Abstract
Background/purpose
Because of the complex anatomical structure of the maxillofacial skeleton, bending plates is necessary during surgery. The fast developing three-dimensional printing (3DP) technology has provided a new method for making personalized craniomaxillofacial bone plates. However, the properties of these bone plates remain unknown. This study evaluates the mechanical, fatigue, and morphological properties of these bone plates, which may provide data supporting future clinical applications.
Materials and methods
The 3DP bone plate was fabricated by selective laser melting (SLM) and electron beam melting (EBM) technologies. Mechanical, surface, and defect analyses were performed to compare their properties with a standard machined sample. One-way analysis of variance was applied, with p < 0.05 considered significant.
Results
The 3DP craniomaxillofacial bone plate had better bending strength than that of the standard machined plate (p < 0.01). Whereas the fatigue resistance of the 3DP bone plate needs to be improved in the future. Surface analysis indicated greater roughness of the 3DP bone plate (p < 0.01). However, the surface roughness could be significantly reduced by polishing the surface, which would meet the needs of clinical application after polishing. Further defect analysis revealed the internal defect inside the plate, which should be avoided to improve the mechanical strength of the printed sample in the future.
Conclusion
The 3DP titanium craniomaxillofacial bone plate has good mechanical performance and surface morphology, meeting the requirements of clinical application. However, poorer fatigue resistance and a high number of internal defects should be modified in the future.
Keywords: Biomechanical properties, Craniomaxillofacial bone plate, Fatigue properties, Morphological properties, Three-dimensional printing
Introduction
A bone plate is necessary for rigid internal fixation in the reparative and reconstructive surgery of maxillofacial trauma, defect, and deformity. Because of the irregular shape and complex anatomical structure of the maxillofacial region, the commercial bone plate must often be bent to fit the bone surface. However, despite repeatedly being bent, the bone plate still cannot be fully fitted. Relevant research indicates that the contact rate between a standard machined bone plate and bone surface is only 53%. Thus, the stress concentration caused by low bone contact can affect bone formation and even lead to loosening of the bone connection.1 Moreover, bending the bone plate during surgery not only increases the operation time but also leads to microcracks and residual stresses. According to animal experiments and clinical studies on this topic, the main reason for plate fracture is the microfissures generated during bending of the plate.2, 3, 4
With the innovation of technology and the increasing demand for individualization, prebent titanium plates have been developed, which eliminate the need to bend and contour during surgery.2 Applying a prebent titanium plate can reduce excessive bending of the titanium plate during surgery, resulting in better adaptation for bony morphology. Furthermore, according to relevant research, better surgical results can also be acquired by applying prebent titanium plates.5,6 However, the prebent titanium plate is manufactured by metal forging, which is cumbersome and time-consuming. Moreover, it is also limited by the need to fabricate complex structures and internal pores. Therefore, personalization is impossible to achieve, and there are many limitations in terms of clinical application.7, 8, 9
With the emergence and development of three-dimensional printing (3DP) technology, the fabrication of personalized titanium alloy craniomaxillofacial bone plates is feasible.10 Many 3DP methods have been developed for metal fabricating.9,11, 12, 13 Among them, selective laser melting (SLM) and electron beam melting (EBM) are the most widely used because of their high efficiency and ability to accurately control internal structures and complex shapes.14, 15, 16 However, the heat sources and manufacturing principles of the two technologies are not the same. There may also be differences in the process parameters of the manufacturing process, such as the scanning pitch and the powder particle size. Therefore, the microstructure, surface roughness, and mechanical properties of the samples fabricated by EBM and SLM are also different.9 Researchers have compared the differences in microstructure, mechanical properties, electrochemical corrosion properties, and biological properties of the two methods.1,15,17, 18, 19, 20, 21, 22, 23
As a long-term intraosseous implant in vivo, the mechanical, morphological, and biological properties of the plate are clearly defined in the American Society of Testing Materials (ASTM) standards. Although many researchers have compared the differences in performance the metal samples made by the two methods, there has been no systematic research on the mechanical performances, surface properties, and internal morphological differences of the craniomaxillofacial plates fabricated by the two methods. In this study, we used the two methods to fabricate a 3DP titanium alloy craniomaxillofacial bone plate and compared the mechanical, fatigue, and morphological differences between the techniques, with the aim of providing data to support future clinical applications.
