Abstract
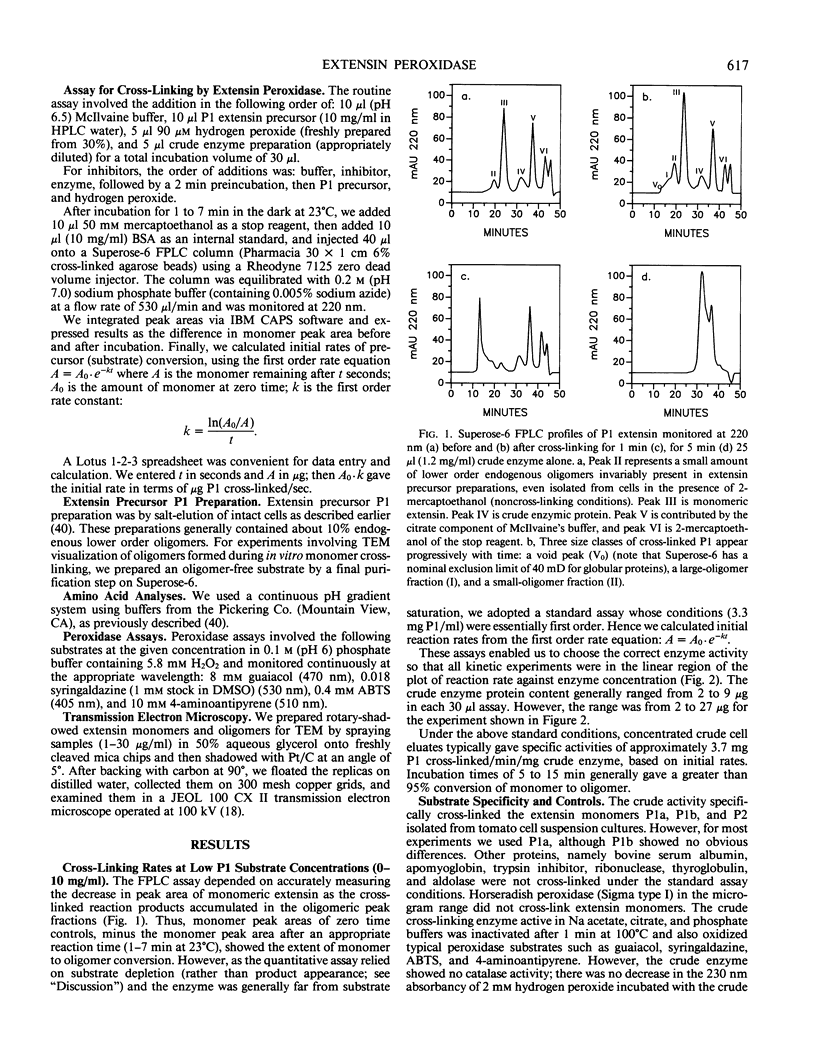
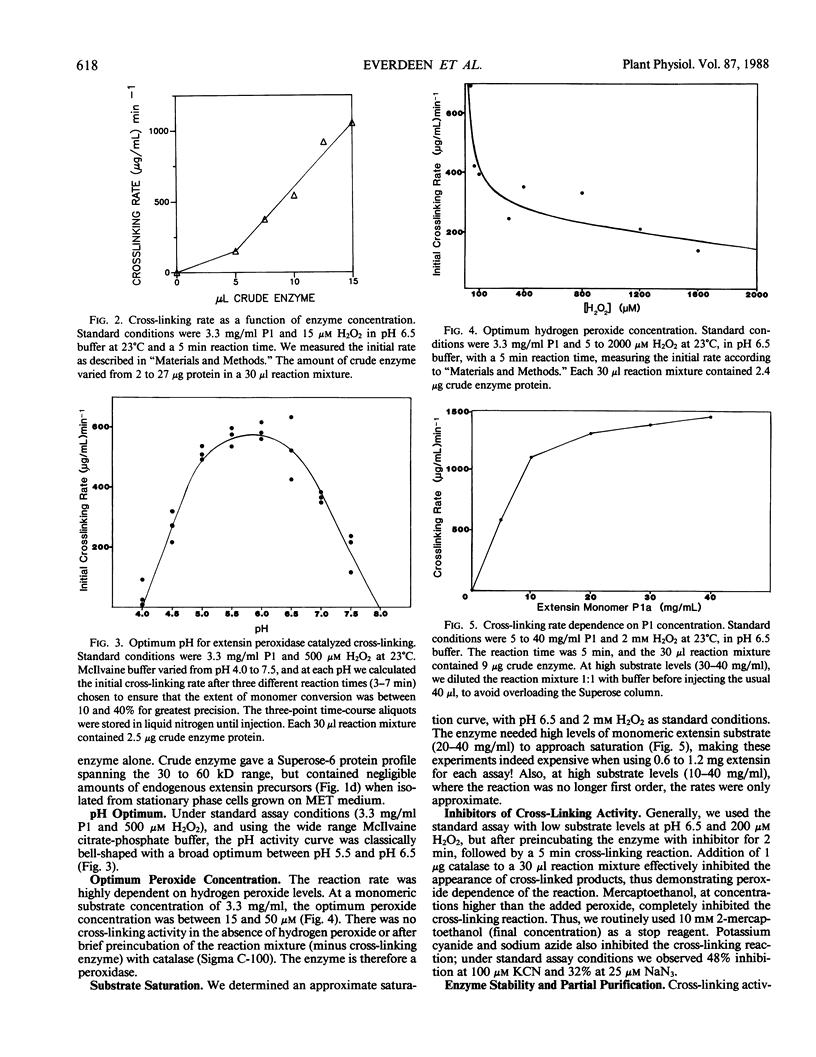
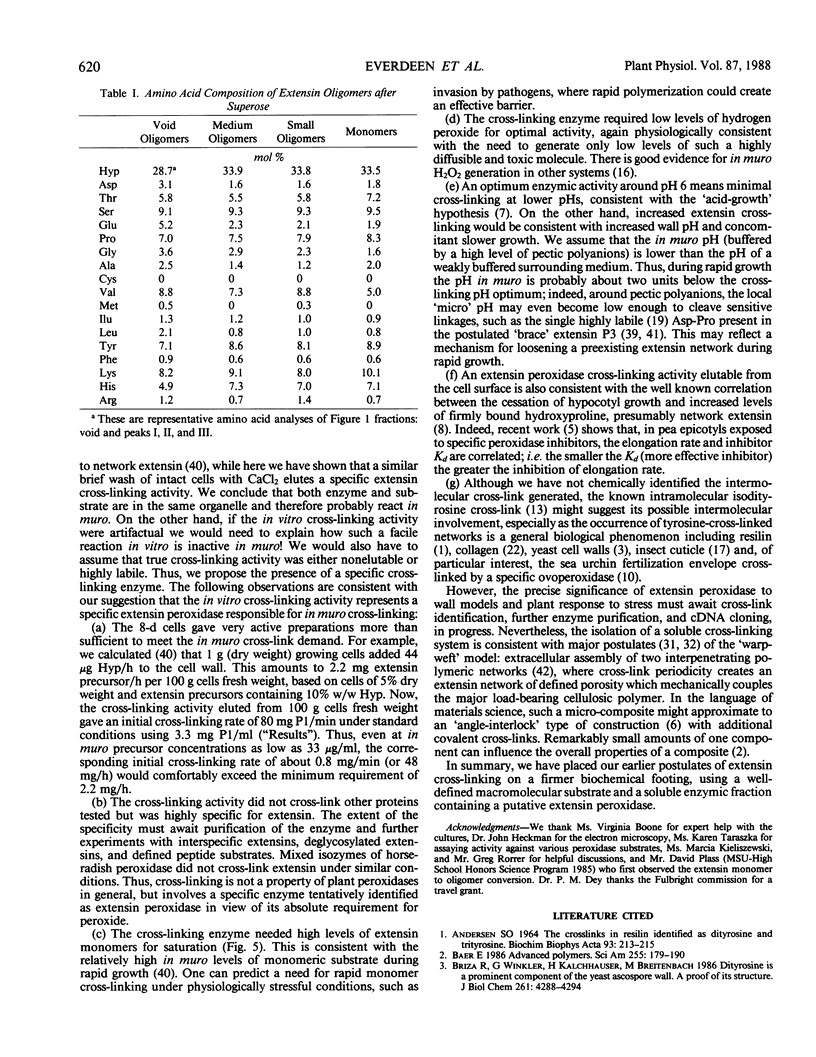
Rapidly growing tomato (Lycopersicon esculentum) cell suspension cultures contain transiently high levels of cell surface, salt-elutable, monomeric precursors to the covalently cross-linked extensin network of the primary cell wall. Thus, we purified a highly soluble monomeric extensin substrate from rapidly growing cells, and devised a soluble in vitro cross-linking assay based on Superose-6 fast protein liquid chromatography separation, which resolved extensin monomers from the newly formed oligomers within 25 minutes. Salt elution of slowly growing (early stationary phase) cells yielded little or no extensin monomers but did give a highly active enzymic preparation that specifically cross-linked extensin monomers in the presence of hydrogen peroxide, judging from: (a) a decrease in the extensin monomer peak on fast protein liquid chromatography gel filtration, (b) appearance of oligomeric peaks, and (c) direct electron microscopical observation of the cross-linked oligomers. The cross-linking reaction had a broad pH optimum between 5.5 and 6.5. An approach to substrate saturation of the enzyme required extensin monomer concentrations of 20 to 40 milligrams per milliliter. Preincubation with catalase completely inhibited the cross-linking reaction, which was highly dependent on hydrogen peroxide and optimal at 15 to 50 micromolar. We therefore identified the cross-linking activity as extensin peroxidase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN S. O. THE CROSS-LINKS IN RESILIN IDENTIFIED AS DITYROSINE AND TRITYROSINE. Biochim Biophys Acta. 1964 Oct 9;93:213–215. doi: 10.1016/0304-4165(64)90289-2. [DOI] [PubMed] [Google Scholar]
- Briza P., Winkler G., Kalchhauser H., Breitenbach M. Dityrosine is a prominent component of the yeast ascospore wall. A proof of its structure. J Biol Chem. 1986 Mar 25;261(9):4288–4294. [PubMed] [Google Scholar]
