and anti-aging genes in immortalized lymphocytes from Alzheimer's disease patients. Nutrients. 2019;11:1764. doi: 10.3390/nu11081764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Reddy P.H., Manczak M., Yin X., Grady M.C., Mitchell A., Tonk S., et al. Protective effects of Indian spice curcumin against amyloid-β in Alzheimer's disease. J Alzheimers Dis. 2018;61:843–866. doi: 10.3233/JAD-170512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Silva R.F.M., Pogačnik L. Polyphenols from food and natural products: neuroprotection and safety. Antioxidants. 2020;9:61. doi: 10.3390/antiox9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fikry H., Saleh L.A., Abdel Gawad S. Neuroprotective effects of curcumin on the cerebellum in a rotenone-induced Parkinson's disease model. CNS Neurosci Ther. 2022;28:732–748. doi: 10.1111/cns.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Uğuz A.C., Öz A., Nazıroğlu M. Curcumin inhibits apoptosis by regulating intracellular calcium release, reactive oxygen species and mitochondrial depolarization levels in SH-SY5Y neuronal cells. J Recept Signal Transduct Res. 2016;36:395–401. doi: 10.3109/10799893.2015.1108337. [DOI] [PubMed] [Google Scholar]
- 244.Maiti P., Dunbar G.L. Comparative neuroprotective effects of dietary curcumin and solid lipid curcumin particles in cultured mouse neuroblastoma cells after exposure to Aβ42. Int J Alzheimer's Dis. 2017;2017 doi: 10.1155/2017/4164872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Maiti P., Dunbar G.L. Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. Int J Mol Sci. 2018;19:1637. doi: 10.3390/ijms19061637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Cui Q., Li X., Zhu H. Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway. Mol Med Rep. 2016;13:1381–1388. doi: 10.3892/mmr.2015.4657. [DOI] [PubMed] [Google Scholar]
- 247.Xu J., Zhou L., Weng Q., Xiao L., Li Q. Curcumin analogues attenuate Aβ25–35-induced oxidative stress in PC12 cells via Keap1/Nrf2/HO-1 signaling pathways. Chem Biol Interact. 2019;305:171–179. doi: 10.1016/j.cbi.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 248.Meitha K., Pramesti Y., Suhandono S. Reactive oxygen species and antioxidants in postharvest vegetables and fruits. Int J Food Sci. 2020;2020 doi: 10.1155/2020/8817778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Caruso G., Godos J., Privitera A., Lanza G., Castellano S., Chillemi A., et al. Phenolic acids and prevention of cognitive decline: polyphenols with a neuroprotective role in cognitive disorders and Alzheimer's disease. Nutrients. 2022;14:819. doi: 10.3390/nu14040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Szwajgier D., Borowiec K., Pustelniak K. The neuroprotective effects of phenolic acids: molecular mechanism of action. Nutrients. 2017;9:477. doi: 10.3390/nu9050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Habtemariam S. Protective effects of caffeic acid and the Alzheimer's brain: an update. Mini Rev Med Chem. 2017;17:667–674. doi: 10.2174/1389557516666161130100947. [DOI] [PubMed] [Google Scholar]
- 252.Colonnello A., Aguilera-Portillo G., Rubio-López L.C., Robles-Bañuelos B., Rangel-López E., Cortez-Núñez S., et al. Comparing the neuroprotective effects of caffeic acid in rat cortical slices and Caenorhabditis elegans: involvement of Nrf2 and SKN-1 signaling pathways. Neurotox Res. 2020;37:326–337. doi: 10.1007/s12640-019-00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Winter A.N., Ross E.K., Khatter S., Miller K., Linseman D.A. Chemical basis for the disparate neuroprotective effects of the anthocyanins, callistephin and kuromanin, against nitrosative stress. Free Radic Biol Med. 2017;103:23–34. doi: 10.1016/j.freeradbiomed.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 254.Guan S., Zhang X.L., Ge D., Liu T.Q., Ma X.H., Cui Z.F. Protocatechuic acid promotes the neuronal differentiation and facilitates survival of phenotypes differentiated from cultured neural stem and progenitor cells. Eur J Pharmacol. 2011;670:471–478. doi: 10.1016/j.ejphar.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 255.Recart V.M., Spohr L., Soares M.S.P., de Mattos B.D.S., Bona N.P., Pedra N.S., et al. Gallic acid protects cerebral cortex, hippocampus, and striatum against oxidative damage and cholinergic dysfunction in an experimental model of manic-like behavior: comparison with lithium effects. Int J Dev Neurosci. 2021;81:167–178. doi: 10.1002/jdn.10086. [DOI] [PubMed] [Google Scholar]