Materials and methods
Fabrication of three-dimensional printing titanium alloy bone plate
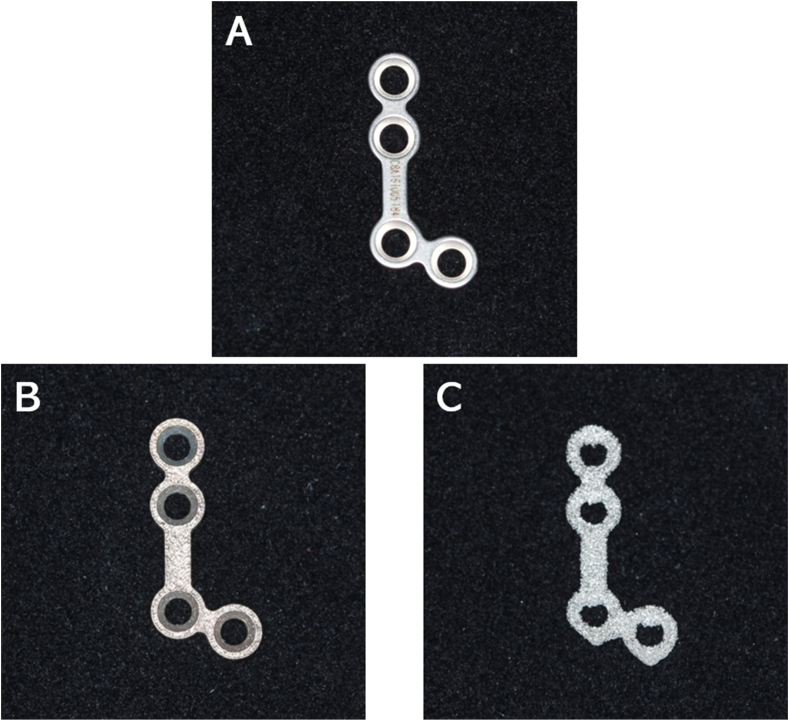
We selected a standard maxillary four-hole titanium plate (YL0.8-12, Cibei, Ningbo, China) as the control sample in this study. The stereolithography format was acquired and imported into a three-dimensional printer. Titanium Ti6Al4V powder (rematitan®CL, Dentaurum, Ispringen, Germany) was applied as the printing material. Two methods, namely selective laser melting (Concept Laser, Lichtenfels, German) and electron beam melting (Arcam, Mölndal, Sweden), were applied in the printing process, respectively. All samples were fabricated in the same direction. The same type of standard titanium plate fabricated by the traditional machined method was selected as the control sample (Fig. 1).
Figure 1.
Bone plate used in the test. (A) Standard plate. (B) Bone plate fabricated by SLM. (C) Bone plate fabricated by EBM.
Mechanical analysis
Three-point bending static mechanical testing
Static mechanical tests were carried out using a three-point bending method according to the ASTM standards. The instrument applied in the test was the ElectroForce 3330 test instrument (Bose ElectroForce, Framingham, MA, USA). The plate was placed in the machine, with the bone interface of the plate upward and in contact with the surface of the inner loading roller shaft. The position of the samples was adjusted so that the inner loading roller shaft was located at the bar section of the L-plates without contacting. After the sample was placed properly, the setting of the instrument was adjusted, and the displacement-controlled test was conducted at 1 mm/min until plastic deformation was reached. After all preparations were completed, the test started, and the maximum bending moment was located under the inner loading roller shaft. The upper surface (the bone interface) was subjected to pressure, whereas the lower surface (the non-bone interface) was under tensional stress. Displacement and load data were collected automatically through the sensors. The stress–strain curve was generated using Origin Pro 2015 Software (Origin Lab, Northampton, NC, USA); meanwhile, the yield and fracture stresses were calculated automatically from the curve. Three samples of each type of plate were selected for testing, and average values were calculated.
Rockwell hardness testing
To evaluate the mechanical properties of the plates, hardness was investigated. Testing was carried out using the Rockwell hardness test method according to the ASTM standards using the HR-150DT metallic Rockwell hardness tester (SIOM, Shanghai, China). Test indentations were generated with a 588N load and a standard indentation time of 15 s. To ensure the test's robustness, the equivalent positions of each sample were tested three times, and all indentations of the test samples were distributed evenly. The average value was taken as the hardness value of the sample, and three samples of each type of plate were also selected for testing. After the test sample was placed properly on the instrument, the test began, and the instrument automatically collected the data through the sensors.
Fatigue-resistance testing
Fatigue-resistance tests were conducted using the three-point bending method in accordance with ASTM standards. Tests were carried out at room temperature, air medium, with the stress ratio set to R = 0.1 using the ElectroForce 3330 test instrument (Bose ElectroForce). The load applied was set as 10%–30% of the yield strength σ calculated in the prior static mechanical test. The load was applied by axial sinusoidal wave, with the test frequency set as 5 Hz, and a maximum number of cycles set at 106. The testing plate was placed in the machine, with the bone interface of the plate upward and in contact with the surface of the inner loading roller shaft surface. The position of the samples was adjusted so that the inner loading roller shaft was located at the bridging part of the L-shaped bone plate without making contact. After the sample was placed properly, the test began. Displacement, load data, and number of cycles were automatically collected through the sensors. The test cycled until plastic deformation or the maximum cycle number was reached. Three samples of each type of plate were selected for testing.
