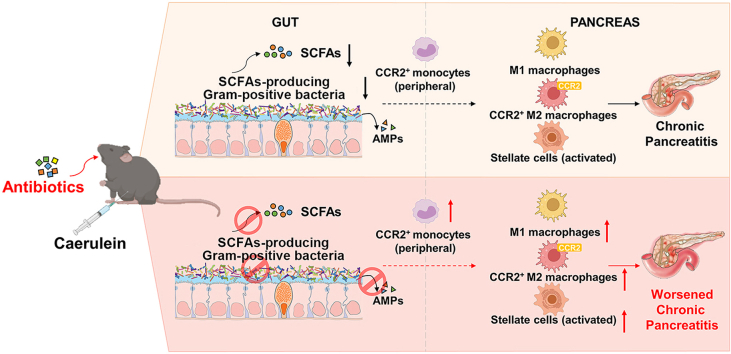
Abstract
Chronic pancreatitis (CP) is a progressive and irreversible fibroinflammatory disorder, accompanied by pancreatic exocrine insufficiency and dysregulated gut microbiota. Recently, accumulating evidence has supported a correlation between gut dysbiosis and CP development. However, whether gut microbiota dysbiosis contributes to CP pathogenesis remains unclear. Herein, an experimental CP was induced by repeated high-dose caerulein injections. The broad-spectrum antibiotics (ABX) and ABX targeting Gram-positive (G+) or Gram-negative bacteria (G–) were applied to explore the specific roles of these bacteria. Gut dysbiosis was observed in both mice and in CP patients, which was accompanied by a sharply reduced abundance for short-chain fatty acids (SCFAs)-producers, especially G+ bacteria. Broad-spectrum ABX exacerbated the severity of CP, as evidenced by aggravated pancreatic fibrosis and gut dysbiosis, especially the depletion of SCFAs-producing G+ bacteria. Additionally, depletion of SCFAs-producing G+ bacteria rather than G– bacteria intensified CP progression independent of TLR4, which was attenuated by supplementation with exogenous SCFAs. Finally, SCFAs modulated pancreatic fibrosis through inhibition of macrophage infiltration and M2 phenotype switching. The study supports a critical role for SCFAs-producing G+ bacteria in CP. Therefore, modulation of dietary-derived SCFAs or G+ SCFAs-producing bacteria may be considered a novel interventive approach for the management of CP.
Key words: Chronic pancreatitis, Antibiotic exposure, Gut microbiota, Short-chain fatty acids-producing bacteria, Pancreatic fibrogenesis, Macrophage responses, Toll-like receptor 4, Roseburia intestinalis
Graphical abstract
Antibiotics aggravate gut dysbiosis and reduce SCFAs-producing Gram-positive bacteria. Depletion of Gram-positive SCFAs-producers causes reduced SCFA production, which accelerates monocyte recruitment, macrophage deregulation and pancreatic fibrosis, promoting the progression of chronic pancreatitis.
1. Introduction
Chronic pancreatitis (CP) is a progressive inflammatory and fibrotic disease of the pancreas with no satisfactory cure1. CP is characterized by persistent inflammation and progressive fibrosis, leading to irreversible morphological changes of the gland, most notably parenchymal atrophy, along with gradual impairment of exocrine and endocrine functions2. To date, the incidence and prevalence of CP have been rising and no effective therapy is available to limit or reverse the inflammatory damage or pancreatic fibrosis associated with CP2. CP reduces patients’ quality of life and is also a significant risk factor for developing pancreatic cancer3. Further understanding of the disease pathogenesis and identification of novel player(s) that contribute to it are required for the development of novel therapeutic strategies for CP.
Accumulating evidence has supported the association of gut dysbiosis with the development of CP. A large number of CP patients have intestinal bacterial overgrowth4, which is closely associated with exocrine insufficiency5. Moreover, small intestinal bacterial overgrowth complicates CP and interferes with the therapy, which is supportive for the involvement of gut dysbiosis in CP4,6,7. An altered intestinal microbiota has been demonstrated in CP patients and experimental CP models8,9. At the genus level, the abundances of Faecalibacterium and Subdoligranulum are reduced, which have been demonstrated to have anti-inflammatory and barrier-protective effects10,11. In contrast, the pro-inflammatory Escherichia-Shigella are the dominant genera in the fecal microbiomes of CP patients12. Alterations in the gut microbiota composition were also observed in experimental CP mice, as evidenced by decreased abundances of Lachnospiraceae_NK4A136, Ruminiclostridium and Roseburia and increased abundance of Bacteroides8. Despite a rather established close association, few studies have looked into specific roles of different gut microbes in the pathogenesis of CP.
Pancreatic fibrosis is a major histological feature of CP, caused by uncontrolled activation and proliferation of pancreatic stellate cells (PSCs)13,14 and dysregulated overproduction of extracellular matrix (ECM) proteins. Under steady-state, PSCs maintain the normal architecture of the pancreas by regulating the synthesis and degradation of ECM proteins14. Macrophages, particularly the alternatively activated or M2 macrophages, are thought to play an important role in regulating PSCs-mediated fibrogenesis. During CP pathogenesis, M2 macrophages secrete massive transforming growth factor-β and platelet-derived growth factor, resulting in aberrant activation of PSCs and massive synthesis of ECMs, eventually causing pancreatic fibrosis15. Thus, inhibition of M2-fibrogenesis represents a promising therapeutic approach for CP.
In the current study, we applied broad-spectrum antibiotics (ABX) and ABX targeting Gram-positive (G+) or Gram-negative (G–) bacteria to explore the specific roles of gut bacteria in CP. Our data reveal a vital role of short-chain fatty acids (SCFAs)-producing G+ bacteria in the modulation of CP and the intervention strategy targeting commensal bacteria for the management of CP.
2. Materials and methods
2.1. Reagents
Caerulein was purchased from Ji Tai Peptide (Yancheng, China). Pentobarbital sodium, acetate, butyrate, propionate and fluorescein isothiocyanate (FITC)-dextran 4000 were purchased from Sigma–Aldrich (Shanghai, China). Vancomycin, neomycin, ampicillin and metronidazole were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). RIPA Lysis Buffer was purchased from Beyotime (Shanghai, China). For immunofluorescence assay, the primary antibody for α-smooth muscle actin (α-SMA, 48938S) was purchased from Cell Signaling Technology (Danvers, MN, USA). Rabbit anti-mouse IgG (H + L) cross-adsorbed secondary antibody (A11059) was purchased from Thermo Fisher Scientific (Waltham, MA, USA). TRIzol reagent was purchased from Life Technologies (Waltham, MA, USA). 4′,6-Diamidino-2-phenylindole (DAPI) was purchased from Solarbio (Beijing, China). Collagenase-P was purchased from Roche (Basel, Basel-City, Switzerland). The following antibodies were used for flow cytometry: PE/Cyanine7 anti-mouse CD45 Antibody (103114), Brilliant Violet 605 anti-mouse/human CD11b Antibody (101257), Brilliant Violet 421 anti-mouse F4/80 antibody (123137), APC anti-mouse CD206 (MMR) antibody (141708), Alexa Fluor 488 anti-mouse Ly-6C antibody (128021) and PE anti-mouse CD192 C–C motif chemokine receptor 2 (CCR2) antibody (150609) from Biolegend (San Diego, CA, USA) and inducible nitric oxide synthase (iNOS) monoclonal antibody (CXNFT), PE from eBioscience (12-5920-80; Waltham, MA, USA).
2.2. Bacterial strains
Roseburia intestinalis DSM 14610 (R. intestinalis) was purchased from Mingzhou Biotechnology Co., Ltd. (Ningbo, China). R. intestinalis was cultured in Modified Reinforced Clostridial Broth for 48–72 h at 37 °C under anaerobic conditions. For further use, bacteria were subsequently collected by centrifugation at 8000×g for 10 min (Centrifuge 5810R; Eppendorf, Hamburg, Germany) and resuspended in 200 μL phosphate buffer saline (PBS).
2.3. Animals and treatments
Male C57BL/6J mice (7–8 weeks old weighing 20 ± 2 g; JOINN Laboratories; Suzhou, China), Tlr4−/− C57BL/6J mice and littermate control wild-type mice (7–8 weeks old weighing 20 ± 2 g; Gempharmatech Co., Ltd.; Nanjing, China) were maintained in a specific pathogen-free environment at the Experimental Animal Center of Jiangnan University (Wuxi, China) with controlled temperature (24 ± 1 °C) and 12 h light/dark cycle and had free access to standard chow (Xietong Pharmaceutical Bio-engineering Co., Ltd.; Nanjing, China). All mice were adjusted to laboratory conditions 1 week before the experiments. All animal care and experimental protocols complied with the guidelines of and were approved by the Animal Ethics Committee of Jiangnan University (Approval Nos.: JN. No20180615c0500930 [97], JN. No20190915c0501208 [202], JN. No20191230c0900331 [375] and JN. No20221015c0501213 [438]).