- 256.Teixeira J., Oliveira C., Amorim R., Cagide F., Garrido J., Ribeiro J.A., et al. Development of hydroxybenzoic-based platforms as a solution to deliver dietary antioxidants to mitochondria. Sci Rep. 2017;7:6842. doi: 10.1038/s41598-017-07272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ojha S., Javed H., Azimullah S., Abul Khair S.B., Haque M.E. Neuroprotective potential of ferulic acid in the rotenone model of Parkinson's disease. Drug Des Dev Ther. 2015;9:5499–5510. doi: 10.2147/DDDT.S90616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Anadón A., Ares I., Martínez-Larrañaga M.R., Martínez M.A. In: Nutraceuticals efficacy, safety and toxicity. 2nd ed. Gupta R.C., Lall R., Srivastava A., editors. Elsevier Inc.; California: 2021. Melatonin: a safe nutraceutical and clinical agent; pp. 537–553. [Google Scholar]
- 259.Claustrat B., Leston J. Melatonin: physiological effects in humans. Neurochirurgie. 2015;61:77–84. doi: 10.1016/j.neuchi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 260.Stebelová K., Anttila K., Mänttäri S., Saarela S., Zeman M. Immunohistochemical definition of MT2 receptors and melatonin in the gastrointestinal tissues of rat. Acta Histochem. 2010;112:26–33. doi: 10.1016/j.acthis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 261.Hardeland R. Melatonin in plants—diversity of levels and multiplicity of functions. Front Plant Sci. 2016;7:198. doi: 10.3389/fpls.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Tan D.X. Melatonin and brain. Curr Neuropharmacol. 2010;8:161. doi: 10.2174/157015910792246263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Alamdari A.F., Rahnemayan S., Rajabi H., Vahed N., Kashani H.R.K., Rezabakhsh A., et al. Melatonin as a promising modulator of aging related neurodegenerative disorders: role of microRNAs. Pharmacol Res. 2021;173 doi: 10.1016/j.phrs.2021.105839. [DOI] [PubMed] [Google Scholar]
- 264.Picca A., Ferri E., Calvani R., Coelho-Júnior H.J., Marzetti E., Arosio B. Age-associated glia remodeling and mitochondrial dysfunction in neurodegeneration: antioxidant supplementation as a possible intervention. Nutrients. 2022;14:2406. doi: 10.3390/nu14122406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zhang H.M., Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res. 2014;57:131–146. doi: 10.1111/jpi.12162. [DOI] [PubMed] [Google Scholar]
- 266.Alghamdi B.S. The neuroprotective role of melatonin in neurological disorders. J Neurosci Res. 2018;96:1136–1149. doi: 10.1002/jnr.24220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Roy J., Wong K.Y., Aquili L., Uddin M.S., Heng B.C., Tipoe G.L., et al. Role of melatonin in Alzheimer's disease: from preclinical studies to novel melatonin-based therapies. Front Neuroendocrinol. 2022;65 doi: 10.1016/j.yfrne.2022.100986. [DOI] [PubMed] [Google Scholar]
- 268.Sun C., Qiu X., Wang Y., Liu J., Li Q., Jiang H., et al. Long-term oral melatonin alleviates memory deficits, reduces amyloid-β deposition associated with downregulation of BACE1 and mitophagy in APP/PS1 transgenic mice. Neurosci Lett. 2020;735 doi: 10.1016/j.neulet.2020.135192. [DOI] [PubMed] [Google Scholar]
- 269.Patki G., Lau Y.S. Melatonin protects against neurobehavioral and mitochondrial deficits in a chronic mouse model of Parkinson's disease. Pharmacol Biochem Behav. 2011;99:704–711. doi: 10.1016/j.pbb.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Jung Y.J., Choi H., Oh E. Melatonin attenuates MPP+-induced apoptosis via heat shock protein in a Parkinson's disease model. Biochem Biophys Res Commun. 2022;621:59–66. doi: 10.1016/j.bbrc.2022.06.099. [DOI] [PubMed] [Google Scholar]
- 271.Willis G.L., Moore C., Armstrong S.M. A historical justification for and retrospective analysis of the systematic application of light therapy in Parkinson's disease. Rev Neurosci. 2012;23:199–226. doi: 10.1515/revneuro-2011-0072. [DOI] [PubMed] [Google Scholar]
- 272.Kato Y., Kubo Y., Iwata D., Kato S., Sudo T., Sugiura T., et al. Gene knockout and metabolome analysis of carnitine/organic cation transporter OCTN1. Pharm Res. 2010;27:832–840. doi: 10.1007/s11095-010-0076-z. [DOI] [PubMed] [Google Scholar]
