Abstract
Ribulose-1,5-bisphosphate (RuBP) carboxylase in lysed spinach (Spinacia oleracea L. cv virtuosa) chloroplasts that had been partly inactivated at low CO2 and Mg2+ by incubating in darkness with 4 millimolar partially purified RuBP was reactivated by light. If purified RuBP was used to inhibit dark activation of the enzyme, reactivation by light was not observed unless fructose-1,6-bisphosphate, ATP, or ADP plus inorganic phosphate were also added. Presumably, ADP plus inorganic phosphate acted as an ATP-generating system with a requirement for the generation of ΔpH across the thylakoid membrane. When the RuBP obtained from Sigma Chemical Co. was used, light did not reactivate the enzyme. There was no direct correlation between ΔpH and activation. Therefore, thylakoids are required in the ribulose-1,5-bisphosphate carboxylase activase system largely to synthesize ATP. Inactivation of RuBP carboxylase in isolated chloroplasts or in the lysed chloroplast system was not promoted simply by a transition from light to dark conditions but was caused by low CO2 and Mg2+.
Full text
PDF
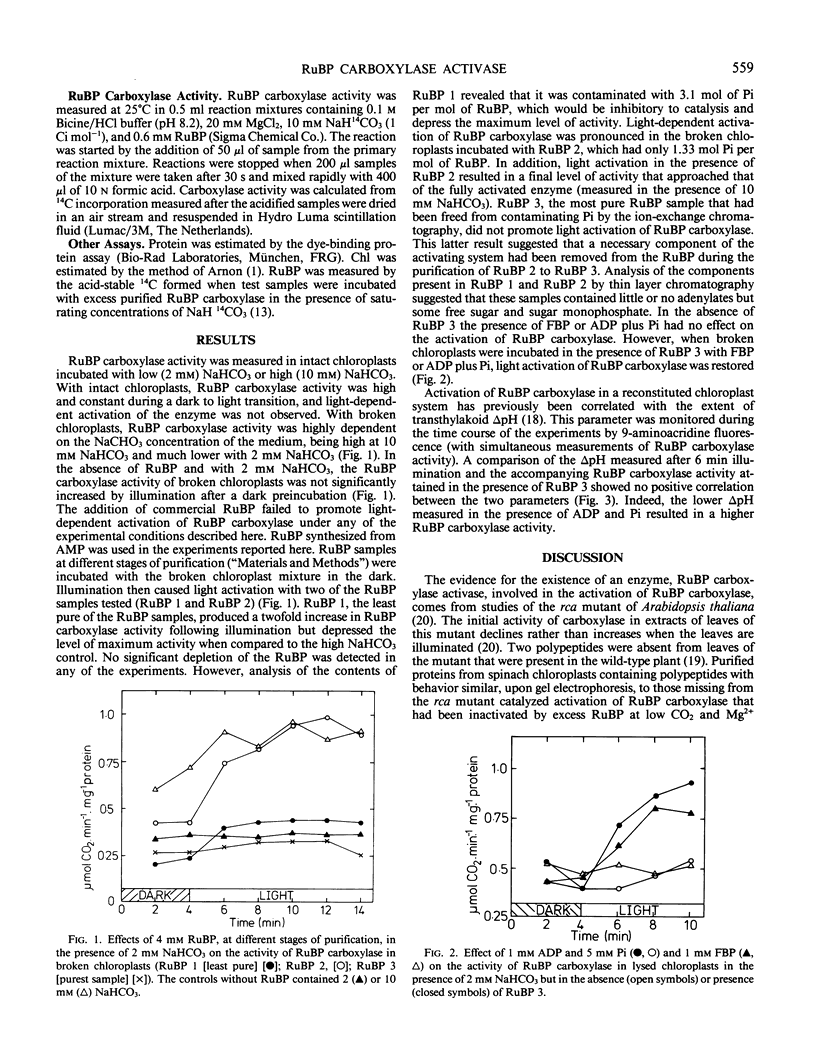

Selected References
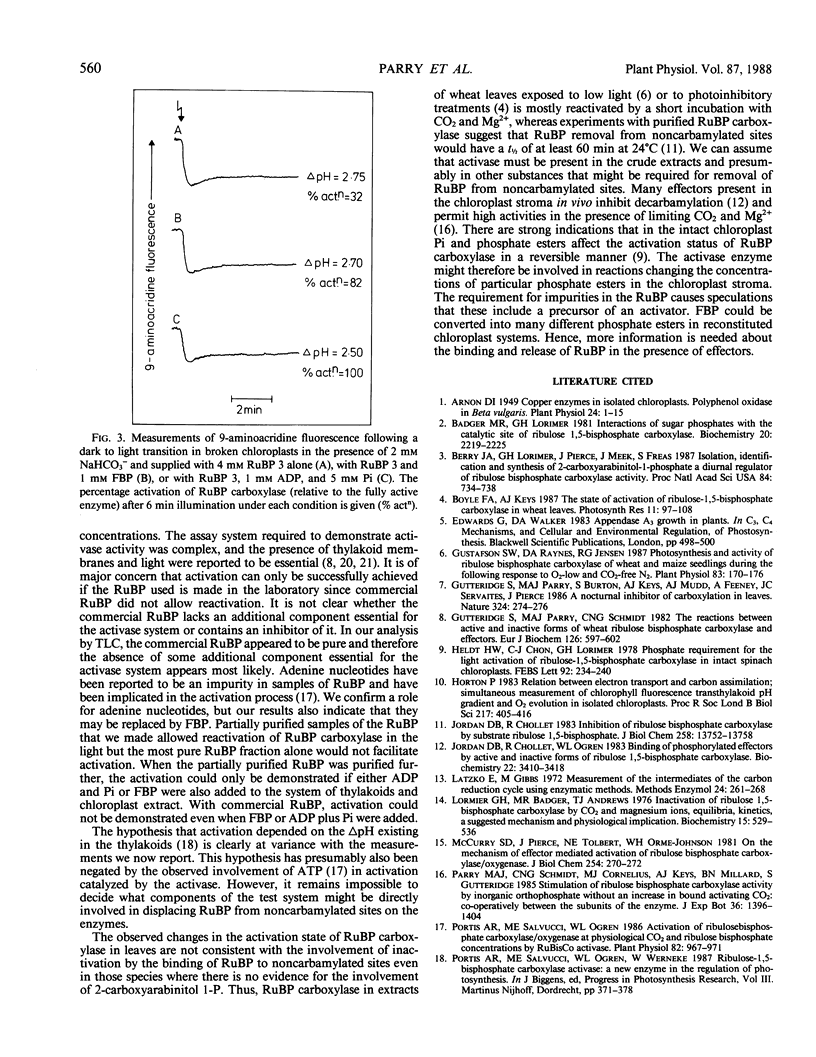
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Lorimer G. H. Interaction of sugar phosphates with the catalytic site of ribulose-1,5-bisphosphate carboxylase. Biochemistry. 1981 Apr 14;20(8):2219–2225. doi: 10.1021/bi00511a023. [DOI] [PubMed] [Google Scholar]
- Berry J. A., Lorimer G. H., Pierce J., Seemann J. R., Meek J., Freas S. Isolation, identification, and synthesis of 2-carboxyarabinitol 1-phosphate, a diurnal regulator of ribulose-bisphosphate carboxylase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):734–738. doi: 10.1073/pnas.84.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson S. W., Raynes D. A., Jensen R. G. Photosynthesis and Activity of Ribulose Bisphosphate Carboxylase of Wheat and Maize Seedlings during and following Exposure to O(2)-Low, CO(2)-Free N(2). Plant Physiol. 1987 Jan;83(1):170–176. doi: 10.1104/pp.83.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge S., Parry M. A., Schmidt C. N. The reactions between active and inactive forms of wheat ribulosebisphosphate carboxylase and effectors. Eur J Biochem. 1982 Sep 1;126(3):597–602. doi: 10.1111/j.1432-1033.1982.tb06822.x. [DOI] [PubMed] [Google Scholar]
