Abstract
Mitochondrial transplantation is a promising therapeutic approach for the treatment of mitochondrial diseases caused by mutations in mitochondrial DNA, as well as several metabolic and neurological disorders. Animal studies have shown that mitochondrial transplantation can improve cellular energy metabolism, restore mitochondrial function, and prevent cell death. However, challenges need to be addressed, such as the delivery of functional mitochondria to the correct cells in the body, and the long-term stability and function of the transplanted mitochondria. Researchers are exploring new methods for mitochondrial transplantation, including the use of nanoparticles or CRISPR gene editing. Mechanisms underlying the integration and function of transplanted mitochondria are complex and not fully understood, but research has revealed some key factors that play a role. While the safety and efficacy of mitochondrial transplantation have been investigated in animal models and human trials, more research is needed to optimize delivery methods and evaluate long-term safety and efficacy. Clinical trials using mitochondrial transplantation have shown mixed results, highlighting the need for further research in this area. In conclusion, although mitochondrial transplantation holds significant potential for the treatment of various diseases, more work is needed to overcome challenges and evaluate its safety and efficacy in human trials.
Keywords: Clinical trials, Isolation, Mitochondria, Preservation, Transplantation
INTRODUCTION
Mitochondrial diseases, caused by mutations in the mitochondrial DNA, represent a growing health challenge. Mitochondrial transplantation is a therapeutic approach that is aimed at delivering healthy mitochondria to cells to alleviate the symptoms of these diseases.
Mitochondrial transplantation has been shown to be a promising approach for the treatment of mitochondrial diseases in animal models. Studies in mouse have demonstrated that mitochondrial transplantation can lead to the improvement of cellular energy metabolism, the restoration of mitochondrial function, and the prevention of cell death. Moreover, mitochondrial transplantation has been found to be safe and effective in the treatment of some mitochondrial diseases, such as Leber’s hereditary optic neuropathy, and Leigh syndrome.
However, there are still several challenges that need to be overcome to translate the results of animal studies to the clinic. One of the main challenges is the delivery of functional mitochondria to the correct cells in the body. In addition, the integration of transplanted mitochondria into host cells, and their long-term stability and function, are important aspects that require further investigation.
To address these challenges, researchers are exploring new methods for mitochondrial transplantation, including the use of nanoparticles or CRISPR gene editing. Nanoparticles have been shown to be effective carriers of functional mitochondria, and can be engineered to target specific cells. CRISPR gene editing technology can be used to modify the DNA of the transplanted mitochondria, for example, to repair mutations that cause mitochondrial diseases.
Therefore, mitochondrial transplantation holds significant potential for the treatment of mitochondrial diseases. However, more research is needed to overcome the current challenges and to evaluate the safety and efficacy of this therapeutic approach in animal models and human trials. Nevertheless, the development of new techniques for mitochondrial transplantation, such as the use of nanoparticles or CRISPR gene editing, is promising, and could pave the way for the development of novel therapies for mitochondrial diseases.
ADVANCEMENTS IN MITOCHONDRIAL ISOLATION AND PRESERVATION TECHNIQUES FOR ENHANCED FUNCTIONAL STUDIES AND THERAPEUTIC APPLICATIONS
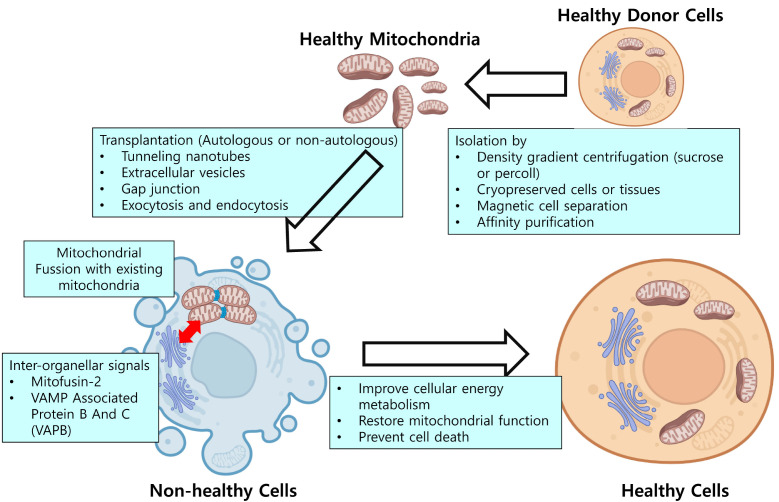
Often referred to as the ‘powerhouses’ of the cell, mitochondria serve as intricate hubs for various metabolic pathways and cellular processes, contributing significantly to the overall health and functionality of the cell. Recent research focused on rescuing mitochondrial function has shown promise through mitochondrial transplantation, which serves as a therapeutic process involving the transfer of functional exogenous mitochondria into target host cells or organs (Fig. 1). The purity and functionality of isolated mitochondria play a crucial role in maintaining mitochondrial function. Therefore, it is essential to rapidly perform and complete the process of mitochondrial isolation under 4°C conditions.