- 273.Graham S.F., Chevallier O.P., Kumar P., Gcaron Türko, Lu O., Bahado-Singh R.O. Metabolomic profiling of brain from infants who died from sudden infant death syndrome reveals novel predictive biomarkers. J Perinatol. 2017;37:91–97. doi: 10.1038/jp.2016.139. [DOI] [PubMed] [Google Scholar]
- 274.Tang R.M.Y., Cheah I.K., Yew T.S.K., Halliwell B. Distribution and accumulation of dietary ergothioneine and its metabolites in mouse tissues. Sci Rep. 2018;8:1601. doi: 10.1038/s41598-018-20021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Halliwell B., Cheah I.K., Tang R.M.Y. Ergothioneine-a diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018;592:3357–3366. doi: 10.1002/1873-3468.13123. [DOI] [PubMed] [Google Scholar]
- 276.Nakamichi N., Tsuzuku S., Shibagaki F. Ergothioneine and central nervous system diseases. Neurochem Res. 2022;47:2513–2521. doi: 10.1007/s11064-022-03665-2. [DOI] [PubMed] [Google Scholar]
- 277.Paul B.D., Snyder S.H. The unusual amino acid l-ergothioneine is a physiologic cytoprotectant. Cell Death Differ. 2010;17:1134–1140. doi: 10.1038/cdd.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Dumitrescu D.G., Gordon E.M., Kovalyova Y., Seminara A.B., Duncan-Lowey B., Forster E.R., et al. A microbial transporter of the dietary antioxidant ergothioneine. Cell. 2022;185:4526–4540.e18. doi: 10.1016/j.cell.2022.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Paul B.D. Ergothioneine: a stress vitamin with antiaging, vascular, and neuroprotective roles?. Antioxidants Redox Signal. 2022;36:1306–1317. doi: 10.1089/ars.2021.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Song T.Y., Lin H.C., Chen C.L., Wu J.H., Liao J.W., Hu M.L. Ergothioneine and melatonin attenuate oxidative stress and protect against learning and memory deficits in C57BL/6J mice treated with d-galactose. Free Radic Res. 2014;48:1049–1060. doi: 10.3109/10715762.2014.920954. [DOI] [PubMed] [Google Scholar]
- 281.Song T.Y., Chen C.L., Liao J.W., Ou H.C., Tsai M.S. Ergothioneine protects against neuronal injury induced by cisplatin both in vitro and in vivo. Food Chem Toxicol. 2010;48:3492–3499. doi: 10.1016/j.fct.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 282.Yang N.C., Lin H.C., Wu J.H., Ou H.C., Chai Y.C., Tseng C.Y., et al. Ergothioneine protects against neuronal injury induced by β-amyloid in mice. Food Chem Toxicol. 2012;50:3902–3911. doi: 10.1016/j.fct.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 283.Feng L., Cheah I.K., Ng M.M.T., Li J., Chan S.M., Lim S.L., et al. The association between mushroom consumption and mild cognitive impairment: a community-based cross-sectional study in Singapore. J Alzheimers Dis. 2019;68:197–203. doi: 10.3233/JAD-180959. [DOI] [PubMed] [Google Scholar]
- 284.Clarke J.D., Hsu A., Williams D.E., Dashwood R.H., Stevens J.F., Yamamoto M., et al. Metabolism and tissue distribution of sulforaphane in Nrf2 knockout and wild-type mice. Pharm Res. 2011;28:3171–3179. doi: 10.1007/s11095-011-0500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Calabrese E.J., Kozumbo W.J. The phytoprotective agent sulforaphane prevents inflammatory degenerative diseases and age-related pathologies via Nrf2-mediated hormesis. Pharmacol Res. 2021;163 doi: 10.1016/j.phrs.2020.105283. [DOI] [PubMed] [Google Scholar]
- 286.Schepici G., Bramanti P., Mazzon E. Efficacy of sulforaphane in neurodegenerative diseases. Int J Mol Sci. 2020;21:8637. doi: 10.3390/ijms21228637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Morroni F., Sita G., Djemil A., D'Amico M., Pruccoli L., Cantelli-Forti G., et al. Comparison of adaptive neuroprotective mechanisms of sulforaphane and its interconversion product erucin in in vitro and in vivo models of Parkinson's disease. J Agric Food Chem. 2018;66:856–865. doi: 10.1021/acs.jafc.7b04641. [DOI] [PubMed] [Google Scholar]