CP was induced by repetitive caerulein injections. In brief, mice were given 6 intraperitoneal injections of caerulein (50 μg/kg body weight) per day at hourly intervals, 3 days per week, for a total of 4 weeks. Control mice were injected with vehicle saline. Mice were then sacrificed and analyzed on Day 3 after the last caerulein injection by a lethal dose of pentobarbital sodium. For the differentially targeted commensal bacterial disruption experiment, the mice were treated with broad-spectrum ABX (0.5 g/L vancomycin, 1 g/L neomycin, 1 g/L ampicillin, and 1 g/L metronidazole)16, G+ ABX (0.5 g/L vancomycin)17 or G– ABX (1 g/L neomycin)18. The mice were given antibiotics (200 μL/mouse) daily by gavage from a week before the caerulein injection to the sacrificed day. For the SCFA supplementation experiment, mice were treated with SCFA mix (67.5 mmol/L acetate, 40 mmol/L butyrate and 25.9 mmol/L propionate) in the drinking water and water solutions were changed daily19. For bacterial supplementation experiment, mice were orally administrated with R. intestinalis at a dose of 1010 colony forming units in 200 μL PBS/mice daily for 5 weeks. The experimental design of CP mice with different treatments was illustrated in Supporting Information Fig. S1.
2.4. Human samples
The recruitment of human subjects was approved by the ethics committee of Changhai Hospital Affiliated with The Second Military Medical University (CHEC2018-132). All subjects provided informed consent before participation and the experiments conformed to the principles set out in the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report. CP patients presented with one of the conditions, including pancreatic calcification, moderate or severe pancreatic ductal changes, abnormal pancreatic function test, endoscopic ultrasound with signs of CP or histological change of CP as described by the Asia–Pacific consensus20.
2.5. Histological analysis
Fresh pancreatic and colonic samples were fixed in 4% paraformaldehyde overnight, washed with running water for 2 h, rehydrated with gradient ethanol and then embedded in paraffin. The skiving machine slicer (Leica; Wetzlar, Hessen, Germany) diced 5 μm sections were stained with hematoxylin and eosin (H&E) or Masson's Trichrome (Sbjbio; Nanjing, China) following the standard procedures. The morphology evaluation was recorded by a digital slice scanner (3DHISTCH Ltd.; Budapest, Hungary). Pancreatic parenchymal rate is the area ratio of pancreas to the visual field, which represents the atrophic degree of the pancreas. Histological scoring of the colon according to the degree of shortening of the villi, 0: none, 1: mild, 2: moderate, 3: marked.
2.6. SCFA measurement
The SCFAs (acetate, propionate and butyrate), present in the colon content, were analyzed by gas chromatography-mass spectrometry as our previous reports21. Freeze-dried colon content (50 mg) was homogenized in 500 μL of saturated NaCl solution and then acidified with 40 μL 10% sulfuric acid. Diethyl ether of 0.8 mL was added to the sample to extract SCFAs. Samples were then centrifuged at 14,000 rpm for 15 min at 4 °C (Centrifuge 5810R; Eppendorf) and the supernatants were used for the analysis of the SCFAs. Supernatants were injected into Rtx-WAX capillary column (30 m × 0.25 mm × 0.25 μm; Shimadzu; Kyoto, Japan) installed on the GC and coupled to the MS detector of GCMS-QP2010 (Shimadzu). The initial oven temperature was 100 °C and increased to 140 °C at a rate of 7.5 °C/min. The temperature was further increased to 200 °C at a rate of 60 °C/min and remained for 3 min. Helium was utilized as the carrier gas at a flow rate of 0.89 mL/min and the column head pressure was 62.7 kPa. The injector was set at 240 °C. The injection mode was split and the ratio was 10:1. For the mass spectrometer, the ion source temperature was 220 °C, the interface temperature was 250 °C and the scan range was from m/z 2 to 100. Real-time analysis software GCMS Postrun (GCMS solution Version 2.72; Shimadzu) was employed to compare the relative concentrations of SCFAs.
2.7. Microbiota composition analysis
Total DNA was extracted from mouse colon content and human feces using the Fast DNA Spin Kit for Feces (MP Biomedicals; Shanghai, China) according to the manufacturer's instructions. The PCR amplification products were separated by 1% agarose gel electrophoresis and the Gel/PCR Extraction Kit (BIOMIGA; San Diego, CA, USA) was used to recover the target fragments. DNA was extracted from samples and the V3–V4 hypervariable region of the 16S rRNA gene was amplified and sequenced using the Illumina MiSeq platform. Taxonomies that were significantly enriched among different groups were identified by LEfSe using default settings. Other analyses including heatmaps and principal coordinates analysis plots were performed with internal scripts.
2.8. Gut permeability assay
For gut permeability assessment, 4 h fasted mice received 400 mg/kg FITC-dextran 4000 by gavage and blood was collected 4 h later. The serum concentration of FITC-dextran was determined by fluorometry (excitation 485 nm, emission 520 nm) on a microplate reader (FLUOstar Optima; BMG Labtech; Offenburg, Baden-Württemberg, Germany).
2.9. Enzyme-linked immunosorbent assays
Pancreatic monocyte chemoattractant protein-1 (MCP-1) level was measured with the Mouse CCL2/JE/MCP-1 Quantikine ELISA Kit from R&D System (Minneapolis, MN, USA) according to the protocols of the manufacturer. Absorbance was measured at 450 nm with a microplate reader Multiclan GO (Thermo Fisher Scientific). Pancreatic samples were homogenized in a saline solution (1:19, w/v). Samples were centrifuged at 4 °C, 10,000×g for 10 min (Centrifuge 5810R; Eppendorf). Protein concentrations were determined by a BCA Protein Assay Kit (Beyotime) during sample preparation to ensure that an equal amount of total protein was applied for cytokine measurements.
2.10. Quantification of mRNA by real-time quantitative PCR (RT-qPCR)
The mRNA levels for Tgfb1, Col1, Acta2, Fn1, Camp, Defb1, Defb2, Reg1 and Reg4 were analyzed by RT-qPCR. Total RNA was extracted from pancreatic tissues using TRIzol Reagent and was subjected to reverse transcription using a PrimeScript RT reagent Kit (TaKaRa Bio; Kyoto, Japan) following the instructions of the manufacturer. SYBR Green RT-qPCR was performed using the RT-qPCR system (Bio-Rad; Berkeley, CA, USA). The relative mRNA levels were normalized to mRNA levels of Actb (housekeeping control) and calculations for fold change of each mRNA were made on the comparative cycle threshold method (2−ΔΔCt). The primers used in this study were provided in Table 1.
Table 1.
Specific primers for RT-qPCR.
| Gene | Forward | Reverse |
|---|---|---|
| Tgfb1 | 5′-CCCTATATTTGGAGCCTGGA-3′ | 5′-CTTGCGACCCACGTAGTAGA-3′ |
| Col1 | 5′-GCTCCTCTTAGGGGCCACT-3′ | 5′-CCACGTCTCACCATTGGGG-3′ |
| Acta2 | 5′-GTCCCAGACATCAGGGAGTAA-3′ | 5′-TCGGATACTTCAGCGTCAGGA-3′ |
| Fn1 | 5′-ATGTGGACCCCTCCTGATAGT-3′ | 5′-GCCCAGTGATTTCAGCAAAGG-3′ |
| Camp | 5′-GCTGTGGCGGTCACTATCA-3′ | 5′-TGTCTAGGGACTGCTGGTTGA-3′ |
| Defb1 | 5′-AGGTGTTGGCATTCTCACAAG-3′ | 5′-GCTTATCTGGTTTACAGGTTCCC-3′ |
| Defb2 | 5′-CTGCTGCTGATATGCTGCCTC-3′ | 5′-TAAACTTCCAACAGCTGGAGTGG-3′ |
| Reg1 | 5′-ATGGCTAGGAACGCCTACTTC-3′ | 5′-CCCAAGTTAAACGGTCTTCAGT-3′ |
| Reg4 | 5′-GGCGTGCGGCTACTCTTAC-3′ | 5′-GGAAGTATCCATAGCAGTGGGA-3′ |
| Actb | 5′-AATCCCATCACCATCTTCCA-3′ | 5′-TGGACTCCACGACGTACTCA-3′ |
2.11. Immunofluorescence
The 5-μm thick tissue sections were cut from paraffin-embedded blocks. Antigen retrieval was performed for 30 min. Incubation with the anti-α-SMA antibody (1:200) was performed overnight at 4 °C. Secondary antibody incubation was performed for 1 h at room temperature. The DAPI staining was conducted according to the protocol provided by the manufacturer. Carl Zeiss microscope (Axio Vert A1; Jena, Thuringia, Germany) was used for the evaluation of immunofluorescence staining.
