Abstract
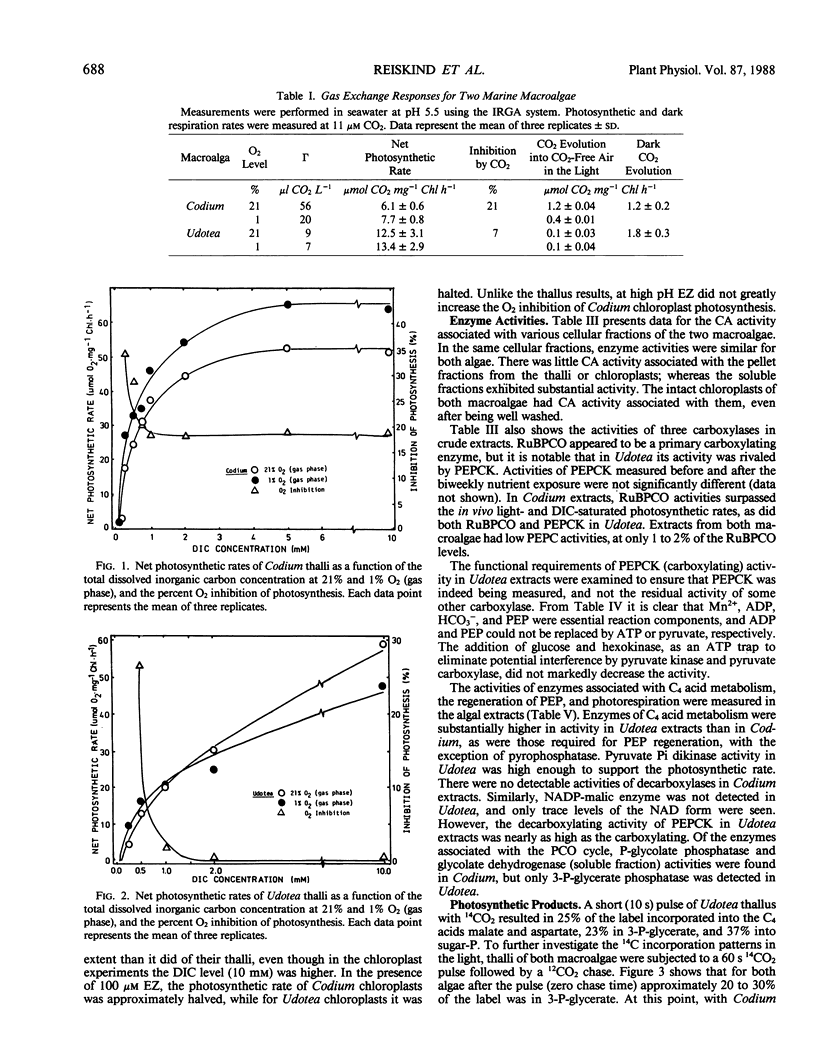
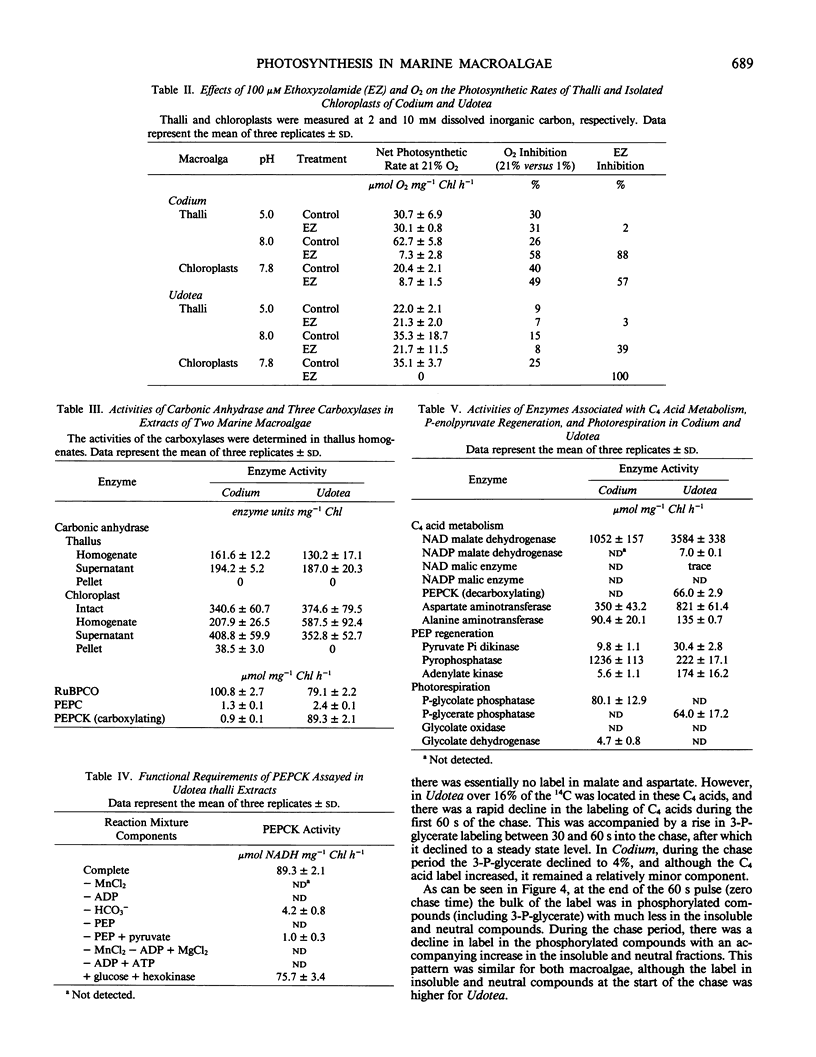
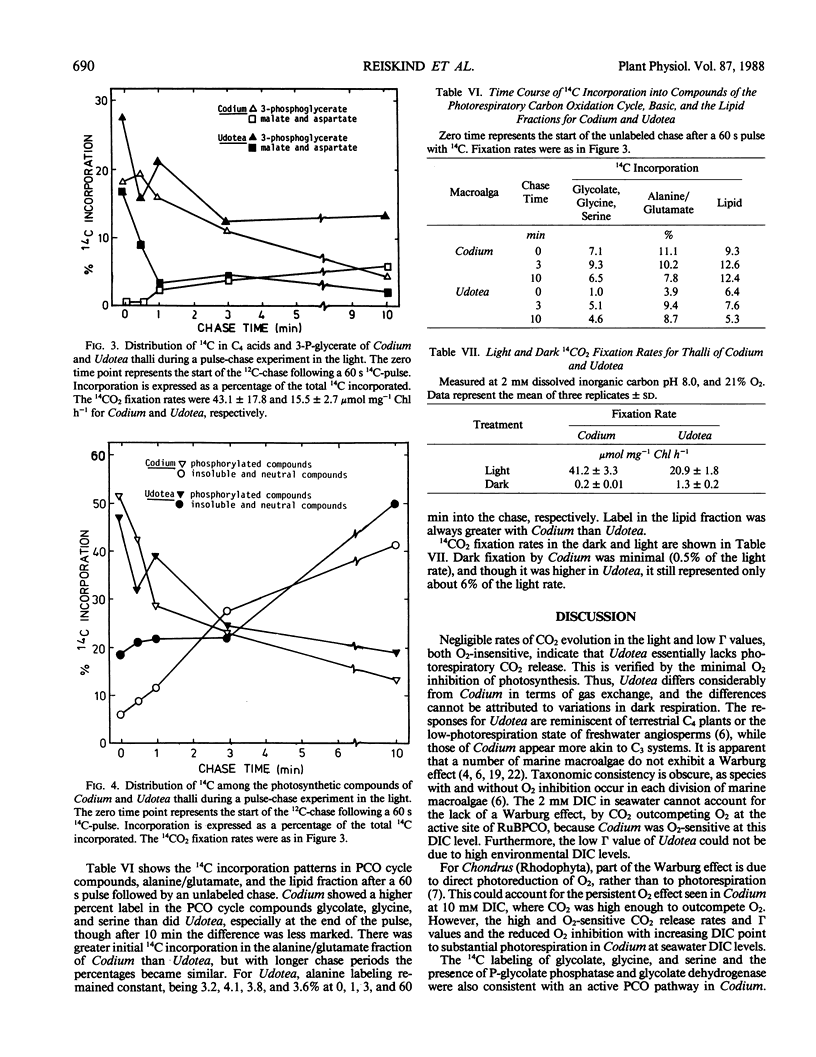
Two green macroalgae, Codium decorticatum and Udotea flabellum, differ photosynthetically. Codium had high O2-sensitive, and Udotea low O2-insensitive, CO2 compensation points; Codium showed a Warburg effect at seawater dissolved inorganic carbon levels and had photorespiratory CO2 release, whereas Udotea did not. Seawater dissolved inorganic carbon levels did not saturate photosynthesis. For Codium, but not Udotea, the Warburg effect was increased by ethoxyzolamide, a carbonic anhydrase inhibitor, at high but not low pH. Isolated chloroplasts from both macroalgae showed a Warburg effect that was ethoxyzolamide-insensitive. In both macroalgae, chloroplastic and extrachloroplastic carbonic anhydrase activity was present. P-enolpyruvate carboxykinase (PEPCK) carboxylating activity in Udotea extracts was equivalent to that of ribulose bisphosphate carboxylase, and enzyme activities for C4 acid metabolism and P-enolpyruvate regeneration were sufficient to operate a limited C4-like system. In Udotea, malate and aspartate were early-labeled photosynthetic products that turned over within 60 seconds. Photorespiratory compounds were much less labeled in Udotea. Low dark fixation rates ruled out Crassulacean acid metabolism. A limited C4-like system, based on PEPCK, is hypothesized to be the mechanism reducing photorespiration in Udotea. Codium showed no evidence of photosynthetic C4 acid metabolism. Marine