Fig. 1.
The diagram of mitochondrial transplantation. Mitochondria isolated from healthy donor cells, either through density gradient centrifugation, magnetic cell separation, or affinity purification, could enhance cellular energy metabolism and prevent cell death.
The first attempt to isolate mitochondria was made by Bensley and Hoerr in the 1930s. They successfully isolated mitochondria from the livers of guinea pigs and rabbits by grinding the liver, and centrifuging the resulting emulsion in a salt solution (1, 2). Subsequently, in the 1940s, differential centrifugation (DC) was introduced for cell fractionation and mitochondrial isolation, based on the differences in sedimentation velocity of cellular organelles (3). While these early studies aimed to separate mitochondria from other cell components and analyze their functions, recent studies have focused on the therapeutic use of functional mitochondria. Consequently, more advanced isolation methods that preserve the metabolic characteristics of mitochondria are required.
Density gradient centrifugation (GC) has been employed to isolate mitochondria and remove contaminants, such as other subcellular components. However, the use of sucrose gradient centrifugation often leads to poor preservation of the metabolic properties of mitochondria (4). Subsequent studies attempted to isolate metabolically active mitochondria from brain tissue using density gradient centrifugation with Percoll gradients, instead of sucrose (5-8). Despite the elimination of synaptosomal contaminants by these methods, the isolated mitochondrial fraction still contained lysosomal and peroxisomal contaminants. Furthermore, these initial isolation methods, which involved several repetitive steps, were time-consuming and difficult to handle for numerous samples, thereby reducing the viability and metabolic function of mitochondria. To address these limitations and preserve the metabolic characteristics of mitochondria, recent approaches have focused on simplifying the process by favoring differential centrifugation (DC).
For the isolation of metabolically active mitochondria, it is crucial to promptly isolate them from fresh tissues or cultured cells. To overcome these limitations, some studies have proposed advanced methods to isolate functional mitochondria from cryopreserved samples. These studies have shown that mitochondrial properties can be preserved if tissues are frozen in a buffer containing cryoprotectants, such as glycerol or DMSO. Tissues stored at −80°C in a frozen buffer containing 20% glycerol (or DMSO) and fatty acid-free bovine serum albumin (BSA) demonstrated no significant differences in membrane potential generation and ATP production, compared to fresh tissue (9, 10).
In recent years, several studies have attempted to address time-consuming methodological challenges by incorporating affinity purification into mitochondrial isolation. Magnetic beads have been reported as a useful tool for the isolation of mitochondria (11-13). These approaches utilize magnetic cell separation (MACS), a cell sorting method. Magnetic beads conjugated with antibodies against TOMM22, a mitochondrial membrane protein, are employed to isolate mitochondria from other cellular compartments. By binding to TOMM22 expressed on the mitochondrial outer membrane, the anti-TOMM22-conjugated magnetic beads label the mitochondria magnetically. Consequently, only the magnetically labeled mitochondria are captured and isolated in the magnetic field of the MACS separator. These MACS-based approaches yield purer mitochondria at a faster rate compared to differential centrifugation (DC) or gradient centrifugation (GC) methods, while resulting in the isolation of intact mitochondria with higher oxygen consumption capacity (12, 13). Other studies utilizing affinity purification have employed an epitope HA-tagged to TOMM20 for mitochondrial isolation (14, 15). These recent advances in affinity-based methods have significantly reduced isolation time, while preserving the integrity and purity of mitochondria.
However, a crucial question remains: do isolated mitochondria retain their metabolic function after transplantation into new host cells or organs? Above all, the successful delivery of well-preserved intact mitochondria to target cells and the maintenance of mitochondrial metabolic capacity are essential. To achieve these goals, the development of a well-established transplantation method will be a key factor.
MECHANISMS OF INTERCELLULAR TRANSFER OF MITOCHONDRIA
Mitochondria are sensitive to cell damage and play a crucial role in maintaining cellular homeostasis. Recent studies have shown that mitochondria can be transferred intercellularly under physiological and pathological conditions (Fig. 1).