2.12. Flow cytometry
Freshly harvested pancreatic tissue samples were digested in 0.75 mg/mL collagenase-P solution at 37 °C for 15 min. Subsequently, tissue was homogenized in gentleMACS Dissociators (Miltenyi Biotec; Cologne, Bergisch Gladbach, Germany) and filtered through a 75-μm filter screen with PBS. Single-cell suspensions were incubated for 15 min at room temperature in PBS with the following antibodies: PE/Cyanine7 anti-mouse CD45, Brilliant Violet 605™ anti-mouse CD11b, Brilliant Violet 421™ anti-mouse F4/80, APC anti-mouse CD206 (MMR), Alexa Fluor® 488 anti-mouse Ly-6C, PE anti-mouse CCR2 and PE anti-mouse iNOS. Gating methods of fluorescence-activated cell sorting were programmed as CD45+CD11b+Ly6ChiCCR2+ for CCR2+ monocytes (Supporting Information Fig. S2A), CD45+CD11b+F4/80+ for macrophages, CD45+CD11b+F4/80+iNOS+ for M1 macrophages, CD45+CD11b+F4/80+CD206+ for M2 macrophages and CD45+CD11b+F4/80+CD206+CCR2+ for CCR2+ M2 macrophages (Fig. S2B). Stained cells were analyzed on an Attune NxT Flow Cytometer (Thermo Fisher Scientific) and data were analyzed using FlowJo software (BD Bioscience; Bergen County, NJ, USA).
2.13. Statistical analysis
The sample size declared in the different experimental groups was the number of independent mice in each group and statistical analysis was performed using these independent values. Data are expressed as mean with standard deviation (SD). For flow cytometry statistical analysis, data were expressed as median with interquartile range. P < 0.05 was considered statistically significant. Differences among three or more groups were determined using analysis of variance (ANOVA) followed by Tukey's post hoc test. All data were analyzed using GraphPad Prism 8 software (San Diego, CA, USA).
3. Results
3.1. Broad-spectrum ABX intensifies gut dysbiosis with decreased SCFAs-producing bacteria in CP
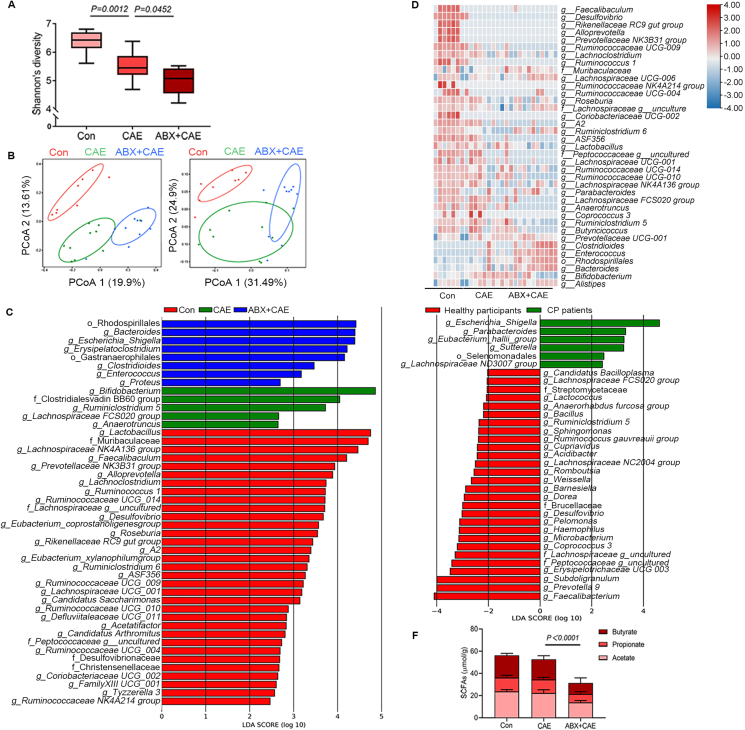
Several studies have demonstrated the association between gut dysbiosis and CP development. Patients and mice with CP both exhibit gut dysbiosis with decreased diversity and richness and taxa-composition changes8,22, 23, 24. Firstly, we examined how gut microbiota depletion by broad-spectrum ABX affected CP pathogenesis. As expected, the Shannon index revealed that the diversity of the intestinal microbiota was markedly decreased in mice with CP (Fig. 1A). Broad-spectrum ABX treatment further reduced the microbiota diversity (Fig. 1A). Moreover, the microbiota communities were remarkably different among control, CP and broad-spectrum ABX-treated CP mice (Fig. 1B). LEfSe analyses of the predominant microbial taxa confirmed altered microbial compositions among the three groups. CP mice and broad-spectrum ABX-treated CP mice exhibited remarkably reduced compositions of the preferential microbial taxa, compared with the control group (Fig. 1C). Interestingly, the predominant microbial taxa in control mice were mostly SCFAs-producers, which were significantly reduced in CP mice and broad-spectrum ABX-treated CP mice (Fig. 1C and Supporting Information Fig. S3, Tables S1 and S2). These results were further evidenced by heatmap analysis of SCFAs-producers in the three groups of mice (Fig. 1D). Compared with control mice, CP mice and broad-spectrum ABX-treated CP mice displayed reduced abundances of predominant SCFAs-producers, especially SCFAs-producing G+ bacteria, including Ruminococcus 1, Ruminococcaceae UCG-009, Peptococcaceae, Lachnospiraceae UCG-001, Lactobacillus, Roseburia and Lachnoclostridium, with a more pronounced effect observed in ABX-treated CP mice (Fig. 1D). LEfSe analyses of the fecal microbiota of 69 healthy participants and 71 CP patients showed that the abundance of SCFAs-producing bacteria was significantly decreased in CP patients compared with healthy participants, especially SCFAs-producing G+ bacteria, including Faecalibacterium, Subdoligranulum, Peptococcaceae and Lachnospiraceae (Fig. 1E and Supporting Information Table S3). These changes were consistent with what was observed in CP mice. Given that SCFAs-producing bacteria were primarily affected during disease progression, we next examined the levels of colonic SCFAs in different groups. Not surprisingly, depletion of predominant SCFAs-producing bacteria in broad-spectrum ABX-treated CP mice was accompanied by decreased levels of colonic SCFAs (acetate, propionate and butyrate) (Fig. 1F). Collectively, broad-spectrum ABX treatment aggravates gut dysbiosis and importantly decreases the abundance of SCFAs-producing commensal bacteria in mice with CP.
Figure 1.
A broad-spectrum antibiotic (ABX) cocktail intensifies gut dysbiosis and reduction of short-chain fatty acids (SCFAs)-producing bacteria in chronic pancreatitis (CP). (A) The diversity shown by Shannon's index (Con, n = 8; CAE, n = 10; ABX + CAE, n = 10). (B) Principal coordinates analysis comparing the microbiome composition in colon content of mice (Con, n = 8; CAE, n = 10; ABX + CAE, n = 10; left: unweighted, right: weighted). (C) The LEfSe analyses of microbiome composition (based on abundance) distribution of three groups of mice, histogram of the LDA scores reveals the most differentially abundant taxa among different treatments (Con, n = 8; CAE, n = 10; ABX + CAE, n = 10). (D) Heatmap representation of SCFAs-producing bacteria (based on absolute count) in three groups of mice (Con, n = 8; CAE, n = 10; ABX + CAE, n = 10). (E) The LEfSe analyses of microbiome composition distribution of healthy participants and CP patients (healthy participants, n = 69; CP patients, n = 71). (F) The levels of SCFAs (acetate, propionate and butyrate) in colon content (n = 6). Data (A) were representative and were the min to the max from three independent experiments. Data (F) are representative and were the mean ± standard deviation (SD) from three independent experiments. P values were calculated by one-way analysis of variance (ANOVA) followed by Tukey's post hoc test for multiple comparisons.
3.2. Broad-spectrum ABX treatment aggravates the severity of CP
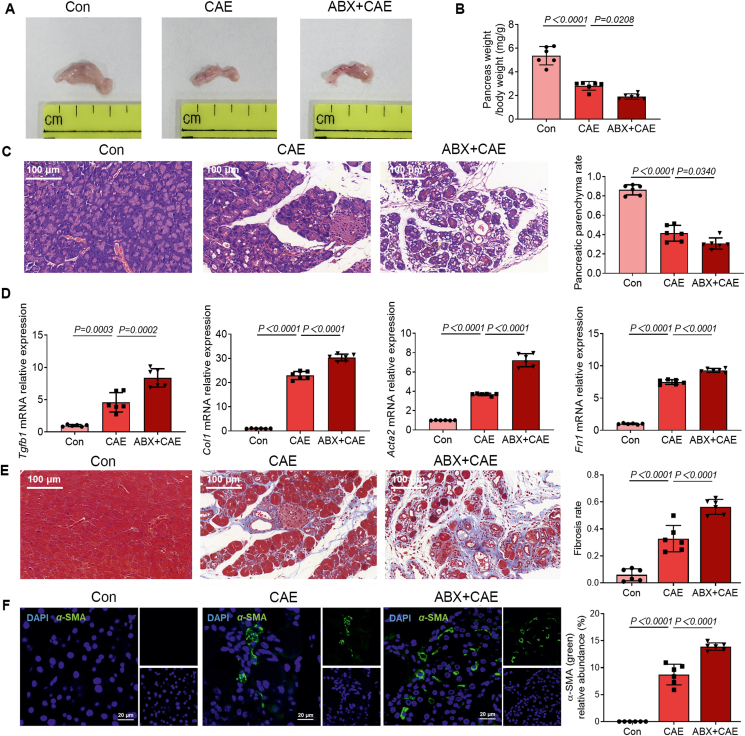
Next, we examined how microbiota depletion by broad-spectrum ABX affected the development of CP. Broad-spectrum ABX worsened pancreatic atrophy and weight loss, which are symptomatic characteristics of CP (Fig. 2A and B). Histological examination of pancreatic sections also confirmed an overall aggravated index of disease severity after broad-spectrum ABX treatment (Fig. 2C). Pancreatic atrophy and/or fibrosis cause the irreversible destruction of pancreatic parenchyma25. CP induction significantly decreased pancreatic parenchyma rate and broad-spectrum ABX further exacerbated the condition (Fig. 2C). Pancreatic fibrosis is a hallmark of CP and contributes to gradual pancreatic insufficiency and is majorly associated with the activation of PSCs25,26. Next, we demonstrated that the mRNA expression of profibrotic growth factors Tgfb1, Col1, Acta2 and Fn1 in the pancreas was highly induced by broad-spectrum ABX treatment compared with the untreated CP group (Fig. 2D). To determine the extent of fibrosis, sections were evaluated by Masson's Trichrome staining, which preferentially labels collagen fibrils with blue color27. Depletion of commensal bacteria aggravated the deposition of collagen fibers in CP (Fig. 2E), which was accompanied by upregulation of α-SMA in the pancreas (Fig. 2F). Together, these results show that microbiota depletion caused by broad-spectrum ABX exacerbates the severity of CP.