- 288.Morroni F., Tarozzi A., Sita G., Bolondi C., Zolezzi Moraga J.M., Cantelli-Forti G., et al. Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson's disease. Neurotoxicology. 2013;36:63–71. doi: 10.1016/j.neuro.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 289.Lee S., Kim J., Seo S.G., Choi B.R., Han J.S., Lee K.W., et al. Sulforaphane alleviates scopolamine-induced memory impairment in mice. Pharmacol Res. 2014;85:23–32. doi: 10.1016/j.phrs.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 290.Kim H.V., Kim H.Y., Ehrlich H.Y., Choi S.Y., Kim D.J., Kim Y. Amelioration of Alzheimer's disease by neuroprotective effect of sulforaphane in animal model. Amyloid. 2013;20:7–12. doi: 10.3109/13506129.2012.751367. [DOI] [PubMed] [Google Scholar]
- 291.Zhang S., Peng B., Chen Z., Yu J., Deng G., Bao Y., et al. Brain-targeting, acid-responsive antioxidant nanoparticles for stroke treatment and drug delivery. Bioact Mater. 2022;16:57–65. doi: 10.1016/j.bioactmat.2022.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Tsai Y.M., Chien C.F., Lin L.C., Tsai T.H. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood–brain barrier penetration. Int J Pharm. 2011;416:331–338. doi: 10.1016/j.ijpharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 293.Vanaja K., Wahl M.A., Bukarica L., Heinle H. Liposomes as carriers of the lipid soluble antioxidant resveratrol: evaluation of amelioration of oxidative stress by additional antioxidant vitamin. Life Sci. 2013;93:917–923. doi: 10.1016/j.lfs.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 294.Sharma N., Nehru B. Beneficial effect of vitamin E in rotenone induced model of PD: behavioural, neurochemical and biochemical study. Exp Neurobiol. 2013;22:214–223. doi: 10.5607/en.2013.22.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhao B., Ren B., Guo R., Zhang W., Ma S., Yao Y., et al. Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway. Food Chem Toxicol. 2017;109:505–516. doi: 10.1016/j.fct.2017.09.050. [DOI] [PubMed] [Google Scholar]
- 296.Wang J., Li L., Wang Z., Cui Y., Tan X., Yuan T., et al. Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J Nutr Biochem. 2018;56:16–25. doi: 10.1016/j.jnutbio.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 297.Huang C., Gan D., Fan C., Wen C., Li A., Li Q., et al. The secretion from neural stem cells pretreated with lycopene protects against tert-butyl hydroperoxide-induced neuron oxidative damage. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/5490218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Sachdeva A.K., Chopra K. Lycopene abrogates Aβ1–42-mediated neuroinflammatory cascade in an experimental model of Alzheimer's disease. J Nutr Biochem. 2015;26:736–744. doi: 10.1016/j.jnutbio.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 299.Prakash A., Kumar A. Implicating the role of lycopene in restoration of mitochondrial enzymes and BDNF levels in β-amyloid induced Alzheimer's disease. Eur J Pharmacol. 2014;741:104–111. doi: 10.1016/j.ejphar.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 300.Chen W., Mao L., Xing H., Xu L., Fu X., Huang L., et al. Lycopene attenuates Aβ1–42 secretion and its toxicity in human cell and Caenorhabditis elegans models of Alzheimer disease. Neurosci Lett. 2015;608:28–33. doi: 10.1016/j.neulet.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 301.Prema A., Janakiraman U., Manivasagam T., Thenmozhi A.J. Neuroprotective effect of lycopene against MPTP induced experimental Parkinson's disease in mice. Neurosci Lett. 2015;599:12–19. doi: 10.1016/j.neulet.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 302.Xu L., Zhu J., Yin W., Ding X. Astaxanthin improves cognitive deficits from oxidative stress, nitric oxide synthase and inflammation through upregulation of PI3K/Akt in diabetes rat. Int J Clin Exp Pathol. 2015;8:6083–6094. [PMC free article] [PubMed] [Google Scholar]
- 303.Lee D.H., Kim C.S., Lee Y.J. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem Toxicol. 2011;49:271–280. doi: 10.1016/j.fct.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Katayama S., Ogawa H., Nakamura S. Apricot carotenoids possess potent anti-amyloidogenic activity in vitro. J Agric Food Chem. 2011;59:12691–12696. doi: 10.1021/jf203654c. [DOI] [PubMed] [Google Scholar]
- 305.Tan D., Yu X., Chen M., Chen J., Xu J. Lutein protects against severe traumatic brain injury through anti-inflammation and antioxidative effects via ICAM-1/Nrf-2. Mol Med Rep. 2017;16:4235–4240. doi: 10.3892/mmr.2017.7040. [DOI] [PubMed] [Google Scholar]