When cells are damaged or mitochondrial dysfunction occurs, adjacent cells transfer healthy mitochondria into the damaged cells that need energy support. This process of transferring functional mitochondria can help restore cellular function and facilitate repair (16, 17). Additionally, mitochondria transfer can occur between tumor cells and non-tumor cells. This transfer might enhance survival (18, 19), invasiveness (20, 21), and metabolic adaptation of cancer cells (22), potentially contributing to cancer progression and metastasis.
Intercellular mitochondrial transfer occurs in various ways, including tunneling nanotubes (TNTs), extracellular vesicles (EVs), gap junctions, and exocytosis and endocytosis of free mitochondria, depending on the cell types and conditions. However, mitochondrial transfer is primarily reported to occur through tunneling nanotubes (TNTs) and extracellular vesicles (EVs).
Tunneling nanotubes (TNTs) are long and thin bridge-like structures between cells composed of plasma membrane and actin filaments. TNTs primarily play a role in intercellular communication by allowing the exchange of various cellular components, including proteins, lipids, and other cargos. Researchers confirmed that TNTs allow mitochondrial transfer by observing the movement of MitoTracker-labeled mitochondria in 2005 (23). Since then, several groups have confirmed intercellular mitochondrial transfer through TNTs (24-27).
Another mechanism of mitochondrial transfer is the use of extracellular vesicles (EVs). EVs deliver various cellular components, including proteins, RNA, miRNA, lipids, and even organelles like mitochondria. EVs, which play an important role in intercellular communication, are classified into three types: exosomes (30-150 nm), microvesicles (0.1-1 μm), and apoptotic bodies (> 1 μm). Microvesicles, relatively large EVs, can carry cellular organelles like mitochondria. It was in 2006 that EV-mediated mitochondrial transfer was presented as a mode to transfer functional mitochondria. This research suggested the potential exchange of functional organelles between cells (28). Another study confirmed that mitochondrial transfer is reduced by inhibiting endocytosis (29).
MECHANISMS OF ACTION AND INTEGRATION OF TRANSPLANTED MITOCHONDRIA
Studies have shown that transplanted mitochondria can integrate into host cells and contribute to the improvement of cellular energy metabolism, restoration of mitochondrial function, and prevention of cell death (30-32). However, the mechanisms underlying these effects are complex, and not fully understood. Nevertheless, research has revealed some key factors that play a role in the integration and function of transplanted mitochondria (Fig. 1).
Both autologous and non-autologous mitochondria can be used for transplantation. Non-autologous mitochondrial transplantation has been demonstrated to improve cellular energy metabolism, similar to the transplantation of autologous mitochondria (30). The study showed that mitochondria isolated from healthy skeletal muscle cells can be successfully transplanted into normal cardiomyocytes without adverse effects; however, it also revealed limitations in transiently enhancing mitochondrial respiration and ATP production in normal cardiomyocytes through mitochondrial transplantation. The study suggested that these transiently enhanced bioenergetics after mitochondrial transplantation reflect the energy demand of recipient cells.
In recipient cells with damaged mitochondria, transplanted mitochondria had a lasting impact on mitochondrial respiration and cell death. Artificially induced mitochondrial dysfunction resulted in decreased mitochondrial oxidation and membrane potential. Mitochondrial pre-treatment was reported to prevent these failures resulting from mitochondrial dysfunction in cardiomyocytes. Furthermore, the study suggested that these myocardial protections are achieved through the inhibition of apoptosis (31). In another study, mitochondrial transplantation was performed using a polypeptide (PEP)-triphenylphosphonium cations (TPP) complex bound to mitochondria (32). According to this research, the targeting polypeptide (PEP) possesses ischemia-sensing properties and dissociates from the PEP-TPP-mitochondria complex, enabling the effective internalization of mitochondria. Thus, injected mitochondria could be translocated into the ischfemic region, and effectively transplanted. Transplanted mitochondria using the PEP-TPP-mitochondria complex improved cardiomyocyte energetics, decreased the pro-inflammatory response, and reduced cell apoptosis. These studies on cardiomyocytes have yielded consistent results, demonstrating that mitochondrial transplantation promotes energy metabolism and reduces cell death.
In addition, the integration of transplanted mitochondria into host cells can be influenced by various molecular and cellular mechanisms, such as the interplay between mitochondria and the endoplasmic reticulum (ER), the regulation of mitochondrial fission and fusion, and the release of inter-organellar signals. The success and effectiveness of mitochondrial transplantation relies on the ability of transplanted mitochondria to become functionally integrated and contribute to the overall cellular function.