Surface analysis
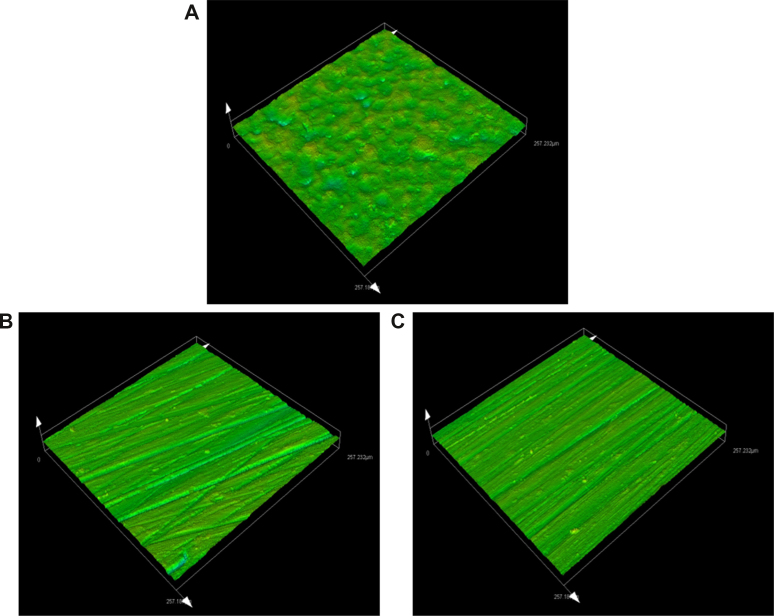
Laser scanning confocal microscopy (OLS5000, OLYMPUS, Tokyo, Japan) was applied in surface topography analysis. Because the bone plate is routinely polished in clinical application,1 we selected a portion of samples for polishing that were further divided into five groups: standard plate, SLM plate without polishing, SLM plate with polishing, EBM plate without polishing, and EBM plate with polishing. None of the samples were subjected to further acid etching or heat treatment. After cleaning the surfaces of the plates, they were placed in the machine and the view adjusted to the bar section of the L-plates. The magnification was set as 1080x and adjusted to the proper view. Subsequently, the test began, and the system automatically generated a 3D model of the area. Meanwhile, the surface roughness was calculated automatically using the system (Fig. 2). For each plate, the bar section of both the bone interface and non-bone interface were tested, and the results were described as Sa. To test the average surface roughness, three samples of each type of plate were selected.
Figure 2.
Surface analysis of the bone plate using laser scanning confocal microscopy. (A) Standard plate. (B) Bone plate fabricated by SLM with polishing. (C) Bone plate fabricated by EBM with polishing.
Defect analysis
Defects are defined as voids within the solid structure, which can reduce the mechanical and fatigue properties. Thus, we used the Zeiss Versa 500 Micro-CT system (Carl Zeiss AG, Jena, Germany) to detect the internal defects. Micro-computed tomography (micro-CT) was operated at an accelerating voltage of 120 kV and power of 10 W. The sample was rotated 360° at a 1-s exposure time with a total of 1600 projections, and images were automatically collected on a charge-coupled device detector. The micro-CT images were then reconstructed using ZEISS XM Reconstructor software (Carl Zeiss AG, Jena, Germany). Moreover, Avizo 8.0 software (Thermo Fisher Scientific, Waltham, MA, USA) was used to analyze the micro-CT data. Based on the micro-CT data, the defect size, count, and distribution were obtained.
Statistical analysis
Data were imported into the SPSS13.0 software package for statistical treatment and analysis. The experimental results were described as¯x ± s, and the statistical method applied was one-way analysis of variance (ANOVA). P < 0.05 was considered to indicate a significant difference. If the differences reached significance, we applied the least significant difference (LSD) test to post hoc multiple comparisons.
Results
Mechanical analysis
Three-point bending strength
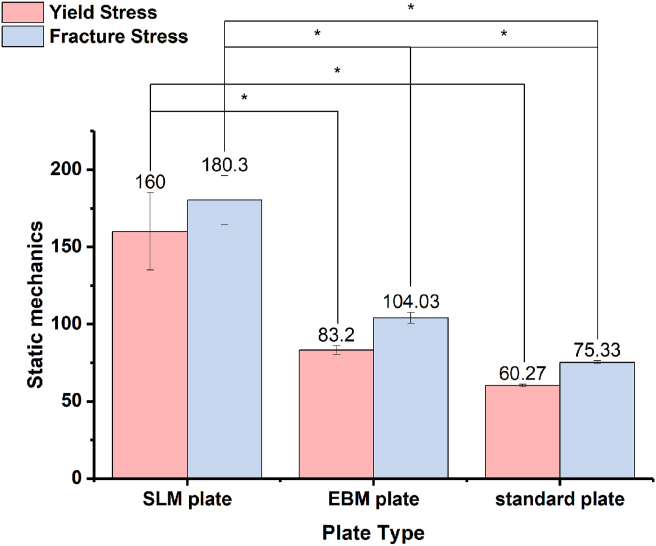
Fig. 3 shows the results of the three-point bending strength under static mechanical testing. The yield stress of the SLM plate was higher than that of the EBM plate, and the standard plate showed the lowest value (p < 0.01). The post hoc LSD test also suggested significant differences between the SLM and EBM plates. In terms of the fracture stress, the SLM plate showed the highest value, followed by the EBM plate, whereas the standard plate was the worst among them. The differences were statistically significant (p < 0.01) according to the analysis of variance. Furthermore, the post hoc LSD test also indicated a significant difference in the pairwise comparison.