Figure 2.
A ABX cocktail aggravates CP. (A) The representative macrographic images of the pancreas. (B) The ratio of pancreatic weight and body weight (n = 6). (C) The representative images and parenchyma rates of the pancreas by hematoxylin and eosin (H&E) staining (n = 6). Scale bar: 100 μm. (D) The fibrosis-associated gene (Tgfb1, Col1, Acta2 and Fn1) expression in the pancreas (n = 6). (E) The representative images and fibrosis rates of the pancreas by Masson's Trichrome staining (n = 6). Scale bar: 100 μm. (F) Localization and expression of α-smooth muscle actin (α-SMA) (green) in the pancreas by immunofluorescent staining (n = 6). Representative photomicrographs of individual and merged staining were shown. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bar: 20 μm. Data (B–F) are representative and were the mean ± SD from three independent experiments. P values were calculated by one-way ANOVA followed by Tukey's post hoc test for multiple comparisons.
3.3. Depletion of G+ bacteria but not G– bacteria diminishes SCFAs-producing G+ bacteria and aggravates the severity of CP
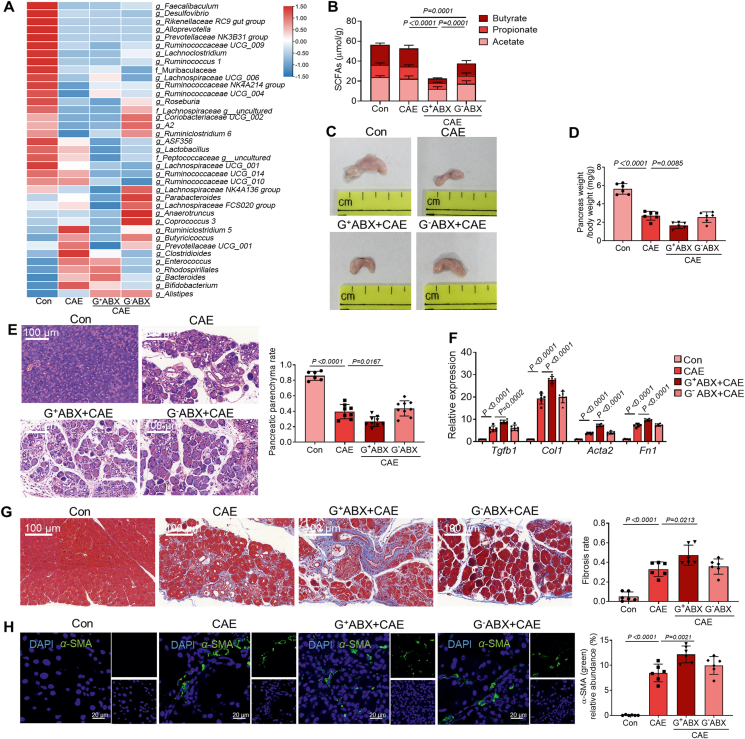
To further reveal the effect of different commensal bacteria on the development of CP, we further applied vancomycin to deplete G+ bacteria and neomycin to eliminate G– bacteria. LEfSe analyses of microbiome composition revealed that both G+ ABX and G– ABX impacted the relative abundances of SCFAs-producing bacteria (Supporting Information Fig. S4 and Table S4). Heatmap representation of distributions of SCFAs-producers in four different groups further showed that G– ABX significantly decreased the relative abundances of SCFAs-producing G– bacteria Bacteroides28 and Rhodospirillales29 (Fig. 3A). G+ ABX significantly reduced the abundances of SCFAs-producing G+ bacteria including Ruminococcus 1, Ruminococcaceae UCG-009, Peptococcaceae, Lachnospiraceae UCG-001, Lactobacillus, Roseburia and Lachnoclostridium (Fig. 3A). Both G– ABX and G+ ABX caused significant decreases in SCFA levels, with a more pronounced effect observed for G+ ABX (Fig. 3B). However, only G+ ABX further aggravated CP-associated intestinal barrier damage (Supporting Information Fig. S5).
Figure 3.
Depletion of Gram-positive bacteria causes decreased abundances of SCFAs-producing Gram-positive bacteria and intensifies CP. (A) Heatmap representation of SCFAs-producing bacteria in four groups of mice. (Con, n = 8; CAE, n = 10; G+ ABX+CAE, n = 10; G– ABX + CAE, n = 10). (B) SCFA concentrations (acetate, propionate and butyrate) in colon content (n = 6). (C) The representative macrographic images of the pancreas. (D) The ratio of pancreatic weight and body weight (n = 6). (E) The representative images and parenchyma rates of the pancreas by H&E staining (n = 6). Scale bar: 100 μm. (F) The fibrosis-associated gene (Tgfb1, Col1, Acta2 and Fn1) expression in the pancreas (n = 6). (G) The representative images and fibrosis rates of the pancreas by Masson's Trichrome staining (n = 6). Scale bar: 100 μm. (H) Localization and expression of α-SMA (green) in the pancreas by immunofluorescent staining (n = 6). Representative photomicrographs of individual and merged staining were shown. Nuclei were stained with DAPI (blue). Scale bar: 20 μm. Data (B, D–H) were representative and were the mean ± SD from three independent experiments. P values were calculated by one-way ANOVA followed by Tukey's post hoc test for multiple comparisons.
G+ ABX but not G– ABX further aggravated pancreatic atrophy (Fig. 3C) and pancreatic weight loss (Fig. 3D). Additionally, depletion of G+, but not G– bacteria exaggerated typical features of CP, as evidenced by an increased index of disease severity (Fig. 3E), pancreatic fibrosis (Fig. 3F and G) and the activation of PSCs (Fig. 3H). Collectively, these results show that disruption of G+ bacteria accelerates the severity of CP, pointing to a critical role of SCFAs-producing G+ bacteria.
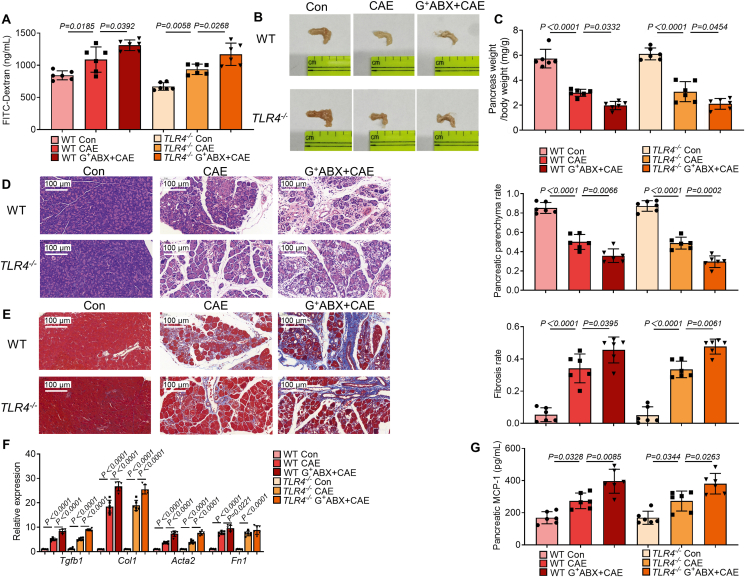
3.4. Toll-like receptor 4 (TLR4) signaling is not involved in G+ ABX-induced exacerbation of CP
TLR4 is the receptor of lipopolysaccharide and plays a vital role in mediating the effects of G– bacteria30. We therefore investigated whether TLR4 is involved in G+ ABX-induced exacerbation of CP by using TLR4 knockout mice (TLR4−/− mice). The results showed no difference in intestinal permeability (Fig. 4A), pancreatic atrophy (Fig. 4B), pancreatic weight loss (Fig. 4C), pancreatic histology (Fig. 4D) and pancreatic fibrosis (Fig. 4E and F) in TLR4−/− mice following CP induction compared with their wild type (WT) controls. Activated PSCs exacerbate CP by secreting large amounts of MCP-1 to recruit its C–C Motif chemokine receptor 2 (CCR2)-positive inflammatory monocytes from peripheral blood to the pancreas31,32. Therefore, next, we examined the expression of MCP-1 and proportions of plasma monocytes and pancreatic macrophages. Compared with control mice, the expression of pancreatic MCP-1 and the populations of CCR2+ monocytes were significantly increased in G+ ABX-treated TLR4−/− and WT with CP (Fig. 4G and Supporting Information Fig. S6A). As a key cell type involved in pancreatic fibrosis, the activation of PSCs facilitates the activation of M2 macrophage polarization and CP progression15. Herein, we found significantly enhanced frequencies of pancreatic macrophages, M1 macrophages, M2 macrophages and CCR2+ M2 macrophages both in ABX-treated TLR4−/− and WT mice with CP (Fig. S6B–S6E). Meanwhile, the deletion of TLR4 had no effect on G+ ABX-exacerbated immune disorders in CP. Collectively, the data suggest that TLR4 is not involved in mediating the worsening of CP caused by G+ ABX.