In recent times, there has been a growing recognition of the significance of the interaction between the endoplasmic reticulum (ER) and mitochondria in facilitating the integration of transplanted mitochondria. Consequently, researchers have directed their attention toward comprehending the role of the ER in the process of mitochondrial transfer. A notable investigation highlighted the ER’s role as a mediator of mitochondrial transfer between proximate cells (33). This exploration was carried out using osteocytes, chosen due to their intricate dendritic structures that establish connections with neighboring cells. The outcomes of this study unveiled the capacity for mitochondria to be exchanged via dendritic extensions linking adjacent osteocytes, with the ER playing a pivotal role in facilitating this intricate process of mitochondrial transfer. Furthermore, the research findings strongly imply a dependence on the ER for both mitochondrial transfer and the subsequent distribution of mitochondria through dendritic processes.
Upon the transfer of mitochondria to recipient cells, a dynamic interaction transpires between the ER of the donor and that of the recipient osteocyte. The donor mitochondria exhibit a close colocalization with the ER along dendrites, and it is precisely these mitochondria in close proximity to the ER that undergo the transfer. Throughout this transfer process, specific proteins localized within the membranes of both the mitochondria and the ER act as tethers, effectively linking the mitochondria to the ER. Consequently, this interaction prompts the migration of mitochondria to neighboring cells. It has been observed that the suppression of the expression of Mitofusin-2 (Mfn2) or VAMP associated protein B and C (VAPB), the membrane proteins found on mitochondria and the ER respectively, results in the inhibition of the mitochondrial transfer process. The findings of this study compellingly demonstrate that the exchange of mitochondria between osteocytes primarily occurs through dendritic processes, facilitated by a complex of tethering proteins that prominently feature Mfn2 and VAPB.
Mitochondria undergo fusion and fission, referred as ‘mitochondrial dynamics’, to maintain their own intact function and cellular homeostasis. Mitochondrial fusion is the process by which two or more mitochondria combine to form a single, larger mitochondrion. On the other hand, mitochondrial fission is the opposite process in which a mitochondrion is divided into two or more smaller mitochondria (34). These processes can also occur in the recipient cells when exogenous mitochondria are introduced. When isolated mitochondria are introduced, they may undergo fusion with existing mitochondria in the recipient cells. These interactions can impact the distribution and functionality of both transplanted and host mitochondria. Studies have shown that the fusion process is observed in the early stages of mitochondrial transplantation. This process might contribute to the function of the mitochondrial population in the recipient cells (35). Additionally, a study reported that the mitochondrial fusion process occurs more frequently than the fission process when isolated mitochondria are transplanted (36). Researchers have suggested that transplanted mitochondria can promote ‘mitochondrial dynamics’ in the recipient cells. However, it’s important to note that the exact dynamics of mitochondrial fusion and fission during transplantation are still an active area of research.
SAFETY AND EFFICACY OF MITOCHONDRIAL TRANSPLANTATION
The safety and efficacy of mitochondrial transplantation have been investigated in a range of animal models and human trials. Studies in animal models have demonstrated that mitochondrial transplantation is a safe and effective approach for treating various mitochondrial diseases. For example, one study utilized a peptide-mediated delivery method by conjugating the cell-penetrating peptide Pep-1 to enhance mitochondrial functionality and delivery efficacy. When co-incubated with Pep-1 in PC12 cells, a Parkinson’s disease (PD) model generated from rat, the mitochondria abolished apoptotic cell death and neurotoxin-induced oxidative stress (37). Another study involved mitochondrial transplantation through intravenous injection of mitochondria isolated from human hepatoma cells (HepG2 cells) into the brains of neurotoxin-induced PD mice (38). This transplantation resulted in the suppression of PD progression by increasing the efficiency of the Electron Transport Chain (ETC), reducing free radical generation, and decreasing apoptotic cells in the transplanted mice. These findings suggest that mitochondrial transplantation holds promise for the treatment of various mitochondrial diseases, including Leber’s hereditary optic neuropathy (LHON), a condition characterized by reduced retinal ganglion cells (RGCs) due to mutations in mtDNA encoding for mitochondrial Complex I subunits.
The Pebay group investigated the use of induced pluripotent stem cells (iPSCs) to model LHON, and created isogenic iPSC controls by substituting LHON mtDNA using cybrid technology. This technique involves fusing a cell that acts as a nuclear donor with a cytoplast from a different source, which serves as a mitochondrial donor, to generate eukaryotic cell lines. The researchers produced mutation-free LHON iPSCs, and differentiated them into retinal ganglion cells (RGCs). After validating the level of apoptosis, the LHON condition, which was corrected using cybrid technology to fix the mutation, exhibited recovery comparable to the control (39).
Mitochondrial transplantation has achieved success in various in vitro and in vivo scenarios. Multiple delivery methods have been employed for mitochondrial transplantation, such as co-incubation, direct injection, centrifugation, magnetomitotransfer, cell-penetrating peptide, biocompatible polymer, photothermal nanoblade, and fluidic force microscopy (FluidFM) (Table 1) (40-48).