- 306.Xiang S., Liu F., Lin J., Chen H., Huang C., Chen L., et al. Fucoxanthin inhibits β-amyloid assembly and attenuates β-amyloid oligomer-induced cognitive impairments. J Agric Food Chem. 2017;65:4092–4102. doi: 10.1021/acs.jafc.7b00805. [DOI] [PubMed] [Google Scholar]
- 307.Ye Q., Ye L., Xu X., Huang B., Zhang X., Zhu Y., et al. Epigallocatechin-3-gallate suppresses 1-methyl-4-phenyl-pyridine-induced oxidative stress in PC12 cells via the SIRT1/PGC-1α signaling pathway. BMC Compl Alternative Med. 2012;12:82. doi: 10.1186/1472-6882-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wang Y., Fu X.T., Li D.W., Wang K., Wang X.Z., Li Y., et al. Cyanidin suppresses amyloid beta-induced neurotoxicity by inhibiting reactive oxygen species-mediated DNA damage and apoptosis in PC12 cells. Neural Regen Res. 2016;11:795–800. doi: 10.4103/1673-5374.182707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Pacheco S.M., Soares M.S.P., Gutierres J.M., Gerzson M.F.B., Carvalho F.B., Azambuja J.H., et al. Anthocyanins as a potential pharmacological agent to manage memory deficit, oxidative stress and alterations in ion pump activity induced by experimental sporadic dementia of Alzheimer's type. J Nutr Biochem. 2018;56:193–204. doi: 10.1016/j.jnutbio.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 310.Khan M.S., Ali T., Kim M.W., Jo M.H., Chung J.I., Kim M.O. Anthocyanins improve hippocampus-dependent memory function and prevent neurodegeneration via JNK/Akt/GSK3β signaling in LPS-treated adult mice. Mol Neurobiol. 2019;56:671–687. doi: 10.1007/s12035-018-1101-1. [DOI] [PubMed] [Google Scholar]
- 311.Wei J., Zhang G., Zhang X., Xu D., Gao J., Fan J., et al. Anthocyanins from black chokeberry (Aroniamelanocarpa elliot) delayed aging-related degenerative changes of brain. J Agric Food Chem. 2017;65:5973–5984. doi: 10.1021/acs.jafc.7b02136. [DOI] [PubMed] [Google Scholar]
- 312.Rehman S.U., Shah S.A., Ali T., Chung J.I., Kim M.O. Anthocyanins reversed d-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats. Mol Neurobiol. 2017;54:255–271. doi: 10.1007/s12035-015-9604-5. [DOI] [PubMed] [Google Scholar]
- 313.Ay M., Luo J., Langley M., Jin H., Anantharam V., Kanthasamy A., et al. Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson's disease. J Neurochem. 2017;141:766–782. doi: 10.1111/jnc.14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Wang D.M., Li S.Q., Wu W.L., Zhu X.Y., Wang Y., Yuan H.Y. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer's disease. Neurochem Res. 2014;39:1533–1543. doi: 10.1007/s11064-014-1343-x. [DOI] [PubMed] [Google Scholar]
- 315.Sachdeva S., Pant S.C., Kushwaha P., Bhargava R., Flora S.J.S. Sodium tungstate induced neurological alterations in rat brain regions and their response to antioxidants. Food Chem Toxicol. 2015;82:64–71. doi: 10.1016/j.fct.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 316.Lakroun Z., Kebieche M., Lahouel A., Zama D., Desor F., Soulimani R. Oxidative stress and brain mitochondria swelling induced by endosulfan and protective role of quercetin in rat. Environ Sci Pollut Res Int. 2015;22:7776–7781. doi: 10.1007/s11356-014-3885-5. [DOI] [PubMed] [Google Scholar]
- 317.Lv C., Hong T., Yang Z., Zhang Y., Wang L., Dong M., et al. Effect of quercetin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson's disease. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/928643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Ma W., Yuan L., Yu H., Ding B., Xi Y., Feng J., et al. Genistein as a neuroprotective antioxidant attenuates redox imbalance induced by β-amyloid peptides 25–35 in PC12 cells. Int J Dev Neurosci. 2010;28:289–295. doi: 10.1016/j.ijdevneu.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 319.Gaballah H.H., Zakaria S.S., Elbatsh M.M., Tahoon N.M. Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson's disease. Chem Biol Interact. 2016;251:10–16. doi: 10.1016/j.cbi.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 320.Shen C., Cheng W., Yu P., Wang L., Zhou L., Zeng L., et al. Resveratrol pretreatment attenuates injury and promotes proliferation of neural stem cells following oxygen-glucose deprivation/reoxygenation by upregulating the expression of Nrf2, HO-1 and NQO1. in vitro. Mol Med Rep. 2016;14:3646–3654. doi: 10.3892/mmr.2016.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Ren J., Fan C., Chen N., Huang J., Yang Q. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in rats. Neurochem Res. 2011;36:2352–2362. doi: 10.1007/s11064-011-0561-8. [DOI] [PubMed] [Google Scholar]