Figure 3.
Static mechanical testing results of different samples.
Rockwell hardness
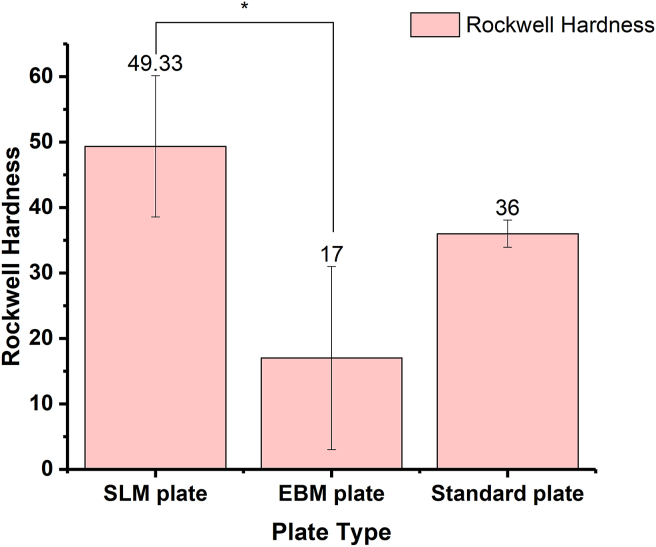
As shown in Fig. 4, the SLM plate had the highest Rockwell hardness value, followed by the standard plate, with the EBM plate having the lowest value. According to the ANOVA, the differences reached significance (p < 0.01). Post hoc LSD test of the pairwise comparison indicated no significant difference between the hardness of the standard plate and the other two kinds of plates. However, the difference in Rockwell hardness between the SLM plate and EBM plate reached significance, according to the result of the post hoc LSD test.
Figure 4.
Rockwell hardness testing results of different samples.
Fatigue-resistance property
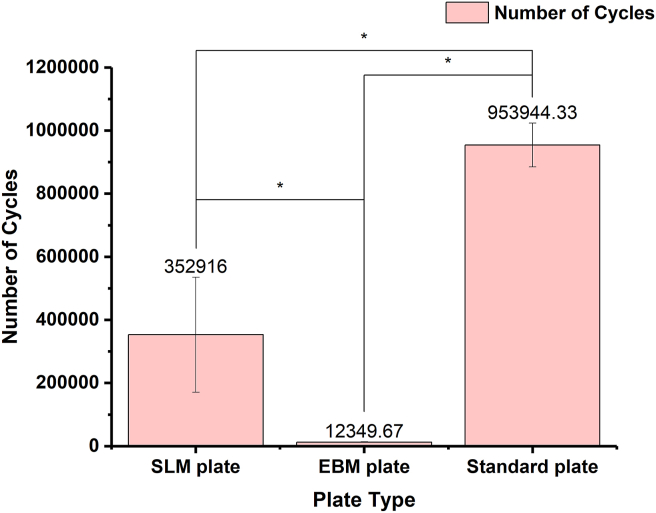
Fig. 5 shows the results of fatigue-resistance testing. The standard plate sustained the maximum cycle number, which shows excellent fatigue-resistance property. The SLM plate was next, whereas the EBM plate was the worst among them (p < 0.01). Furthermore, the post hoc LSD test also indicated a significant difference in the pairwise comparison.
Figure 5.
Fatigue-resistance testing results of different samples.
Surface analysis
Table 1 shows the results of the surface analysis of the five types of plates with and without polishing. For the 3DP plates without polishing, regardless of whether they were SLM or EBM plates, discontinuous and irregular pores were observed on the surface of the samples. Compared with the EBM plates, the SLM plates had a statistically significant smoother surface (p < 0.01). Polishing the bone plates can significantly reduce surface roughness, resulting in conformation with industry standards. The ANOVA showed no significant difference in surface roughness between the SLM, EBM, and standard plate (p > 0.05).
Table 1.
Surface roughness of 3DP titanium alloy craniomaxillofacial bone plates.
| Surface roughness (Sa, μm) |
||
|---|---|---|
| Non-bone interface | Bone interface | |
| SLM plate without polishing | 5.63 ± 1.35 | 14.86 ± 1.11 |
| SLM plate with polishing | 0.35 ± 0.12 | 0.36 ± 0.07 |
| EBM plate without polishing | 28.23 ± 5.70 | 24.80 ± 3.58 |
| EBM plate with polishing | 0.26 ± 0.09 | 0.23 ± 0.06 |
| Standard plate | 0.32 ± 0.05 | 0.33 ± 0.04 |
Abbreviations: 3DP, three-dimensional printing; SLM, selective laser melting; EBM, electron beam melting.
