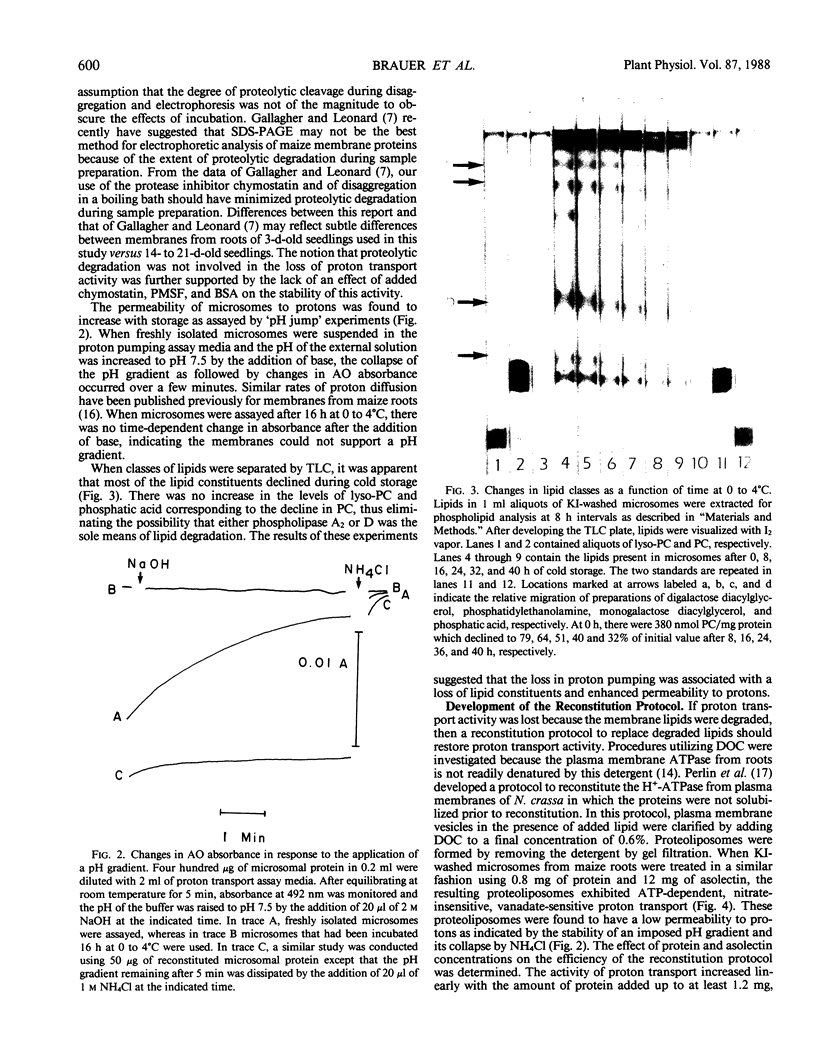
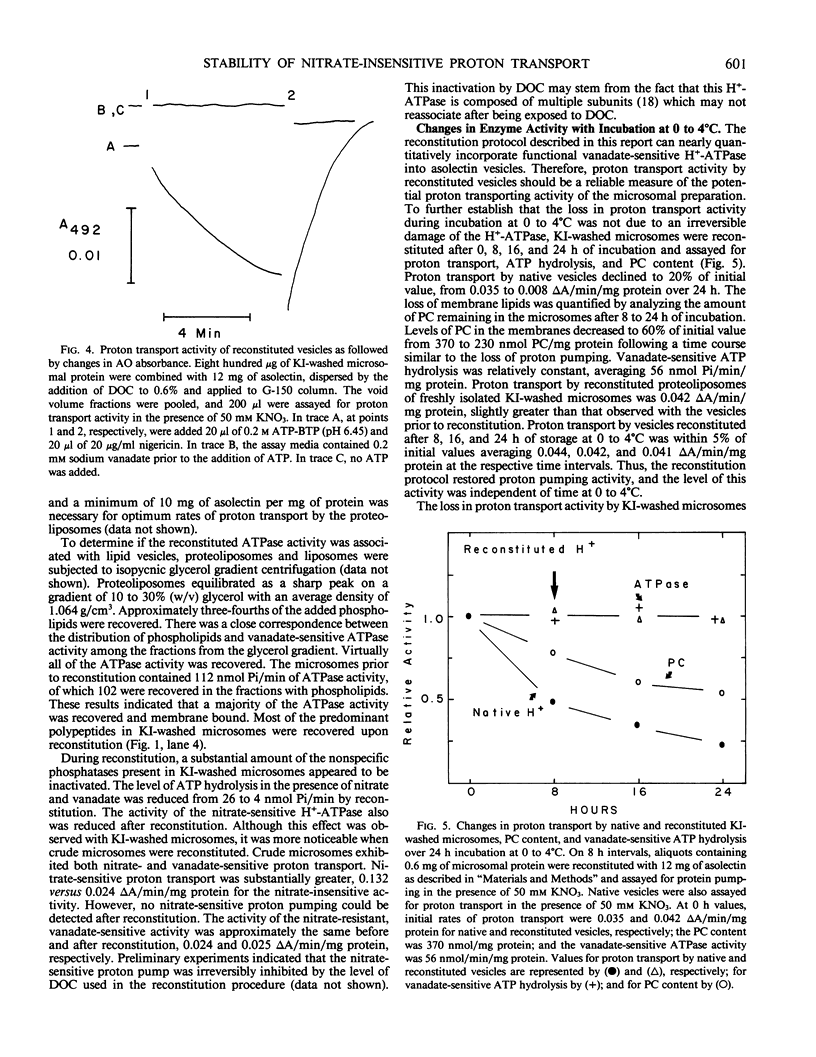
Abstract
Proton transport catalyzed by the nitrate-insensitive, vanadate-sensitive H+-ATPase in microsomes from maize (Zea mays L.) roots washed with 0.25 molar KI decreased as a function of time at 0 to 4°C. The rate of proton transport was approximately one-half of that by freshly isolated microsomes after 6 to 18 hours of cold storage. The decrease in proton transport coincided with losses in membrane phosphatidylcholine and was not associated with a change in vanadate-sensitive ATP hydrolysis. A technique based on a protocol developed for the reconstitution of Neurospora crassa plasma membrane H+-ATPase (DS Perlin, K Kasamo, RJ Brooker, CW Slayman 1984 J Biol Chem 259: 7884-7892) was employed to restore proton transport activity to maize microsomes. These results indicated that the decline in proton transport by maize root membranes during cold storage was not due to degradation of the protein moiety of the H+-ATPase, but was due to the loss of phospholipids.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison R., Scarborough G. A. Solubilization and purification of the Neurospora plasma membrane H+-ATPase. J Biol Chem. 1981 Dec 25;256(24):13165–13171. [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Salt tolerance in suspension cultures of sugar beet : induction of na/h antiport activity at the tonoplast by growth in salt. Plant Physiol. 1987 Apr;83(4):884–887. doi: 10.1104/pp.83.4.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Michelis M. I., Spanswick R. M. H-pumping driven by the vanadate-sensitive ATPase in membrane vesicles from corn roots. Plant Physiol. 1986 Jun;81(2):542–547. doi: 10.1104/pp.81.