Table 1.
Studies of mitochondrial transplantation in human sample for each mitochondria delivery method
| Donor cell/tissue | Recipient cell/tissue | Delivery method | Reference |
|---|---|---|---|
| Human MSCs | MDA-MB-231 (human breast cancer cell line) | Co-incubation | (41) |
| Rectus abdominis muscle (in pediatric patients with refractory cardiogenic shock after ischemia-reperfusion injury [IRI]) | Myocardium (in pediatric patients with refractory cardiogenic shock after ischemia-reperfusion injury [IRI]) | Direct injection (autologus transplantation) | (42) |
| Human UC-MSCs | Human UC-MSCs | Centrifugation | (43) |
| MRC-5 (human fetal lung fibroblast cell line) | MRC-5 | Magnetomitotransfer | (44) |
| 143B cells (human osteosarcoma cell line) | MERRF B2 cybrid cells (fused 143B ρ° cells with enucleated skin fibroblasts from MERRF syndrome patient) | Cell-penetrating peptide | (45) |
| HeLa cells | MDA-MB-231 | Biocompatible polymer | (46) |
| MDA-MB-453 (human breast cancer cell line) | 143BTK ρ° cells (human osteosarcoma cell line) | Photothermal nanoblade | (47) |
| HeLa cells | HeLa cells | Fluidic force microscope (FluidFM) | (48) |
Nevertheless, further research is necessary to evaluate the safety and efficacy of mitochondrial transplantation in human trials. To date, only a limited number of clinical trials have been conducted, with mixed results. While some trials have demonstrated the safety and efficacy of mitochondrial transplantation, others have indicated the need for more research to optimize delivery methods and assess the long-term safety and efficacy of this approach. Additionally, the transplantation of isolated mitochondria from exogenous sources requires careful consideration and exploration in human trials.
CLINICAL TRIALS THROUGH MITOCHONDRIAL TRANSPLANTATION
Mitochondrial transplantation is being studied in preclinical and clinical trials as a potential treatment for various diseases related to mitochondrial dysfunction. These diseases include neurodegenerative diseases, brain and heart ischemia, obesity, diabetes, and non-alcoholic fatty liver disease.
One example of a clinical trial involving mitochondrial transplantation is that being conducted by the McCully group, who performed autologous mitochondria transplantation to treat dysfunction after ischemia-reperfusion (IR) injury. Myocardial IR injury is a significant contributor to negative cardiovascular outcomes following instances of myocardial ischemia, cardiac surgery, or circulatory arrest. This condition arises due to an imbalance between the heart’s oxygen demand and supply when blood flow is restricted (referred to as ischemia), causing damage or dysfunction in cardiac tissue. Rapid restoration of blood flow is crucial to prevent further tissue damage, typically achieved through thrombolytic therapy or percutaneous coronary intervention. However, the process of reperfusion, where blood flow is reintroduced to the ischemic heart tissue, can also lead to additional injury, known as myocardial IR injury. The McCully group isolated mitochondria from the rectus abdominis muscle of patients, and directly injected them into the IR-injured myocardium. In all cases, ventricular function had improved within a few days. Additionally, markers of systemic inflammatory response syndrome were not found before or after injection, confirming the safety and efficacy of autologous mitochondrial transplantation (49).
Walker’s group is another research team that is investigating the use of autologous mitochondrial transplantation. They are currently recruiting subjects for a first-in-human-brain trial to confirm the safety of mitochondrial transplantation for cerebral ischemia. Brain ischemia occurs when the brain receives inadequate blood flow to meet its metabolic needs. This results in insufficient oxygen supply, leading to cerebral hypoxia and ultimately the death of brain tissue, known as cerebral infarction or ischemic stroke. This condition is a subtype of stroke, which also includes subarachnoid hemorrhage and intracerebral hemorrhage. In this trial, they will isolate the mitochondria from muscle tissue adjacent to the surgical site, and infuse them into the brain artery via micro-catheter during reperfusion with endovascular treatment (NCT04998357, Table 2).
Table 2.