Defect analysis
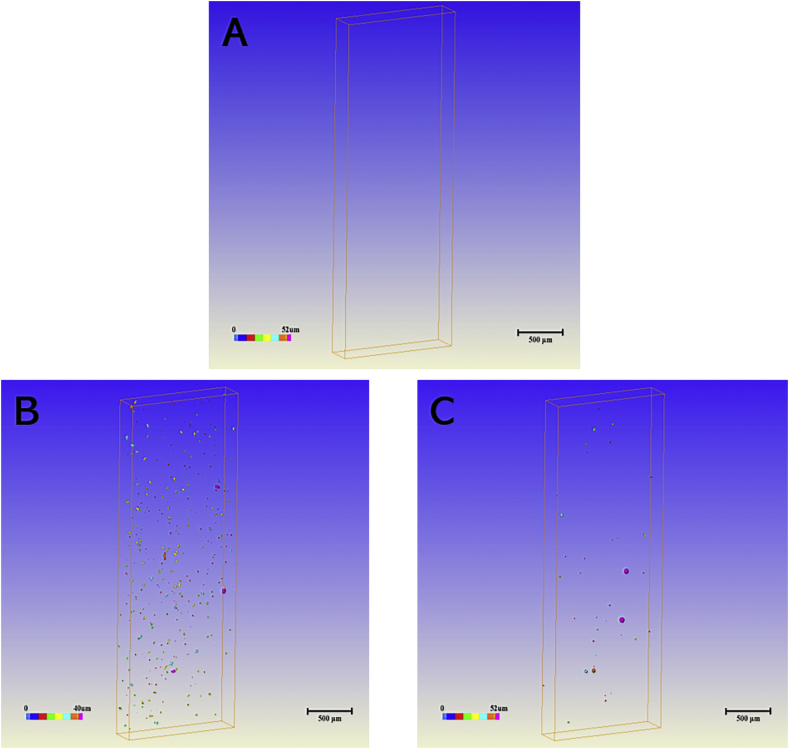
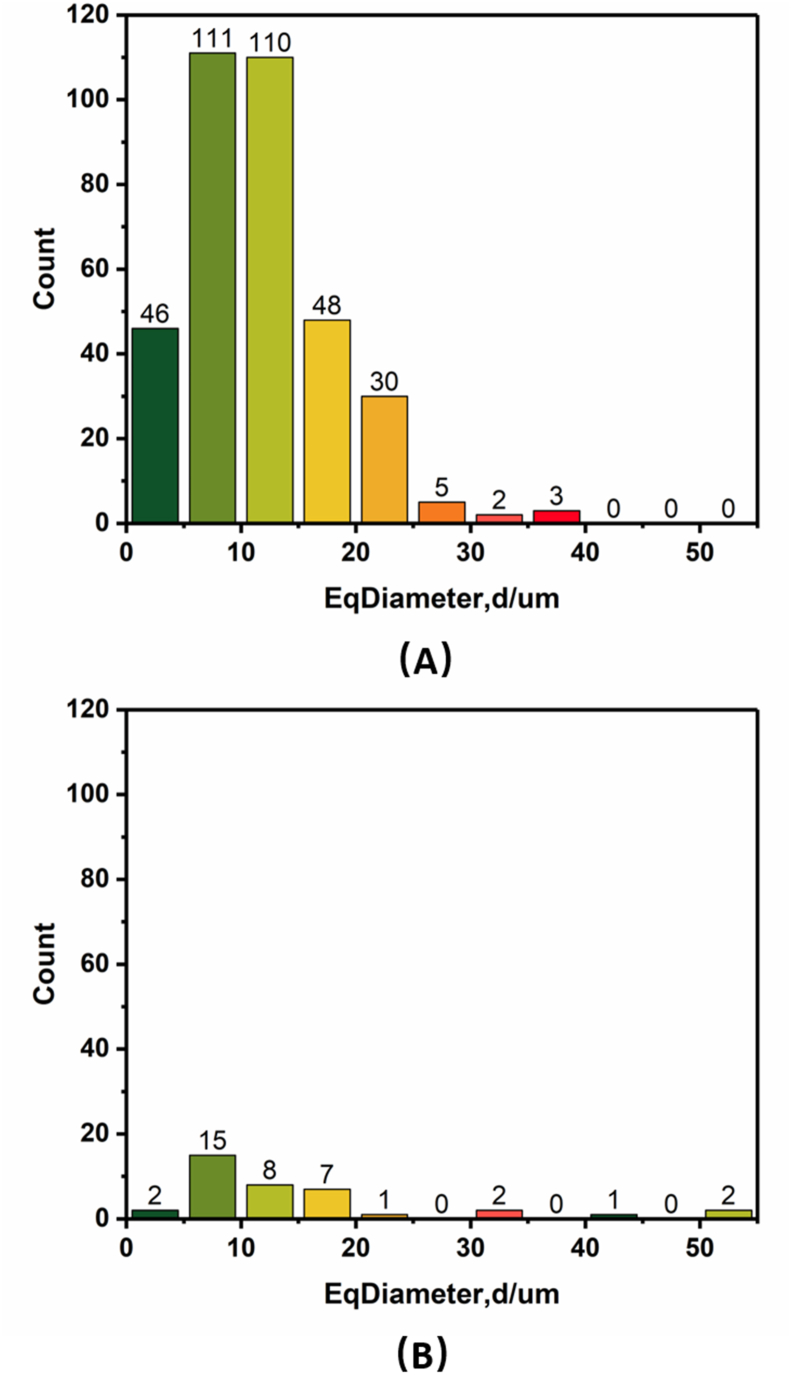
Defects are defined as voids within the solid structure that can reduce the mechanical and fatigue properties. To visualize and quantify the defects, we used micro-CT to study the samples. Fig. 6 shows micro-CT images of three kinds of samples. The results indicated that as compared with the complete solid structure of the standard machined sample, there were a great number of irregular pore structures inside the SLM-produced plate and EBM-produced plate. Furthermore, nearly all defects in EBM-produced plate were spherical in shape, whereas those in the SLM-produced plate tended to be irregular, including some cylinder-shaped defects. Fig. 7 shows the distribution of the defects in the SLM and EBM plates according to Fig. 6b and c, respectively. The results implied that the number of defects inside the EBM plate was lower than that of SLM plate. However, the EBM plate had more large-magnitude defects compared with the SLM plate.