- 322.Oyagbemi A.A., Omobowale T.O., Saba A.B., Olowu E.R., Dada R.O., Akinrinde A.S. Gallic acid ameliorates cyclophosphamide-induced neurotoxicity in Wistar rats through free radical scavenging activity and improvement in antioxidant defense system. J Diet Suppl. 2016;13:402–419. doi: 10.3109/19390211.2015.1103827. [DOI] [PubMed] [Google Scholar]
- 323.Lenzi J., Rodrigues A.F., Rós Ade S., de Castro A.B., de Lima D.D., Magro D.D., et al. Ferulic acid chronic treatment exerts antidepressant-like effect: role of antioxidant defense system. Metab Brain Dis. 2015;30:1453–1463. doi: 10.1007/s11011-015-9725-6. [DOI] [PubMed] [Google Scholar]
- 324.Nagarajan S., Chellappan D.R., Chinnaswamy P., Thulasingam S. Ferulic acid pretreatment mitigates MPTP-induced motor impairment and histopathological alterations in C57BL/6 mice. Pharm Biol. 2015;53:1591–1601. doi: 10.3109/13880209.2014.993041. [DOI] [PubMed] [Google Scholar]
- 325.Zhang Z., Li G., Szeto S.S.W., Chong C.M., Quan Q., Huang C., et al. Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radic Biol Med. 2015;84:331–343. doi: 10.1016/j.freeradbiomed.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 326.Tsai S.J., Yin M.C. Anti-glycative and anti-inflammatory effects of protocatechuic acid in brain of mice treated by d-galactose. Food Chem Toxicol. 2012;50:3198–3205. doi: 10.1016/j.fct.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 327.Shi G.F., An L.J., Jiang B., Guan S., Bao Y.M. Alpinia protocatechuic acid protects against oxidative damage in vitro and reduces oxidative stress in vivo. Neurosci Lett. 2006;403:206–210. doi: 10.1016/j.neulet.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 328.Guan S., Bao Y.M., Jiang B., An L.J. Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur J Pharmacol. 2006;538:73–79. doi: 10.1016/j.ejphar.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 329.Solís-Chagoyán H., Domínguez-Alonso A., Valdés-Tovar M., Argueta J., Sánchez-Florentino Z.A., Calixto E., et al. Melatonin rescues the dendrite collapse induced by the pro-oxidant toxin okadaic acid in organotypic cultures of rat hilar hippocampus. Molecules. 2020;25:5508. doi: 10.3390/molecules25235508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Promyo K., Iqbal F., Chaidee N., Chetsawang B. Aluminum chloride-induced amyloid β accumulation and endoplasmic reticulum stress in rat brain are averted by melatonin. Food Chem Toxicol. 2020;146 doi: 10.1016/j.fct.2020.111829. [DOI] [PubMed] [Google Scholar]
- 331.Sim M., Hong S., Jung S., Kim J.S., Goo Y.T., Chun W.Y., et al. Vitamin C supplementation promotes mental vitality in healthy young adults: results from a cross-sectional analysis and a randomized, double-blind, placebo-controlled trial. Eur J Nutr. 2022;61:447–459. doi: 10.1007/s00394-021-02656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Fitzgerald K.C., O'Reilly Éj, Fondell E., Falcone G.J., McCullough M.L., Park Y., et al. Intakes of vitamin C and carotenoids and risk of amyotrophic lateral sclerosis: pooled results from 5 cohort studies. Ann Neurol. 2013;73:236–245. doi: 10.1002/ana.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Yang F., Wolk A., Håkansson N., Pedersen N.L., Wirdefeldt K. Dietary antioxidants and risk of Parkinson's disease in two population-based cohorts. Mov Disord. 2017;32:1631–1636. doi: 10.1002/mds.27120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Hughes K.C., Gao X., Kim I.Y., Rimm E.B., Wang M., Weisskopf M.G., et al. Intake of antioxidant vitamins and risk of Parkinson's disease. Mov Disord. 2016;31:1909–1914. doi: 10.1002/mds.26819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Agarwal P., Wang Y., Buchman A.S., Holland T.M., Bennett D.A., Morris M.C. Dietary antioxidants associated with slower progression of parkinsonian signs in older adults. Nutr Neurosci. 2022;25:550–557. doi: 10.1080/1028415X.2020.1769411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Polidori M.C., Praticó D., Mangialasche F., Mariani E., Aust O., Anlasik T., et al. High fruit and vegetable intake is positively correlated with antioxidant status and cognitive performance in healthy subjects. J Alzheimers Dis. 2009;17:921–927. doi: 10.3233/JAD-2009-1114. [DOI] [PubMed] [Google Scholar]