Registered studies for mitochondrial transplantation on ClinicalTrials.gov
| Condition/disease | Donor | Recipient | Phase | Status | NCT number |
|---|---|---|---|---|---|
| Cerebral Ischemia | Muscle tissue | Brain artery | Phase 1 | Recruiting | NCT04998357 |
| Extracorporeal Membrane Oxygenation Complication | Skeletal muscle | Myocardium | N/A | Recruiting | NCT02851758 |
| Repetition Failure | MSCs | Oocyte | N/A | Unknown | NCT03639506 |
| Myocardial Infarction, Myocardial Ischemia, Myocardial Stunning | MSCs-derived exosomes | Myocardium | Phase 1 | Recruiting | NCT05669144 |
| Phase 2 | |||||
| Polymyositis, Dermatomyositis | Allogeneic Umbilical Cord-derived MSCs (PN-101) | - | Phase 1 | Enrolling by invitation | NCT04976140 |
| Phase 2 | |||||
| Infertility | Ovarian stem cells | Oocyte | N/A | Completed | NCT02586298 |
N/A: not applicable.
Since 2021, Paean Biotechnology Inc. has been actively engaged in clinical trials centered around mitochondrial transplantation, sourced from allogeneic umbilical cord-derived mesenchymal stem cells, targeting refractory cases of polymyositis (PM) and dermatomyositis (DM) conditions (NCT04976140). Polymyositis (PM) or dermatomyositis (DM) are complex inflammatory disorders affecting muscles, skin, and various organs. Diagnosis hinges on specific criteria, including muscle weakness, elevated muscle enzyme levels, electromyography alterations, distinctive muscle biopsy findings, and observable skin manifestations. While the precise origins of these conditions remain uncertain, autoimmune mechanisms are believed to be involved. In PM, muscle fibers fall victim to attacks from CD8+ T-cells, culminating in muscle cell demise. Conversely, in DM, early activation of complement triggers capillary and muscle damage, leading to ischemia. The challenge of targeted immunotherapy stems from unidentified autoantigens. However, nonspecific immunotherapeutic drugs have displayed promise in enhancing patient outcomes. Present treatments fail to directly address the underlying autoimmune processes in PM or the complement-related complications in DM. In the clinical study, participants will undergo intravenous administration of mitochondria. Initial safety assessments include monitoring the development of dose-limiting toxicities (DLT) in subjects during the first two weeks following mitochondrial administration.
However, as previously mentioned, there are several limitations to the use of mitochondrial transplantation in clinical research involving human. Numerous studies have emphasized the need to isolate mitochondria rapidly and under low-temperature conditions, as they are fragile, and quickly lose their viability. Additionally, mitochondria cannot be stored permanently. Therefore, ensuring the maintenance of mitochondrial activity, stability, and long-term safety and efficacy is critical for their use in human applications. Furthermore, it is essential to select an appropriate delivery mechanism that aligns with the treatment approach to accurately target the specific tissue (50).
CONCLUSION
Overall, while mitochondrial transplantation holds great promise, continued research and development are needed to overcome existing challenges, optimize techniques, and establish the safety and efficacy of this therapeutic approach in the treatment of mitochondrial diseases. The advancements in delivery methods, preservation techniques, and mechanistic insights discussed in this manuscript pave the way for future breakthroughs and the development of novel therapies for mitochondrial diseases.
Funding Statement
ACKNOWLEDGEMENTS This research was supported by the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (KGM5392313) and NRF-2021R1A2C1093421.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Bensley RR, Hoerr NL. Studies on cell structure by the freezing-drying method. VI. The preparation and properties of mitochondria. Anat Rec. 1934;60:449–455. doi: 10.1002/ar.1090600408. [DOI] [Google Scholar]
- 2.Ernster L, Schatz G. Mitochondria: a historical review. J Cell Biol. 1981;91(3 Pt 2):227s–255s. doi: 10.1083/jcb.91.3.227s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claude A. Fractionation of mammalian liver cells by differential centrifugation; experimental procedures and results. J Exp Med. 1946;84:61–89. doi: 10.1084/jem.84.1.61. [DOI] [PubMed] [Google Scholar]
- 4.Clark JB, Nicklas WJ. The metabolism of rat brain mitochondria. Preparation and characterization. J Biol Chem. 1970;245:4724–31. doi: 10.1016/S0021-9258(18)62854-6. [DOI] [PubMed] [Google Scholar]
- 5.Rendon A, Masmoudi A. Purification of non-synaptic and synaptic mitochondria and plasma membranes from rat brain by a rapid Percoll gradient procedure. J Neurosci Methods. 1985;14:41–51. doi: 10.1016/0165-0270(85)90113-X. [DOI] [PubMed] [Google Scholar]