- Jordan D. B., Chollet R. Inhibition of ribulose bisphosphate carboxylase by substrate ribulose 1,5-bisphosphate. J Biol Chem. 1983 Nov 25;258(22):13752–13758. [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Measurement of the intermediates of the photosynthetic carbon reduction cycle, using enzymatic methods. Methods Enzymol. 1972;24:261–268. doi: 10.1016/0076-6879(72)24073-3. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Portis A. R., Salvucci M. E., Ogren W. L. Activation of Ribulosebisphosphate Carboxylase/Oxygenase at Physiological CO(2) and Ribulosebisphosphate Concentrations by Rubisco Activase. Plant Physiol. 1986 Dec;82(4):967–971. doi: 10.1104/pp.82.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M. E., Portis A. R., Ogren W. L. Light and CO(2) Response of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase Activation in Arabidopsis Leaves. Plant Physiol. 1986 Mar;80(3):655–659. doi: 10.1104/pp.80.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J. R., Berry J. A., Freas S. M., Krump M. A. Regulation of ribulose bisphosphate carboxylase activity in vivo by a light-modulated inhibitor of catalysis. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8024–8028. doi: 10.1073/pnas.82.23.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C. Binding of a Phosphorylated Inhibitor to Ribulose Bisphosphate Carboxylase/Oxygenase during the Night. Plant Physiol. 1985 Aug;78(4):839–843. doi: 10.1104/pp.78.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Parry M. A., Gutteridge S., Keys A. J. Species variation in the predawn inhibition of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol. 1986 Dec;82(4):1161–1163. doi: 10.1104/pp.82.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C. R., Portis A. R., Ogren W. L. A Mutant of Arabidopsis thaliana Which Lacks Activation of RuBP Carboxylase In Vivo. Plant Physiol. 1982 Aug;70(2):381–387. doi: 10.1104/pp.70.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu J. C., Allen L. H., Bowes G. Dark/Light modulation of ribulose bisphosphate carboxylase activity in plants from different photosynthetic categories. Plant Physiol. 1984 Nov;76(3):843–845. doi: 10.1104/pp.76.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]