Figure 6.
Distribution of internal defects as analyzed by micro-CT: (A) standard plate, (B) bone plate fabricated by SLM, (C) bone plate fabricated by EBM.
Figure 7.
Size and count distribution of the defects inside the bone plates as a function of equivalent diameter: (A) bone plate fabricated by SLM and (B) bone plate fabricated by EBM.
Discussion
The use of bone plate for rigid internal fixation of the skeleton after trauma is often indispensable. Rigid fixation refers to any form of fixation directly applied to the skeleton, which is strong enough to prevent the displacement between fracture segments during active functional movements.24 Due to the tremendous stress, bone plate loosening and fracture are occasionally reported in clinical application.3 During fracture healing, the bone plate must have sufficient strength to avoid flexion of the fracture site and to reduce the relative movement between the bone segments so that tissue repair and fracture healing can proceed successfully.25 Therefore, the biomechanical properties of the bone plate have become an important evaluation criterion for bone plate quality. Numerous studies have compared the biomechanical properties of 3DP technology. Yan et al.20 reported that EBM-fabricated titanium mesh scaffolds have sufficient compressive strength, which could meet the needs of clinical applications. Zhao et al.15 compared the mechanical properties of titanium alloy materials produced by SLM and EBM and found that the SLM samples had greater tensile strength but poor ductility. Liu et al.1 fabricated locking compression plates (LCP plate) with EBM technology and found their mechanical strength was significantly greater than that of standard machined LCP plates. Zhao et al.19 reported that SLM plates have a hardness similar to that of standard forged plates, whereas EBM plates have higher hardness.
Our static mechanical testing demonstrates that, as compared with the standard machined plate, the values of the 3DP plates were significantly higher, and the mechanical strength of the SLM plates was better than that of the EBM plates, in line with previous reports. The results were further plotter into stress–strain curves, which indicated that the SLM plates had larger elastic modules, followed by EBM plates, whereas the standard plates had smaller elastic modules. These results demonstrate that the 3DP plate can resist large stress without deformation. Results of the Rockwell hardness test imply that the hardness of the SLM-produced plates was higher than that of the standard machined plates, and the value of the EBM-produced plate was the smallest. However, no significant differences were found between the standard and 3DP plates. These results are different from the findings of previous research, perhaps because of the difference in details and parameter settings in the 3DP process of 3DP. In addition, the difference in test methods and instruments may have caused variance as well.
The bone plate in the maxillofacial region is subjected to cyclic stress during functional application, which can lead to stress fatigue.26 Therefore, the bone plate applied in the maxillofacial region must not only resist the stress immediately after fixation but also bear a long-term chronic cyclic load until the fracture heals. For this reason, fatigue resistance is also an important evaluation criterion of bone plate quality.27 Some related studies evaluated the fatigue performance of metals, but the results varied. Li et al.28 reported that the 3DP titanium alloy with surface treatment has nice fatigue-resistance performance and that surface treatment can significantly improve fatigue performance. Beretta et al.29 conducted a literature review and found that the fatigue properties of the 3DP titanium alloy after stress release were not significantly different from that of the traditional metals. However, Zhao et al.15 took the opposite attitude, namely, that the 3DP titanium alloy has poor fatigue properties because of its high porosity. In this study, we explored the fatigue performance of three kinds of titanium plates. The results indicated that the standard machined plate had better fatigue-resistance performance the 3DP plate did, and the EBM plate had poorer fatigue-resistance performance than did the SLM plate. Differences exist in the results of different studies, for many possible reasons may contribute to it. First, different parameter settings and details in the 3DP process may have affected the performance. In addition, different test methods and instruments could have caused diversity. Finally, different surface treatment technologies might also affect fatigue performance.