- 337.Feart C., Letenneur L., Helmer C., Samieri C., Schalch W., Etheve S., et al. Plasma carotenoids are inversely associated with dementia risk in an elderly French cohort. J Gerontol A Biol Sci Med Sci. 2016;71:683–688. doi: 10.1093/gerona/glv135. [DOI] [PubMed] [Google Scholar]
- 338.Min J.Y., Min K.B. Serum lycopene, lutein and zeaxanthin, and the risk of Alzheimer's disease mortality in older adults. Dement Geriatr Cogn Disord. 2014;37:246–256. doi: 10.1159/000356486. [DOI] [PubMed] [Google Scholar]
- 339.Kesse-Guyot E., Andreeva V.A., Ducros V., Jeandel C., Julia C., Hercberg S., et al. Carotenoid-rich dietary patterns during midlife and subsequent cognitive function. Br J Nutr. 2014;111:915–923. doi: 10.1017/S0007114513003188. [DOI] [PubMed] [Google Scholar]
- 340.Mohn E., Vishwanathan R., Schalch W., Lichtenstein A., Matthan N., Poon L., et al. Proceedings of the annual meeting at experimental biology. FASEB J; Boston, MA: 2013. The relationship of lutein and DHA in age-related cognitive function. [Google Scholar]
- 341.Liu X., Dhana K., Furtado J.D., Agarwal P., Aggarwal N.T., Tangney C., et al. Higher circulating α-carotene was associated with better cognitive function: an evaluation among the MIND trial participants. J Nutr Sci. 2021;10:e64. doi: 10.1017/jns.2021.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Ying A.F., Khan S., Wu Y., Jin A., Wong A.S., Tan E.K., et al. Dietary antioxidants and risk of Parkinson's disease in the Singapore Chinese health study. Mov Disord. 2020;35:1765–1773. doi: 10.1002/mds.28173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Igwe E.O., Roodenrys S., Probst Y.C., do Rosario V., Netzel M.E., Hong H.T., et al. Low anthocyanin plum nectar does not impact cognition, blood pressure and gut microbiota in healthy older adults: a randomized crossover trial. Nutr Res. 2020;82:74–87. doi: 10.1016/j.nutres.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 344.Coe S., Collett J., Izadi H., Wade D.T., Clegg M., Harrison J.M., et al. A protocol for a randomised double-blind placebo-controlled feasibility study to determine whether the daily consumption of flavonoid-rich pure cocoa has the potential to reduce fatigue in people with relapsing and remitting multiple sclerosis (RRMS) Pilot Feasibility Stud. 2018;4:35. doi: 10.1186/s40814-018-0230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Levin J., Maaß S., Schuberth M., Respondek G., Paul F., Mansmann U., et al. The PROMESA-protocol: progression rate of multiple system atrophy under EGCG supplementation as anti-aggregation-approach. J Neural Transm. 2016;123:439–445. doi: 10.1007/s00702-016-1507-8. [DOI] [PubMed] [Google Scholar]
- 346.Brickman A.M., Khan U.A., Provenzano F.A., Yeung L.K., Suzuki W., Schroeter H., et al. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci. 2014;17:1798–1803. doi: 10.1038/nn.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Katagiri M., Satoh A., Tsuji S., Shirasawa T. Effects of astaxanthin-rich Haematococcus pluvialis extract on cognitive function: a randomised, double-blind, placebo-controlled study. J Clin Biochem Nutr. 2012;51:102–107. doi: 10.3164/jcbn.D-11-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Wightman E.L., Haskell C.F., Forster J.S., Veasey R.C., Kennedy D.O. Epigallocatechin gallate, cerebral blood flow parameters, cognitive performance and mood in healthy humans: a double-blind, placebo-controlled, crossover investigation. Hum Psychopharmacol. 2012;27:177–186. doi: 10.1002/hup.1263. [DOI] [PubMed] [Google Scholar]
- 349.Small G.W., Siddarth P., Li Z., Miller K.J., Ercoli L., Emerson N.D., et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatr. 2018;26:266–277. doi: 10.1016/j.jagp.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 350.Wightman E.L., Haskell-Ramsay C.F., Reay J.L., Williamson G., Dew T., Zhang W., et al. The effects of chronic trans-resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br J Nutr. 2015;114:1427–1437. doi: 10.1017/S0007114515003037. [DOI] [PubMed] [Google Scholar]