- Brysk M. M., Chrispeels M. J. Isolation and partial characterization of a hydroxyproline-rich cell wall glycoprotein and its cytoplasmic precursor. Biochim Biophys Acta. 1972 Feb 29;257(2):421–432. doi: 10.1016/0005-2795(72)90295-4. [DOI] [PubMed] [Google Scholar]
- Cleland R., Karlsnes A. M. A possible role of hydroxyproline-containing proteins in the cessation of cell elongation. Plant Physiol. 1967 May;42(5):669–671. doi: 10.1104/pp.42.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. B., Varner J. E. Cross-linking of soluble extensin in isolated cell walls. Plant Physiol. 1984 Oct;76(2):414–417. doi: 10.1104/pp.76.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deits T., Farrance M., Kay E. S., Medill L., Turner E. E., Weidman P. J., Shapiro B. M. Purification and properties of ovoperoxidase, the enzyme responsible for hardening the fertilization membrane of the sea urchin egg. J Biol Chem. 1984 Nov 10;259(21):13525–13533. [PubMed] [Google Scholar]
- Dougall D. K., Shimbayashi K. Factors Affecting Growth of Tobacco Callus Tissue and Its Incorporation of Tyrosine. Plant Physiol. 1960 May;35(3):396–404. doi: 10.1104/pp.35.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry S. C. Isodityrosine, a new cross-linking amino acid from plant cell-wall glycoprotein. Biochem J. 1982 May 15;204(2):449–455. doi: 10.1042/bj2040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. W., Terhune B. T., Lamport D. T. Characterization of native and modified extensin monomers and oligomers by electron microscopy and gel filtration. Plant Physiol. 1988 Mar;86(3):848–856. doi: 10.1104/pp.86.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauregui-Adell J., Marti J. Acidic cleavage of the aspartyl-proline band and the limitations of the reaction. Anal Biochem. 1975 Dec;69(2):468–473. doi: 10.1016/0003-2697(75)90148-7. [DOI] [PubMed] [Google Scholar]
- KOLLAR S. J., JARAI M. Biochemical chlorination in Streptomvces aureofaciens. Nature. 1960 Nov 19;188:665–665. doi: 10.1038/188665a0. [DOI] [PubMed] [Google Scholar]