Figure 4.
Toll-like receptor 4 (TLR4) deficiency does not influence the severity of CP. (A) Intestinal permeability was assessed by measuring fluorescein isothiocyanate (FITC)-dextran (n = 6). (B) The representative macrographic images of the pancreas. (C) The ratio of pancreatic weight and body weight (n = 6). (D) The representative images and parenchyma rates of the pancreas by H&E staining (n = 6). Scale bar: 100 μm. (E) The representative images and fibrosis rates of the pancreas by Masson's Trichrome staining as indicated by collagen-rich scars (blue color) (n = 6). Scale bar: 100 μm. (F) The fibrosis-associated gene (Tgfb1, Col1, Acta2 and Fn1) expression in the pancreas (n = 6). (G) The monocyte chemoattractant protein-1 (MCP-1) levels in the pancreas (n = 6). Data (A, C–G) are representative and were the mean ± SD from three independent experiments. P values were calculated by one-way ANOVA followed by Tukey's post hoc test for multiple comparisons.
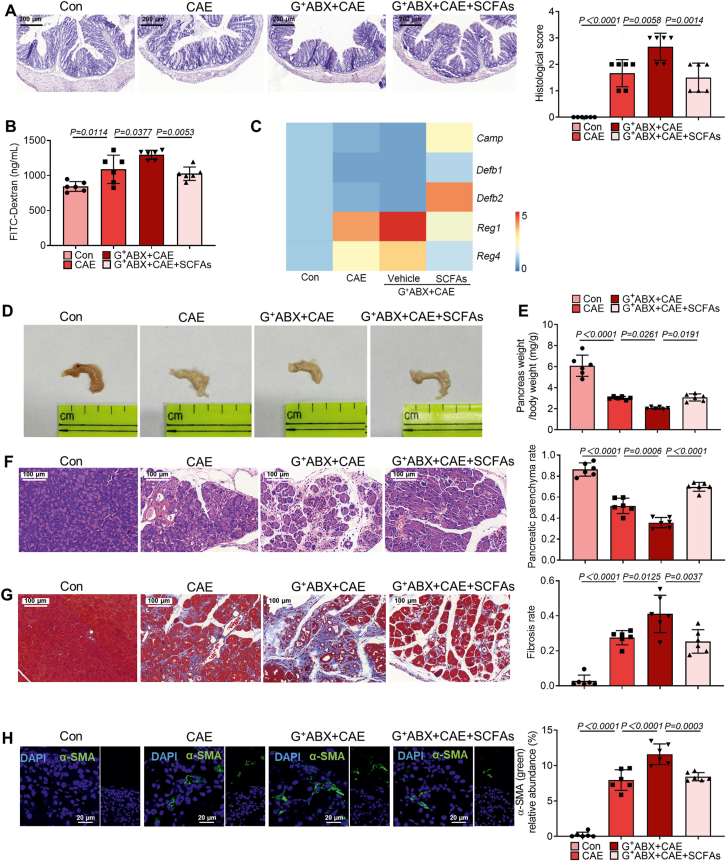
3.5. SCFAs rebuild the barrier function and protect CP
Altered gut microbiota composition has been reported to be associated with alterations in gut barrier integrity in patients with CP33. As inducers of intestinal antimicrobial peptides, SCFAs have been shown to indirectly regulate gut barrier34. Thus, we next explored whether SCFA supplementation could improve intestinal barrier function and protect CP.
Our data show that SCFAs improved the colonic damage caused by CP (Supporting Information Fig. S7A). Importantly, compared with CP mice, SCFA supplementation enhanced the expression of Camp, Defb1 and Defb2, while decreasing the expression of Reg1 and Reg4 (Fig. S7B). These antimicrobial peptides have been proved to regulate gut barrier function and host immune responses16,35. Meanwhile, the severity of CP was inhibited by SCFA supplementation as evidenced by increased pancreas weight and alleviated pancreatic fibrosis (Fig. S7C–S7E). Notably, the depletion of G+ bacteria intensified CP, accompanied by gut barrier dysfunction (Fig. 5A–C). SCFA supplementation relived gut barrier damage caused by G+ ABX treatment (Fig. 5A–C). Besides, pancreatic atrophy and pancreas weight loss caused by the depletion of G+ bacteria were attenuated by exogenous SCFA supplementation (Fig. 5D–F). Not only pancreatic fibrosis, but also the activation of PSCs was alleviated by SCFA supplementation (Fig. 5G and H). Together, SCFA supplementation improves gut barrier dysfunction in CP mice and has a protective effect on CP.
Figure 5.
SCFAs mitigate the intensified effects of the depletion of Gram-positive bacteria on CP. (A) The representative images and histological scores of the colon by H&E staining (n = 6). Scale bar: 200 μm. (B) Intestinal permeability assessment by measuring FITC-dextran (n = 6). (C) The mRNA expression of antimicrobial peptides (Camp, Defb1, Defb2, Reg1 and Reg4, n = 6). (D, E) The representative macroscopic images of the pancreas (D) and the ratio of pancreatic weight and body weight (E) (n = 6). (F) The representative images and parenchyma rates of the pancreas by H&E staining (n = 6). Scale bar: 100 μm. (G) The representative images and fibrosis rates of the pancreas by Masson's Trichrome staining (n = 6). Scale bar: 100 μm. (H) Localization and expression of α-SMA (green) in the pancreas by immunofluorescent staining (n = 6). Representative photomicrographs of individual and merged staining were shown. Nuclei were stained with DAPI (blue). Scale bar: 20 μm. Data are representative and were the mean ± SD from three independent experiments. P values were calculated by one-way ANOVA followed by Tukey's post hoc test for multiple comparisons.
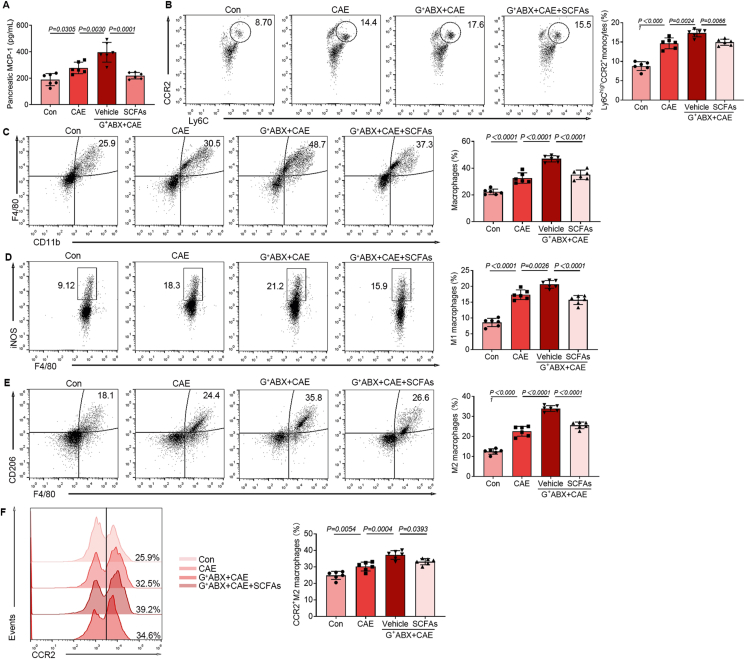
3.6. SCFAs alleviate monocyte recruitment and pancreatic macrophage deregulation in mice with CP
Accumulating studies have reported the crucial roles of SCFAs in regulating immune cell responses36,37. We previously confirmed that exogenous SCFAs could alleviate the symptoms of CP in mice (Fig. 5). However, the mechanisms underlying the protective effects of SCFAs on CP mice remain unclear. In this context, we examined how SCFAs regulate the recruitment of monocytes and the infiltration and polarization of pancreatic macrophages of CP mice treated with G+ ABX. The expression of MCP-1, the populations of CCR2+ monocytes, macrophages, M1 macrophages, M2 macrophages and CCR2+ M2 macrophages were all enhanced in CP mice (Fig. 6A–F). Moreover, the expression of pancreatic MCP-1, the recruitment of monocytes and macrophage infiltration and polarization in CP were potentiated by G+ ABX treatment, but alleviated by SCFA supplementation (Fig. 6A–F). Collectively, the recruitment of CCR2+ monocytes, the infiltration of inflammation-associated M1 macrophages, fibrosis-associated M2 macrophages and CCR2+ M2 macrophages are accumulated in mice with CP and G+ ABX-treated CP mice and exogenous SCFAs treatment significantly alleviates the condition.