- 351.Turner R.S., Thomas R.G., Craft S., van Dyck C.H., Mintzer J., Reynolds B.A., et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85:1383–1391. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Wightman E.L., Reay J.L., Haskell C.F., Williamson G., Dew T.P., Kennedy D.O. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: a randomised, double-blind, placebo-controlled, cross-over investigation. Br J Nutr. 2014;112:203–213. doi: 10.1017/S0007114514000737. [DOI] [PubMed] [Google Scholar]
- 353.Witte A.V., Kerti L., Margulies D.S., Flöel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. 2014;34:7862–7870. doi: 10.1523/JNEUROSCI.0385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Ringman J.M., Frautschy S.A., Teng E., Begum A.N., Bardens J., Beigi M., et al. Oral curcumin for Alzheimer's disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer's Res Ther. 2012;4:43. doi: 10.1186/alzrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Kudoh C., Hori T., Yasaki S., Ubagai R., Tabira T. Effects of ferulic acid and Angelica archangelica extract (Feru-guard®) on mild cognitive impairment: a multicenter, randomized, double-blind, placebo-controlled prospective trial. J Alzheimers Dis Rep. 2020;4:393–398. doi: 10.3233/ADR-200211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Saitou K., Ochiai R., Kozuma K., Sato H., Koikeda T., Osaki N., et al. Effect of chlorogenic acids on cognitive function: a randomized, double-blind, placebo-controlled trial. Nutrients. 2018;10:1337. doi: 10.3390/nu10101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Kato M., Ochiai R., Kozuma K., Sato H., Katsuragi Y. Effect of chlorogenic acid intake on cognitive function in the elderly: a pilot study. Evid Based Complement Alternat Med. 2018;2018 doi: 10.1155/2018/8608497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Kim J.W., Byun M.S., Yi D., Lee J.H., Jeon S.Y., Jung G., et al. Coffee intake and decreased amyloid pathology in human brain. Transl Psychiatry. 2019;9:270. doi: 10.1038/s41398-019-0604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Agudelo-Ochoa G.M., Pulgarín-Zapata I.C., Velásquez-Rodriguez C.M., Duque-Ramírez M., Naranjo-Cano M., Quintero-Ortiz M.M., et al. Coffee consumption increases the antioxidant capacity of plasma and has no effect on the lipid profile or vascular function in healthy adults in a randomized controlled trial. J Nutr. 2016;146:524–531. doi: 10.3945/jn.115.224774. [DOI] [PubMed] [Google Scholar]
- 360.Solfrizzi V., Panza F., Imbimbo B.P., D'Introno A., Galluzzo L., Gandin C., et al. Coffee consumption habits and the risk of mild cognitive impairment: the Italian longitudinal study on aging. J Alzheimers Dis. 2015;47:889–899. doi: 10.3233/JAD-150333. [DOI] [PubMed] [Google Scholar]
- 361.Camfield D.A., Silber B.Y., Scholey A.B., Nolidin K., Goh A., Stough C. A randomised placebo-controlled trial to differentiate the acute cognitive and mood effects of chlorogenic acid from decaffeinated coffee. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Xu L., Yu H., Sun H., Hu B., Geng Y. Dietary melatonin therapy alleviates the lamina cribrosa damages in patients with mild cognitive impairments: a double-blinded, randomized controlled study. Med Sci Mon Int Med J Exp Clin Res. 2020;26 doi: 10.12659/MSM.923232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Cruz-Aguilar M.A., Ramírez-Salado I., Guevara M.A., Hernández-González M., Benitez-King G. Melatonin effects on EEG activity during sleep onset in mild-to-moderate Alzheimer's disease: a pilot study. J Alzheimers Dis Rep. 2018;2:55–65. doi: 10.3233/ADR-170019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Wade A.G., Farmer M., Harari G., Fund N., Laudon M., Nir T., et al. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer's disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clin Interv Aging. 2014;9:947–961. doi: 10.2147/CIA.S65625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Rondanelli M., Opizzi A., Faliva M., Mozzoni M., Antoniello N., Cazzola R., et al. Effects of a diet integration with an oily emulsion of DHA-phospholipids containing melatonin and tryptophan in elderly patients suffering from mild cognitive impairment. Nutr Neurosci. 2012;15:46–54. doi: 10.1179/1476830511Y.0000000032. [DOI] [PubMed] [Google Scholar]