- Keegstra K., Talmadge K. W., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: III. A Model of the Walls of Suspension-cultured Sycamore Cells Based on the Interconnections of the Macromolecular Components. Plant Physiol. 1973 Jan;51(1):188–197. doi: 10.1104/pp.51.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPORT D. T. CELL SUSPENSION CULTURES OF HIGHER PLANTS: ISOLATION AND GROWTH ENERGETICS. Exp Cell Res. 1964 Jan;33:195–206. doi: 10.1016/s0014-4827(64)81026-0. [DOI] [PubMed] [Google Scholar]
- LAMPORT D. T. Oxygen fixation into hydroxyproline of plant cell wall protein. J Biol Chem. 1963 Apr;238:1438–1440. [PubMed] [Google Scholar]
- LaBella F., Waykole P., Queen G. Formation of insoluble gels and dityrosine by the action of peroxidase on soluble collagens. Biochem Biophys Res Commun. 1968 Feb 26;30(4):333–338. doi: 10.1016/0006-291x(68)90746-8. [DOI] [PubMed] [Google Scholar]
- Liu E. H., Lamport D. T. An Accounting of Horseradish Peroxidase Isozymes Associated with the Cell Wall and Evidence that Peroxidase Does Not Contain Hydroxyproline. Plant Physiol. 1974 Dec;54(6):870–876. doi: 10.1104/pp.54.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort A. J., Lamport D. T. Anhydrous hydrogen fluoride deglycosylates glycoproteins. Anal Biochem. 1977 Oct;82(2):289–309. doi: 10.1016/0003-2697(77)90165-8. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Lee Y. M., Brown W. D. Interactions of heme proteins with hydrogen peroxide: protein crosslinking and covalent binding of benzo[a]pyrene and 17 beta-estradiol. Arch Biochem Biophys. 1983 Mar;221(2):417–427. doi: 10.1016/0003-9861(83)90160-1. [DOI] [PubMed] [Google Scholar]
- Stuart D. A., Varner J. E. Purification and Characterization of a Salt-extractable Hydroxyproline-rich Glycoprotein from Aerated Carrot Discs. Plant Physiol. 1980 Nov;66(5):787–792. doi: 10.1104/pp.66.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]