The macrostructure of materials, including surface roughness and internal defects, has a crucial impact on their mechanical and biological properties. Surface roughness can directly affect the biological properties of the bone plates by changing cell adhesion and proliferation. Ponader et al.30 found that the osteoblasts can enter the proliferation stage on either compact smooth or rough-textured surfaces, if the Ra value does not exceed 24.9 μm. When the surface roughness of the material is greater than 56.9 μm, it is not conducive for cell proliferation. Furthermore, internal defects are another important element that is defined as voids within the solid structure. The existence of internal defects will reduce the mechanical and fatigue properties of the bone plates and become an important source of fatigue fracture of the bone plates.3 Some previous researchers have studied the surface and internal defects of 3DP samples. Zhao et al.15 conducted a surface analysis using scanning electron microscopy and found that both SLM samples and EBM samples have unmelted powder particles attached on the surface. Because the particle size of the EBM samples was larger, its surface was rougher. Further micro-CT scans showed that 3DP samples have a larger porosity than that of standard forged metal. Liu et al.1 fabricated an LCP plate using EBM technology and found it to be rougher than that of a standard machined plate.
In this study, we conducted surface analyses of the 3DP bone plates. Our results indicate that 3DP plates have rougher surfaces, which can be significantly reduced with surface polishing. Thus, for metal 3DP bone implants, surface polishing is recommended to increase the biological performance.31 On the other hand, the results of micro-CT imply that more defects exist in 3DP bone plates. Moreover, compared with SLM plates, there are more large-magnitude defects in EBM plates, which may significantly reduce their fatigue resistance. Future research should focus on reducing internal defects so as to increase their fatigue–resistance properties.
In conclusion, the 3DP titanium craniomaxillofacial bone plate has good mechanical performance and surface morphology, which meet the requirements of clinical application. However, the poorer fatigue resistance and a great number of internal defects need to be further studied and modified.
Declaration of competing interest
The authors have no conflict of interest to report, and the funding agencies did not interfere with either the design of the study or the interpretation of the result.
Acknowledgements
This project was sponsored in part by the National Key Research and Development Program of China (project No. 2017YFB1104100), and the Clinical Research Plan of SHDC (project No. SHDC12019103), and Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (project No. 20152225).
Contributor Information
Lei Zhang, Email: oral66@126.com.
Xudong Wang, Email: xudongwang70@hotmail.com.
References
- 1.Liu P.C., Yang Y.J., Liu R., et al. A study on the mechanical characteristics of the EBM-printed Ti-6Al-4 V LCP plates in vitro. J Orthop Surg Res. 2014;9:106. doi: 10.1186/s13018-014-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauria A., Medeiros R., Rodrigues D.C., Sato F.R.L., Moreira R.W.F. Evaluation of cyclic and linear mechanical resistance of prebent and manually-bent plates used for maxillary advancement in orthognathic surgery. Br J Oral Maxillofac Surg. 2016;54:987–991. doi: 10.1016/j.bjoms.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Katakura A., Shibahara T., Noma H., Yoshinari M. Material analysis of AO plate fracture cases. J Oral Maxillofac Surg. 2004;62:348–352. doi: 10.1016/j.joms.2003.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Martola M., Lindqvist C., Hänninen H., Al-Sukhun J. Fracture of titanium plates used for mandibular reconstruction following ablative tumor surgery. J Biomed Mater Res B Appl Biomater. 2007;80:345–352. doi: 10.1002/jbm.b.30603. [DOI] [PubMed] [Google Scholar]
- 5.Lye K.W., Waite P.D., Wang D., Sittitavornwong S. Predictability of prebent advancement plates for use in maxillomandibular advancement surgery. J Oral Maxillofac Surg. 2008;66:1625–1629. doi: 10.1016/j.joms.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 6.Coskunses F.M., Kan B., Mutlu I., Cilasun U., Celik T. Evaluation of prebent miniplates in fixation of Le Fort I advancement osteotomy with the finite element method. J Cranio-Maxillo-Fac Surg. 2015;43:1505–1510. doi: 10.1016/j.jcms.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Chia H.N., Wu B.M. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015;9:4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Do A.V., Khorsand B., Geary S.M., Salem A.K. 3D printing of scaffolds for tissue regeneration applications. Adv Healthc Mater. 2015;4:1742–1762. doi: 10.1002/adhm.201500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei H.P., Wang X.D. Advances in titanium bone implants made by rapid prototyping technology. Chinese J Tissue Eng Res. 2017;21:3583–3588. [Google Scholar]