- 6.Sims NR. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem. 1990;55:698–707. doi: 10.1111/j.1471-4159.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- 7.Anderson MF, Sims NR. Improved recovery of highly enriched mitochondrial fractions from small brain tissue samples. Brain Res Brain Res Protoc. 2000;5:95–101. doi: 10.1016/S1385-299X(99)00060-4. [DOI] [PubMed] [Google Scholar]
- 8.Sims NR, Anderson MF. Isolation of mitochondria from rat brain using Percoll density gradient centrifugation. Nat Protoc. 2008;3:1228–1239. doi: 10.1038/nprot.2008.105. [DOI] [PubMed] [Google Scholar]
- 9.Kuznetsov AV, Kunz WS, Saks V, et al. Cryopreservation of mitochondria and mitochondrial function in cardiac and skeletal muscle fibers. Anal Biochem. 2003;319:296–303. doi: 10.1016/S0003-2697(03)00326-9. [DOI] [PubMed] [Google Scholar]
- 10.Valenti D, de Bari L, De Filippis B, Ricceri L, Vacca RA. Preservation of mitochondrial functional integrity in mitochondria isolated from small cryopreserved mouse brain areas. Anal Biochem. 2014;444:25–31. doi: 10.1016/j.ab.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Hornig-Do HT, Günther G, Bust M, Lehnarts P, Bosio A, Wiesner R. Isolation of functional pure mitochondria by superparamagnetic microbeads. Anal Biochem. 2009;389:1–5. doi: 10.1016/j.ab.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 12.Franko A, Baris OR, Bergschneider E, et al. Efficient isolation of pure and functional mitochondria from mouse tissues using automated tissue disruption and enrichment with anti-TOM22 magnetic beads. PLoS One. 2013;8:e82392. doi: 10.1371/journal.pone.0082392.c4ffcc322db246078027a6b3eea701fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebbard WB, Harwood CL, Prajapati P, Springer JE, Saatman KE, Sullivan PG. Fractionated mitochondrial magnetic separation for isolation of synaptic mitochondria from brain tissue. Sci Rep. 2019;9:9656. doi: 10.1038/s41598-019-45568-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen WW, Freinkman E, Wang T, Birsoy K, Sabatini DM. Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell. 2016;166:1324–1337. doi: 10.1016/j.cell.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahier A, Dai CY, Tweedie A, Bezawork-Geleta A, Kirmes I, Zuryn S. Affinity purification of cell-specific mitochondria from whole animals resolves patterns of genetic mosaicism. Nat Cell Biol. 2018;20:352–360. doi: 10.1038/s41556-017-0023-x. [DOI] [PubMed] [Google Scholar]
- 16.Jiang D, Gao F, Zhang Y, et al. Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis. 2016;7:e2467. doi: 10.1038/cddis.2016.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li CJ, Chen PK, Sun LY, Pang CY. Enhancement of mitochondrial transfer by antioxidants in human mesenchymal stem cells. Oxid Med Cell Longev. 2017;2017:8510805. doi: 10.1155/2017/8510805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasquier J, Guerrouahen BS, Al Thawadi H, et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med. 2013;11:94. doi: 10.1186/1479-5876-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moschoi R, Imbert V, Nebout M, et al. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood. 2016;128:253–264. doi: 10.1182/blood-2015-07-655860. [DOI] [PubMed] [Google Scholar]
- 20.Lou E, Fujisawa S, Morozov A, et al. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS One. 2012;7:e33093. doi: 10.1371/journal.pone.0033093.7ef94fc94a9e4b128b4193da820d765e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu J, Zheng X, Li F, et al. Tunneling nanotubes promote intercellular mitochondria transfer followed by increased invasiveness in bladder cancer cells. Oncotarget. 2017;8:15539–15552. doi: 10.18632/oncotarget.14695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin HY, Liou CW, Chen SD, et al. Mitochondrial transfer from Wharton's jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion. 2015;22:31–44. doi: 10.1016/j.mito.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96:1039–1041. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- 24.Onfelt B, Nedvetzki S, Benninger RK, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;12:8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 25.Acquistapace A, Bru T, Lesault PF, et al. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells. 2011;29:812–824. doi: 10.1002/stem.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu K, Ji K, Guo L, et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc Res. 2014;92:10–18. doi: 10.1016/j.mvr.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Jackson MV, Morrison TJ, Doherty DF, et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34:2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinclair KA, Yerkovich ST, Hopkins PM, Chambers DC. Characterization of intercellular communication and mitochondrial donation by mesenchymal stromal cells derived from the human lung. Stem Cell Res Ther. 2016;7:91. doi: 10.1186/s13287-016-0354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali Pour P, Kenney MC, Kheradvar A. Bioenergetics consequences of mitochondrial transplantation in cardiomyocytes. J Am Heart Assoc. 