Figure 6.
SCFAs alleviate monocyte recruitment and pancreatic macrophage deregulation in mice with CP. (A) The MCP-1 levels in the pancreas (n = 6). (B) The frequency of Ly6Chi C–C Motif chemokine receptor 2 (CCR2)+ monocytes among CD45+CD11b+ population (n = 6). (C) The frequency of CD11b+F4/80+ macrophages among CD45+ population (n = 6). (D) The frequency of inducible nitric oxide synthase (iNOS)+ M1 macrophages among CD45+CD11b+F4/80+ population (n = 6). (E) The frequency of F4/80+CD206+ M2 macrophages among CD45+CD11b+ population (n = 6). (F) The frequency of CCR2+ M2 macrophages among CD45+CD11b+ F4/80+CD206+ population (n = 6). Data (A) are representative and were the mean ± SD from three independent experiments. Data (B–F) were representative and were the median ± interquartile range from three independent experiments. P values were calculated by one-way ANOVA followed by Tukey's post hoc test for multiple comparisons.
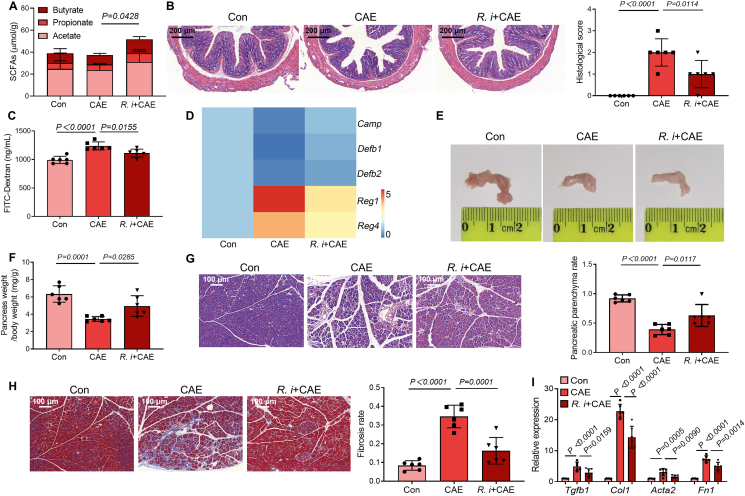
3.7. Supplementation of SCFAs-producing G+ bacteria alleviates CP
Our previous results indicate an essential role of G+ SCFAs-producing bacteria in the prevention or treatment of CP. Finally, we performed an in vivo experiment to verify their effects in CP mice. Herein, the available isolated strain Roseburia intestinalis DSM 14610 (R. intestinalis) of the G+ SCFAs-producer Roseburia that were significantly decreased in CP mice, broad-spectrum ABX-treated CP mice and G+ ABX-treated CP mice was chosen38. Not surprisingly, R. intestinalis treatment significantly enhanced the colonic SCFA content and alleviated CP-associated intestinal barrier dysfunction (Fig. 7A–C). Accordingly, the expression levels of colonic antimicrobial peptides including Camp, Defb1, Defb2 were increased and Reg1 and Reg4 were decreased after treatment with R. intestinalis (Fig. 7D). In addition, R. intestinalis treated CP mice exhibited alleviated pancreatic atrophy and weight loss (Fig. 7E and F). Importantly, R. intestinalis attenuated pancreatic injury and fibrosis caused by CP, as marked by improved pancreatic histological damage, and reduced the extent of fibrosis of pancreatic sections and mRNA expression of fibrosis markers (Fig. 7G–I). Together, our results support that treatment with SCFAs-producing G+ bacteria can relieve the severity of CP.
Figure 7.
Oral treatment with a specific G+ SCFAs-producer Roseburia intestinalis (R. intestinalis) attenuates the severity of CP. (A) The levels of SCFAs (acetate, propionate and butyrate) in colon content (n = 6). (B) The representative images and histological scores of the colon by H&E staining (n = 6). Scale bar: 200 μm. (C) Intestinal permeability assessment by measuring FITC-dextran (n = 6). (D) The mRNA expression of antimicrobial peptides (Camp, Defb1, Defb2, Reg1 and Reg4, n = 6). (E, F) The representative macroscopic images of the pancreas (E) and the ratio of pancreatic weight and body weight (F) (n = 6). (G) The representative images and parenchyma rates of the pancreas by H&E staining (n = 6). Scale bar: 100 μm. (H) The representative images and fibrosis rates of the pancreas by Masson's Trichrome staining (n = 6). Scale bar: 100 μm. (I) The mRNA expression of markers relevant to pancreatic fibrosis (n = 6). Data (A–D and F–I) are representative and were the mean ± SD from three independent experiments. P values were calculated by one-way ANOVA followed by Tukey's post hoc test for multiple comparisons.
4. Discussion
Our study demonstrates that gut dysbiosis in particular depletion of SCFAs-producing bacteria promotes the progression of CP. Disruption of G+ bacteria decreases the levels of SCFAs and compromises gut barrier function, which results in aggravated pancreatic fibrosis, monocyte recruitment and M2 macrophage polarization. SCFA supplementation improves intestinal barrier dysfunction and alleviates monocyte recruitment and pancreatic macrophage deregulation, thereby protecting against the development of CP.
A healthy and stable gut microbiota community is vital in maintaining homeostatic balance and metabolism39. The imbalance of gut microbiota or called gut dysbiosis has recently been found to be associated with pathogenesis of many diseases including CP8. A potential role of gut dysbiosis in progression of CP has, however, not been clearly demonstrated. To establish a causal role of alterations to the gut microbial community composition in the development of CP, we applied a broad-spectrum antibiotic cocktail to disturb the microbial community in mice with CP. Notably, broad-spectrum ABX treatment intensified gut dysbiosis, in particular decreased abundances of SCFAs-producing bacteria, including Ruminococcus 1, Ruminococcaceae UCG-009, Peptococcaceae, Lachnospiraceae UCG-001, Lactobacillus, Roseburia and Lachnoclostridium. These bacteria have demonstrated beneficial effects on human health and immune system40. All these findings suggest that SCFAs-producing bacteria may play a critical role in the progression of CP. To further specify these SCFAs-producing bacteria, we next used ABX targeting the disruption of G+ or G– bacteria. We observed that G+ ABX treatment exacerbated the CP development, as evidenced by aggravated pancreatic fibrosis, monocyte recruitment, pancreatic macrophage deregulation, gut dysbiosis and the depletion of SCFAs-producing G+ bacteria. Importantly, G+ ABX diminished major SCFAs-producing G+ bacteria including Ruminococcus 1, Ruminococcaceae UCG-009, Peptococcaceae, Lachnospiraceae UCG-001, Lactobacillus, Roseburia and Lachnoclostridium, resulting in significantly decreased SCFA production and disruption of barrier integrity, contributing to the overall worsening effect on CP development. In contrast, although G– ABX caused depletion of SCFAs-producing G– bacteria Bacteroides and Rhodospirillales, its effects on gut barrier dysfunction were insignificant, which was in agreement with its overall non-effect on CP progression.
CP is a disease characterized by malabsorption and impaired exocrine pancreatic function, accompanied by altered intestinal microbiota composition, as marked by depletion of anaerobes and significant enrichment of G– and opportunistic pathogenic bacteria (such as Bacteroides and Prevotella)41. Intriguingly, as the receptor of lipopolysaccharide, TLR4 deficiency in G+ ABX-treated mice did not influence CP, suggesting that mechanisms other than via TLR4 may play a part in the pathogenesis of CP. In addition, among G– SCFAs-producers, both barrier-protective species (Bacteroides fragilis, Bacteroides vulgatus and Bacteroides dorei) from genera Bacteroides and barrier-damaging species (Prevotella intestinalis, Prevotella histicola and Prevotella copri) from genera Prevotella have been reported42, 43, 44, 45, 46, which explained the end outcome of G− ABX treatment on CP.