macroalgae, like terrestrial angiosperms, seem to have diverse photosynthetic modes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell R. G., Levin W. B., Shephard D. C. Photosynthesis, Photorespiration and Respiration of Chloroplasts From Acetabularia mediterrania. Plant Physiol. 1969 Jul;44(7):946–954. doi: 10.1104/pp.44.7.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechignac F., Andre M. Oxygen Uptake and Photosynthesis of the Red Macroalga, Chondrus crispus, in Seawater: Effects of Oxygen Concentration. Plant Physiol. 1985 Jul;78(3):545–550. doi: 10.1104/pp.78.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb A. H. The relationship of purity to photosynthetic activity in preparations of Codium fragile chloroplasts. Protoplasma. 1977;92(1-2):137–146. doi: 10.1007/BF01280206. [DOI] [PubMed] [Google Scholar]
- Elrifi I. R., Turpin D. H. The Path of Carbon Flow during NO(3)-Induced Photosynthetic Suppression in N-Limited Selenastrum minutum. Plant Physiol. 1987 Jan;83(1):97–104. doi: 10.1104/pp.83.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick S. E., Gruber P. J., Tolbert N. E. The occurrence of glycolate dehydrogenase and glycolate oxidase in green plants: an evolutionary survey. Plant Physiol. 1973 Oct;52(4):318–323. doi: 10.1104/pp.52.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D. An assat for PEP carboxykinase in crude tissue extracts. Anal Biochem. 1973 Mar;52(1):280–285. doi: 10.1016/0003-2697(73)90350-3. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Mau S. L. Activity, location, and role of asparate aminotransferase and alanine aminotransferase isoenzymes in leaves with C4 pathway photosynthesis. Arch Biochem Biophys. 1973 May;156(1):195–206. doi: 10.1016/0003-9861(73)90357-3. [DOI] [PubMed] [Google Scholar]
- Holdsworth E. S., Bruck K. Enzymes concerned with beta-carboxylation in marine phytoplankter. Purification and properties of phosphoenolpyruvate carboxykinase. Arch Biochem Biophys. 1977 Jul;182(1):87–94. doi: 10.1016/0003-9861(77)90286-7. [DOI] [PubMed] [Google Scholar]
- Moroney J. V., Togasaki R. K., Husic H. D., Tolbert N. E. Evidence That an Internal Carbonic Anhydrase Is Present in 5% CO(2)-Grown and Air-Grown Chlamydomonas. Plant Physiol. 1987 Jul;84(3):757–761. doi: 10.1104/pp.84.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M. E., Bowes G. Two photosynthetic mechanisms mediating the low photorespiratory state in submersed aquatic angiosperms. Plant Physiol. 1983 Oct;73(2):488–496. doi: 10.1104/pp.73.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van T. K., Haller W. T., Bowes G. Comparison of the photosynthetic characteristics of three submersed aquatic plants. Plant Physiol. 1976 Dec;58(6):761–768. doi: 10.1104/pp.58.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu C. V., Allen L. H., Bowes G. Effects of Light and Elevated Atmospheric CO(2) on the Ribulose Bisphosphate Carboxylase Activity and Ribulose Bisphosphate Level of Soybean Leaves. Plant Physiol. 1983 Nov;73(3):729–734. doi: 10.1104/pp.73.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]