2020;9:e014501. doi: 10.1161/JAHA.119.014501.06e3641394ce4ef1b15b0e938c1935a8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang A, Liu Y, Pan J, et al. Delivery of mitochondria confers cardioprotection through mitochondria replenishment and metabolic compliance. Mol Ther. 2023;31:1468–1479. doi: 10.1016/j.ymthe.2023.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X, Chen H, Gao R, et al. Intravenous transplantation of an ischemic-specific peptide-TPP-mitochondrial compound alleviates myocardial ischemic reperfusion injury. ACS Nano. 2023;17:896–909. doi: 10.1021/acsnano.2c05286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao J, Qin A, Liu D, et al. Endoplasmic reticulum mediates mitochondrial transfer within the osteocyte dendritic network. Sci Adv. 2019;5:eaaw7215. doi: 10.1126/sciadv.aaw7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rafelski SM. Mitochondrial network morphology: building an integrative, geometrical view. BMC Biol. 2013;11:71. doi: 10.1186/1741-7007-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kesner EE, Saada-Reich A, Lorberboum-Galski H. Characteristics of mitochondrial transformation into human cells. Sci Rep. 2016;6:26057. doi: 10.1038/srep26057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JM, Hwang JW, Kim MJ, et al. Mitochondrial transplantation modulates inflammation and apoptosis, alleviating tendinopathy both in vivo and in vitro. Antioxidants (Basel) 2021;10:696. doi: 10.3390/antiox10050696.50521083f1214ce28c184dbd557ddd3b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang JC, Wu SL, Liu KH, et al. Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson's disease: restoration of mitochondria functions and attenuation of 6-hydroxydopamine-induced neurotoxicity. Transl Res. 2016;170:40–56. doi: 10.1016/j.trsl.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Shi X, Zhao M, Fu C, Fu A. Intravenous administration of mitochondria for treating experimental Parkinson's disease. Mitochondrion. 2017;34:91–100. doi: 10.1016/j.mito.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Wong RCB, Lim SY, Hung SSC, et al. Mitochondrial replacement in an iPSC model of Leber's hereditary optic neuropathy. Aging (Albany NY) 2017;9:1341–1350. doi: 10.18632/aging.101231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang TG, Miao CY. Mitochondrial transplantation as a promising therapy for mitochondrial diseases. Acta Pharm Sin B. 2023;13:1028–1035. doi: 10.1016/j.apsb.2022.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caicedo A, Fritz V, Brondello JM, et al. MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci Rep. 2015;5:9073. doi: 10.1038/srep09073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guariento A, Piekarski BL, Doulamis IP, et al. Autologous mitochondrial transplantation for cardiogenic shock in pediatric patients following ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2021;162:992–1001. doi: 10.1016/j.jtcvs.2020.10.151. [DOI] [PubMed] [Google Scholar]
- 43.Kim MJ, Hwang JW, Yun CK, Lee Y, Choi YS. Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function. Sci Rep. 2018;8:3330. doi: 10.1038/s41598-018-21539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macheiner T, Fengler VH, Agreiter M, et al. Magnetomitotransfer: an efficient way for direct mitochondria transfer into cultured human cells. Sci Rep. 2016;6:35571. doi: 10.1038/srep35571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang JC, Liu KH, Li YC, et al. Functional recovery of human cells harbouring the mitochondrial DNA mutation MERRF A8344G via peptide-mediated mitochondrial delivery. Neurosignals. 2013;21:160–173. doi: 10.1159/000341981.c0b29780493649ef83e12a59ed1c3fca [DOI] [PubMed] [Google Scholar]
- 46.Wu S, Zhang A, Li S, et al. Polymer functionalization of isolated mitochondria for cellular transplantation and metabolic phenotype alteration. Adv Sci (Weinh) 2018;5:1700530. doi: 10.1002/advs.201700530.9ee13898597143bba0afb42b468b904b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu TH, Sagullo E, Case D, et al. Mitochondrial transfer by photothermal nanoblade restores metabolite profile in mammalian cells. Cell Metab. 2016;23:921–929. doi: 10.1016/j.cmet.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gabelein CG, Feng Q, Sarajlic E, et al. Mitochondria transplantation between living cells. PLoS Biol. 2022;20:e3001576. doi: 10.1371/journal.pbio.3001576.1a1a01f6939344d3bba7c6ce9f3308fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emani SM, Piekarski BL, Harrild D, Del Nido PJ, McCully JD. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2017;154:286–289. doi: 10.1016/j.jtcvs.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 50.Park A, Oh M, Lee SJ, et al. Mitochondrial transplantation as a novel therapeutic strategy for mitochondrial diseases. Int J Mol Sci. 2021;22:4793. doi: 10.3390/ijms22094793.d9151cde0c2c400dbb5efb751db2cb1f [DOI] [PMC free article] [PubMed] [Google Scholar]