Given that SCFAs-producing G+ bacteria were critically involved in the development of CP, we next addressed whether SCFAs could alleviate CP. Indeed, SCFA supplementation protected intestinal barrier integrity, attenuated CP-associated monocyte recruitment, pancreatic macrophage deregulation and pancreatic fibrosis, thus protecting the development of CP. The protective effects of SCFAs are associated with upregulated expressions of Camp and Defb2 and downregulated expressions of Reg1 and Reg4. The antimicrobial peptides cathelicidin-related antimicrobial peptide and defensin beta-2 contribute to the antimicrobial barrier function of the intestinal mucosa47,48. In contrast, REG proteins are constitutively synthesized by pancreatic acinar cells and upregulated dramatically during acute pancreatic inflammation49. Accordantly, we observed that downregulated expressions of Reg1 and Reg4 were associated with alleviated inflammation caused by SCFA supplementation. SCFAs are microbially produced metabolites, as crucial executors of diet-based microbial influence on the host. By binding to the G-protein-coupled receptors, they exert profound effects on inflammatory responses50,51. Also, SCFAs have been reported with multiple functions including regulating gut microbiota composition and inducing the production of antimicrobial peptides34,52. Previous reports showed that phytochemicals such as curcumin targeting PSCs as potential anti-fibrotic agents for CP53. Intriguingly, supplementation with curcumin could also enhance the abundance of SCFAs-producing bacteria and SCFA content in the mouse colon54. These findings imply that targeting SCFAs maybe a potential solution for CP therapy.
Accordingly, herein, our study found that administration of SCFAs not only improved CP-associated gut barrier dysfunction, but also alleviated the infiltration and polarization of macrophages by decreasing the recruitment of CCR2+ monocytes in G+ ABX-treated CP mice.
Macrophages can be divided into two spectra of major types: M1, which are correlated with inflammation; and M2, which are dominant in mouse and human CP15. M2 macrophages have been observed in close proximity to PSCs and promote pancreatic fibrosis in CP15. Activated PSCs can secrete MCP-1, which further promotes the recruitment of monocytes and the infiltration and polarization of macrophages into pancreatic microenvironment31. Macrophages enter the pancreas in response to an upregulated level of the chemokine MCP-1 acting on the macrophage receptor CCR255. Thus, CCR2/MCP-1 axis-mediated monocyte migration and M2 macrophage polarization in the pancreas are critically involved in CP56. These responses were exacerbated by G+ ABX-treatment and were significantly alleviated by SCFA supplementation. Similar to SCFA supplementation, specific SCFAs-producing G+ bacteria (i.e., R. intestinalis) treatment also significantly attenuated the severity of CP. These intriguing findings further indicated a critical role of SCFAs-producing G+ bacteria and SCFAs in CP development. Consequently, our data support the supplementation of a combined SCFAs-producing G+ bacteria or SCFAs in the management of CP.
5. Conclusions
Our study demonstrates, for the first time, a critical role of SCFAs-producing G+ bacteria in maintaining intestinal barrier, regulating monocyte/macrophage homeostasis and pancreatic fibrosis. Depletion of G+ bacteria contributes to decreased levels of SCFAs, intensified intestinal barrier dysfunction and monocyte/macrophage deregulation, thereby aggravating the progression of CP. In summary, our study points to the potential therapeutic interventions of dietary-derived SCFAs or targeting SCFAs-producing G+ bacteria in the prevention or treatment of CP.
Acknowledgments
The authors greatly acknowledge Prof. Xiaoyan Wang from the Third Xiangya Hospital, Central South University for intellectual input. The study was supported by funds from the National Natural Science Foundation of China (Grant Nos. 82070666 and 82122068), the Natural Science Foundation for Distinguished Young Scholars of Jiangsu Province (Grant No. BK20200026, China), the Collaborative Innovation Center of Food Safety and Quality Control of Jiangsu Province, the Fundamental Research Funds for the Central Universities (Grant Nos. JUSRP221037 and JUSRP22007, China), the China Postdoctoral Science Foundation (Grant No. 2022M721366), the Excellent Postdoctoral Program of Jiangsu Province (Grant No. 2023ZB168, China), Wuxi City’s first “double hundred” young and middle-aged medical and health talents (Grant No: BJ2020045, China) and Wuxi Social Development Science and Technology Demonstration Project (Grant No: N20201003, China).
Author contributions
Li-Long Pan, Zheng-Nan Ren, Jun Yang and Bin-Bin Li: conceptualization, methodology, investigation, validation, writing-original draft and writing-reviewing and editing. Yi-Wen Huang, Dong-Xiao Song, Xuan Li and Jia-jia Xu: methodology and investigation. Madhav Bhatia: writing-reviewing and editing. Duo-Wu Zou and Chun-Hua Zhou: methodology and resources. Jia Sun: conceptualization, supervision, writing-reviewing and editing and funding acquisition.
Availability of data and material
The datasets supporting the conclusions of this article were available in the NCBI (PRJNA794722, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA794722; PRJNA513244, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA513244). The authors declare that all data supporting the results of this study were available within the article and Supplemental material. Further information and requests for resources and reagents should be directed to and will be fulfilled by the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2023.08.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Beyer G., Habtezion A., Werner J., Lerch M.M., Mayerle J. Chronic pancreatitis. Lancet. 2020;396:499–512. doi: 10.1016/S0140-6736(20)31318-0. [DOI] [PubMed] [Google Scholar]
- 2.Kleeff J., Whitcomb D.C., Shimosegawa T., Esposito I., Lerch M.M., Gress T., et al. Chronic pancreatitis. Nat Rev Dis Prim. 2017;3 doi: 10.1038/nrdp.2017.60. [DOI] [PubMed] [Google Scholar]
- 3.Tamura K., Yu J., Hata T., Suenaga M., Shindo K., Abe T., et al. Mutations in the pancreatic secretory enzymes CPA1 and CPB1 are associated with pancreatic cancer. Proc Natl Acad Sci U S A. 2018;115:4767–4772. doi: 10.1073/pnas.1720588115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capurso G., Signoretti M., Archibugi L., Stigliano S., Fave G.D. Systematic review and meta-analysis: small intestinal bacterial overgrowth in chronic pancreatitis. United European Gastroenterol J. 2016;4:697–705. doi: 10.1177/2050640616630117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hm N.C., Bashir Y., Dobson M., Ryan B.M., Duggan S.N., Conlon K.C. The prevalence of small intestinal bacterial overgrowth in non-surgical patients with chronic pancreatitis and pancreatic exocrine insufficiency (PEI) Pancreatology. 2018;18:379–385. doi: 10.1016/j.pan.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 6.El Kurdi B., Babar S., El Iskandarani M., Bataineh A., Lerch M.M., Young M., et al. Factors that affect prevalence of small intestinal bacterial overgrowth in chronic pancreatitis: a systematic review, meta-analysis, and meta-regression. Clin Transl Gastroenterol. 2019;10 doi: 10.14309/ctg.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan L.L., Li B.B., Pan X.H., Sun J. Gut microbiota in pancreatic diseases: possible new therapeutic strategies. Acta Pharmacol Sin. 2021;42:1027–1039. doi: 10.1038/s41401-020-00532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han M.M., Zhu X.Y., Peng Y.F., Lin H., Liu D.C., Li L. The alterations of gut microbiota in mice with chronic pancreatitis. Ann Transl Med. 2019;7:464. doi: 10.21037/atm.2019.08.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jandhyala S.M., Madhulika A., Deepika G., Rao G.V., Reddy D.N., Subramanyam C., et al. Altered intestinal microbiota in patients with chronic pancreatitis: implications in diabetes and metabolic abnormalities. Sci Rep. 2017;7 doi: 10.1038/srep43640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokol H., Pigneur B., Watterlot L., Lakhdari O., Bermúdez-Humarán L.G., Gratadoux J.J., et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas L.V., Ockhuizen T., Suzuki K. Exploring the influence of the gut microbiota and probiotics on health: a symposium report. Br J Nutr. 2014;112(Suppl 1):S1–S18. doi: 10.1017/S0007114514001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frost F., Weiss F.U., Sendler M., Kacprowski T., Rühlemann M., Bang C., et al. The gut microbiome in patients with chronic pancreatitis is characterized by significant dysbiosis and overgrowth by opportunistic pathogens. Clin Transl Gastroenterol. 2020;11 doi: 10.14309/ctg.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh V.K., Yadav D., Garg P.K. Diagnosis and management of chronic pancreatitis: a review. JAMA. 2019;322:2422–2434. doi: 10.1001/jama.2019.19411. [DOI] [PubMed] [Google Scholar]
- 14.Apte M., Pirola R., Wilson J. The fibrosis of chronic pancreatitis: new insights into the role of pancreatic stellate cells. Antioxidants Redox Signal. 2011;15:2711–2722. doi: 10.1089/ars.2011.4079. [DOI] [PubMed] [Google Scholar]