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. R., Carroll E. J., Leonard R. T. A sensitive diffusion plate assay for screening inhibitors of protease activity in plant cell fractions. Plant Physiol. 1986 Jul;81(3):869–874. doi: 10.1104/pp.81.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Electrophoretic characterization of a detergent-treated plasma membrane fraction from corn roots. Plant Physiol. 1987 Feb;83(2):265–271. doi: 10.1104/pp.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi G., Baker A. F. beta-Glucosidase Activity in Corn Roots: Problems in Subcellular Fractionation. Plant Physiol. 1984 Dec;76(4):861–864. doi: 10.1104/pp.76.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliyath G., Thompson J. E. Calcium- and calmodulin-regulated breakdown of phospholipid by microsomal membranes from bean cotyledons. Plant Physiol. 1987 Jan;83(1):63–68. doi: 10.1104/pp.83.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin D. S., Kasamo K., Brooker R. J., Slayman C. W. Electrogenic H+ translocation by the plasma membrane ATPase of Neurospora. Studies on plasma membrane vesicles and reconstituted enzyme. J Biol Chem. 1984 Jun 25;259(12):7884–7892. [PubMed] [Google Scholar]
- Randall S. K., Sze H. Properties of the partially purified tonoplast H+-pumping ATPase from oat roots. J Biol Chem. 1986 Jan 25;261(3):1364–1371. [PubMed] [Google Scholar]
- Tu S. I., Nagahashi G., Brouillette J. N. Proton pumping kinetics and origin of nitrate inhibition of tonoplast-type H+-ATPase. Arch Biochem Biophys. 1987 Aug 1;256(2):625–637. doi: 10.1016/0003-9861(87)90620-5. [DOI] [PubMed] [Google Scholar]
- Xie X. S., Tsai S. J., Stone D. K. Lipid requirements for reconstitution of the proton-translocating complex of clathrin-coated vesicles. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8913–8917. doi: 10.1073/pnas.83.23.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]