- 10.Lin X.Z., Xiao X.L., Wang Y.M., et al. Biocompatibility of bespoke 3D-printed titanium alloy plates for treating acetabular fractures. BioMed Res Int. 2018:2053486. doi: 10.1155/2018/2053486. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bose S., Vahabzadeh S., Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today. 2013;16:496–504. [Google Scholar]
- 12.Choi J.W., Kim N. Clinical application of three-dimensional printing technology in craniofacial plastic surgery. Arch Plast Surg. 2015;42:267–277. doi: 10.5999/aps.2015.42.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerea M., Dolcini G.A. Custom-made direct metal laser sintering titanium subperiosteal Implants: a retrospective clinical study on 70 patients. BioMed Res Int. 2018:5420391. doi: 10.1155/2018/5420391. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewandowski J.J., Seifi M. Metal additive manufacturing: a review of mechanical properties. Annu Rev Mater Sci. 2016;46:151–186. [Google Scholar]
- 15.Zhao X., Li S., Zhang M., et al. Comparison of the microstructures and mechanical properties of Ti-6Al-4V fabricated by selective laser melting and electron beam melting. Mater Des. 2016;95:21–31. [Google Scholar]
- 16.Zhai Y., Galarraga H., Lados D.A. Microstructure evolution, tensile properties, and fatigue damage mechanisms in Ti-6Al-4V alloys fabricated by two additive manufacturing techniques. Procedia Eng. 2015;114:658–666. [Google Scholar]
- 17.Murr L.E., Esquivel E.V., Quinones S.A., et al. Microstructures and mechanical properties of electron beam-rapid manufactured Ti–6Al–4V biomedical prototypes compared to wrought Ti–6Al–4V. Mater Char. 2009;60:96–105. [Google Scholar]
- 18.Murr L.E., Quinones S.A., Gaytan S.M., et al. Microstructure and mechanical behavior of Ti-6Al-4V produced by rapid-layer manufacturing, for biomedical applications. J Mech Behav Biomed Mater. 2009;2:20–32. doi: 10.1016/j.jmbbm.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Zhao B.J., Wang H., Yan R.Z., Wang C., Niu Y., Hu M. Properties evaluation of a Ti–6Al–4V alloy scaffold fabricated by electron beam melting and selective laser melting for bone tissue engineering. J Biomater Tissue Eng. 2016;6:832–842. [Google Scholar]
- 20.Yan R.Z., Li Y.F., Wang C., Li R.X., Liu Z.W., Hu M. A preliminary study on the mechanical characteristics of the titanium scaffolds with three-dimensional mesh structure fabricated by electron beam melting. Chin J Stomatol. 2016;51:656–660. doi: 10.3760/cma.j.issn.1002-0098.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Zhao B., Wang H., Qiao N., Wang C., Hu M. Corrosion resistance characteristics of a Ti-6Al-4V alloy scaffold that is fabricated by electron beam melting and selective laser melting for implantation in vivo. Mater Sci Eng C Mater Biol Appl. 2017;70:832–841. doi: 10.1016/j.msec.2016.07.045. [DOI] [PubMed] [Google Scholar]
- 22.Wang H., Zhao B., Liu C., Wang C., Tan X., Hu M. A comparison of biocompatibility of a titanium alloy fabricated by electron beam melting and selective laser melting. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao B.J., Wang H., Liu C.K., et al. Evaluation of Ti–6Al–4V alloy biocompatibility fabricated by electron beam melting in vitro and in vivo. J Biomater Tiss Eng. 2016;6:574–581. [Google Scholar]
- 24.Ellis Edward. Rigid skeletal fixation of fractures. J Oral Maxillofac Surg. 1993;51:163–173. doi: 10.1016/s0278-2391(10)80016-3. [DOI] [PubMed] [Google Scholar]
- 25.Jazayeri H.E., Khavanin N., Yu J.W., et al. Fixation points in the treatment of traumatic zygomaticomaxillary complex fractures: a systematic review and meta-analysis. J Oral Maxillofac Surg. 2019;77:2064–2073. doi: 10.1016/j.joms.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Dong S., Wang C., Qi B., et al. Parametric study on material selection and effect of pre-tightening force for humerus implants. Chin J Bone Joint Surg. 2014;7:35–39. [Google Scholar]
- 27.Probst F.A., Mast G., Ermer M., et al. MatrixMANDIBLE preformed reconstruction plates--a two-year two-institution experience in 71 patients. J Oral Maxillofac Surg. 2012;70:e657–e666. doi: 10.1016/j.joms.2012.06.175. [DOI] [PubMed] [Google Scholar]
- 28.Li P., Warner D.H., Fatemi A., Phan N. Critical assessment of the fatigue performance of additively manufactured Ti–6Al–4V and perspective for future research. Int J Fatig. 2016;85:130–143. [Google Scholar]
- 29.Beretta S., Romano S. A comparison of fatigue strength sensitivity to defects for materials manufactured by AM or traditional processes. Int J Fatig. 2017;94:178–191. [Google Scholar]
- 30.Ponader S., Vairaktaris E., Heinl P., et al. Effects of topographical surface modifications of electron beam melted Ti-6Al-4V titanium on human fetal osteoblasts. J Biomed Mater Res. 2008;84:1111–1119. doi: 10.1002/jbm.a.31540. [DOI] [PubMed] [Google Scholar]
- 31.Giannuzzi L.A., Phifer D., Giannuzzi N.J., Capuano M.J. Two-dimensional and 3-dimensional analysis of bone/dental implant interfaces with the use of focused ion beam and electron microscopy. J Oral Maxillofac Surg. 2007;65:737–747. doi: 10.1016/j.joms.2006.10.025. [DOI] [PubMed] [Google Scholar]