- 15.Xue J., Sharma V., Hsieh M.H., Chawla A., Murali R., Pandol S.J., et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158. doi: 10.1038/ncomms8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J., Xu M., Ortsater H., Lundeberg E., Juntti-Berggren L., Chen Y.Q., et al. Cathelicidins positively regulate pancreatic beta-cell functions. Faseb J. 2016;30:884–894. doi: 10.1096/fj.15-275826. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal H., Pathak P., Singh V., Kumar Y., Shankar M., Das B., et al. Vancomycin-induced modulation of Gram-positive gut bacteria and metabolites remediates insulin resistance in iNOS knockout mice. Front Cell Infect Microbiol. 2022;11 doi: 10.3389/fcimb.2021.795333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasseville D. Neomycin. Dermatitis. 2010;21:3–7. [PubMed] [Google Scholar]
- 19.Tian Y., Xu Q., Sun L., Ye Y., Ji G. Short-chain fatty acids administration is protective in colitis-associated colorectal cancer development. J Nutr Biochem. 2018;57:103–109. doi: 10.1016/j.jnutbio.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Tandon R.K., Sato N., Garg P.K., Group C.S. Chronic pancreatitis: Asia–Pacific consensus report. J Gastroenterol Hepatol. 2002;17:508–518. doi: 10.1046/j.1440-1746.2002.02762.x. [DOI] [PubMed] [Google Scholar]
- 21.Ren Z., Pan L.L., Huang Y., Chen H., Liu Y., Liu H., et al. Gut microbiota-CRAMP axis shapes intestinal barrier function and immune responses in dietary gluten-induced enteropathy. EMBO Mol Med. 2021;13 doi: 10.15252/emmm.202114059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C., Li M., Chen W. Characteristics of gut microbiota in cerulein-induced chronic pancreatitis. Diabetes Metab Syndr Obes. 2021;14:285–294. doi: 10.2147/DMSO.S291822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Memba R., Duggan S.N., Ni Chonchubhair H.M., Griffin O.M., Bashir Y., O'Connor D.B., et al. The potential role of gut microbiota in pancreatic disease: a systematic review. Pancreatology. 2017;17:867–874. doi: 10.1016/j.pan.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Akshintala V.S., Talukdar R., Singh V.K., Goggins M. The gut microbiome in pancreatic disease. Clin Gastroenterol Hepatol. 2019;17:290–295. doi: 10.1016/j.cgh.2018.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamin O., Lappin S.L. StatPearls; Treasure Island (FL): 2022. Chronic pancreatitis. [PubMed] [Google Scholar]
- 26.Reding T., Bimmler D., Perren A., Sun L.K., Fortunato F., Storni F., et al. A selective COX-2 inhibitor suppresses chronic pancreatitis in an animal model (WBN/Kob rats): significant reduction of macrophage infiltration and fibrosis. Gut. 2006;55:1165–1173. doi: 10.1136/gut.2005.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan L., Yu H., Fu J., Hu J., Xu H., Zhang Z., et al. Berberine ameliorates chronic kidney disease through inhibiting the production of gut-derived uremic toxins in the gut microbiota. Acta Pharm Sin B. 2023;13:1537–1553. doi: 10.1016/j.apsb.2022.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reichardt N., Duncan S.H., Young P., Belenguer A., McWilliam Leitch C., Scott K.P., et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8:1323–1335. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z., He S., Cao X., Ye Y., Yang L., Wang J., et al. Potential prebiotic activities of soybean peptides Maillard reaction products on modulating gut microbiota to alleviate aging-related disorders in d-galactose-induced ICR mice. J Funct Foods. 2020;65 [Google Scholar]
- 30.Park B.S., Lee J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45:e66. doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan J., Duan L., Wu N., Xu X., Xin J., Jiang S., et al. Baicalin ameliorates pancreatic fibrosis by inhibiting the activation of pancreatic stellate cells in mice with chronic pancreatitis. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.607133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuji Y., Watanabe T., Kudo M., Arai H., Strober W., Chiba T. Sensing of commensal organisms by the intracellular sensor NOD1 mediates experimental pancreatitis. Immunity. 2012;37:326–338. doi: 10.1016/j.immuni.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talukdar R., Karvellas C.J., Talukdar R., Talukdar R., Talukdar R., Jandhyala S.M., et al. Altered gut microbiota in patients with chronic pancreatitis is associated with gut barrier dysfunction and metabolic abnormalities. Clin Gastroenterol Hepatol. 2017;15:153. [Google Scholar]
- 34.Sun J., Furio L., Mecheri R., van der Does Anne M., Lundeberg E., Saveanu L., et al. Pancreatic β-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity. 2015;43:304–317. doi: 10.1016/j.immuni.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Lorchner H., Hou Y., Adrian-Segarra J.M., Kulhei J., Detzer J., Gunther S., et al. Reg proteins direct accumulation of functionally distinct macrophage subsets after myocardial infarction. Cardiovasc Res. 2018;114:1667–1679. doi: 10.1093/cvr/cvy126. [DOI] [PubMed] [Google Scholar]
- 36.Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 37.Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly Y.M., et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan L., Han P., Ma S., Peng R., Wang C., Kong W., et al. Abnormal metabolism of gut microbiota reveals the possible molecular mechanism of nephropathy induced by hyperuricemia. Acta Pharm Sin B. 2020;10:249–261. doi: 10.1016/j.apsb.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang G., Zhang S., Zhou C., Tang X., Li C., Wang C., et al. Influence of Eimeria falciformis infection on gut microbiota and metabolic pathways in mice. Infect Immun. 2018;86 doi: 10.1128/IAI.00073-18. 000733-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frost F., Kacprowski T., Ruhlemann M., Bulow R., Kuhn J.P., Franke A., et al. Impaired exocrine pancreatic function associates with changes in intestinal microbiota composition and diversity. Gastroenterology. 2019;156:1010–1015. doi: 10.1053/j.gastro.2018.10.047. [DOI] [PubMed] [Google Scholar]
- 42.Iljazovic A., Roy U., Galvez E.J.C., Lesker T.R., Zhao B., Gronow A., et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 2021;14:113–124. doi: 10.1038/s41385-020-0296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida N., Emoto T., Yamashita T., Watanabe H., Hayashi T., Tabata T., et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138:2486–2498. doi: 10.1161/CIRCULATIONAHA.118.033714. [DOI] [PubMed] [Google Scholar]
- 45.Marietta E.V., Murray J.A., Luckey D.H., Jeraldo P.R., Lamba A., Patel R., et al. Suppression of inflammatory arthritis by human gut-derived Prevotella histicola in humanized mice. Arthritis Rheumatol. 2016;68:2878–2888. doi: 10.1002/art.39785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scher J.U., Sczesnak A., Longman R.S., Segata N., Ubeda C., Bielski C., et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2 doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahuja M., Schwartz D.M., Tandon M., Son A., Zeng M., Swaim W., et al. Orai1-mediated antimicrobial secretion from pancreatic acini shapes the gut microbiome and regulates gut innate immunity. Cell Metabol. 2017;25:635–646. doi: 10.1016/j.cmet.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fusco A., Savio V., Donniacuo M., Perfetto B., Donnarumma G. Antimicrobial peptides human beta-defensin-2 and -3 protect the gut during Candida albicans infections enhancing the intestinal barrier integrity: in vitro study. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.666900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L., Bachem M.G., Zhou S., Sun Z., Chen J., Siech M., et al. Pancreatitis-associated protein inhibits human pancreatic stellate cell MMP-1 and -2, TIMP-1 and -2 secretion and RECK expression. Pancreatology. 2009;9:99–110. doi: 10.1159/000178880. [DOI] [PubMed] [Google Scholar]
- 50.Maslowski K.M., Vieira A.T., Ng A., Kranich J., Sierro F., Yu D., et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song M., Wang J., Sun Y., Pang J., Li X., Liu Y., et al. Inhibition of gasdermin D-dependent pyroptosis attenuates the progression of silica-induced pulmonary inflammation and fibrosis. Acta Pharm Sin B. 2022;12:1213–1224. doi: 10.1016/j.apsb.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Priyadarshini M., Wicksteed B., Schiltz G.E., Gilchrist A., Layden B.T. SCFA receptors in pancreatic beta cells: novel diabetes targets?. Trends Endocrinol Metabol. 2016;27:653–664. doi: 10.1016/j.tem.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu Y., Ouyang Z., Du H., Wang M., Wang J., Sun H., et al. New opportunities and challenges of natural products research: when target identification meets single-cell multiomics. Acta Pharm Sin B. 2022;12:4011–4039. doi: 10.1016/j.apsb.2022.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li S., You J., Wang Z., Liu Y., Wang B., Du M., et al. Curcumin alleviates high-fat diet-induced hepatic steatosis and obesity in association with modulation of gut microbiota in mice. Food Res Int. 2021;143 doi: 10.1016/j.foodres.2021.110270. [DOI] [PubMed] [Google Scholar]
- 55.Nakanishi Y., Sato T., Ohteki T. Commensal Gram-positive bacteria initiates colitis by inducing monocyte/macrophage mobilization. Mucosal Immunol. 2015;8:152–160. doi: 10.1038/mi.2014.53. [DOI] [PubMed] [Google Scholar]
- 56.Zigmond R.E., Echevarria F.D. Macrophage biology in the peripheral nervous system after injury. Prog Neurobiol. 2019;173:102–121. doi: 10.1016/j.pneurobio.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article were available in the NCBI (PRJNA794722, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA794722; PRJNA513244, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA513244). The authors declare that all data supporting the results of this study were available within the article and Supplemental material. Further information and requests for resources and reagents should be directed to and will be fulfilled by the corresponding author.