Abstract
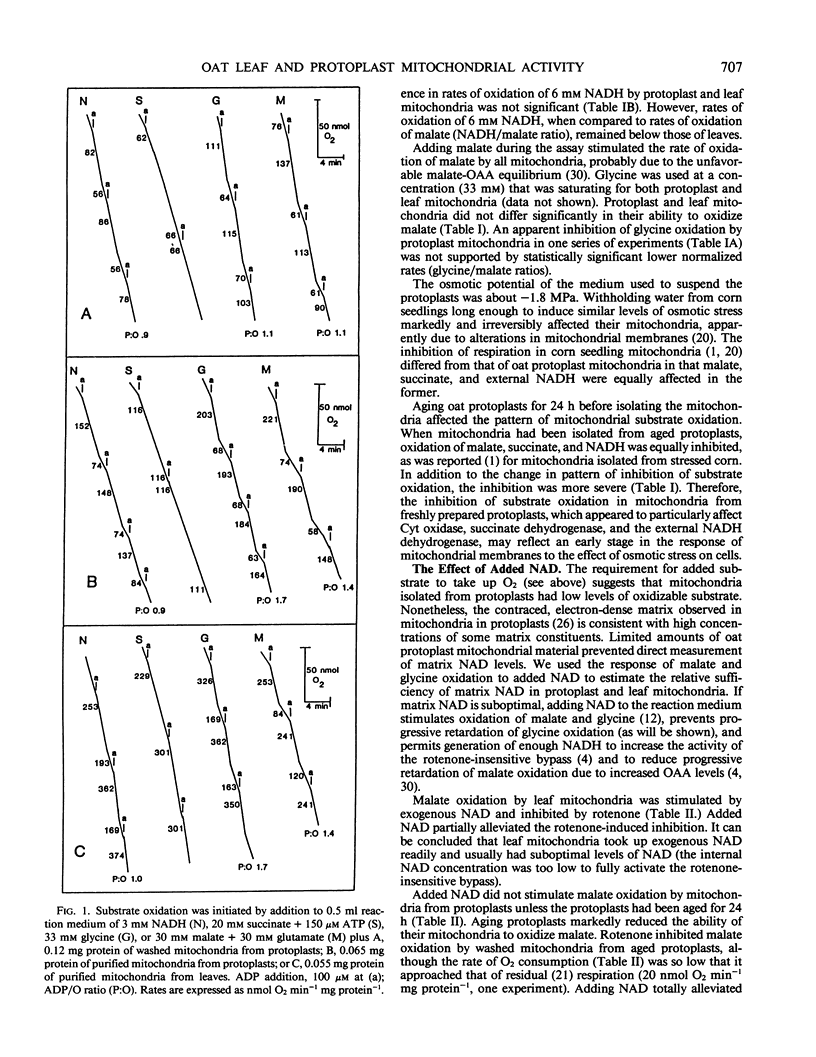
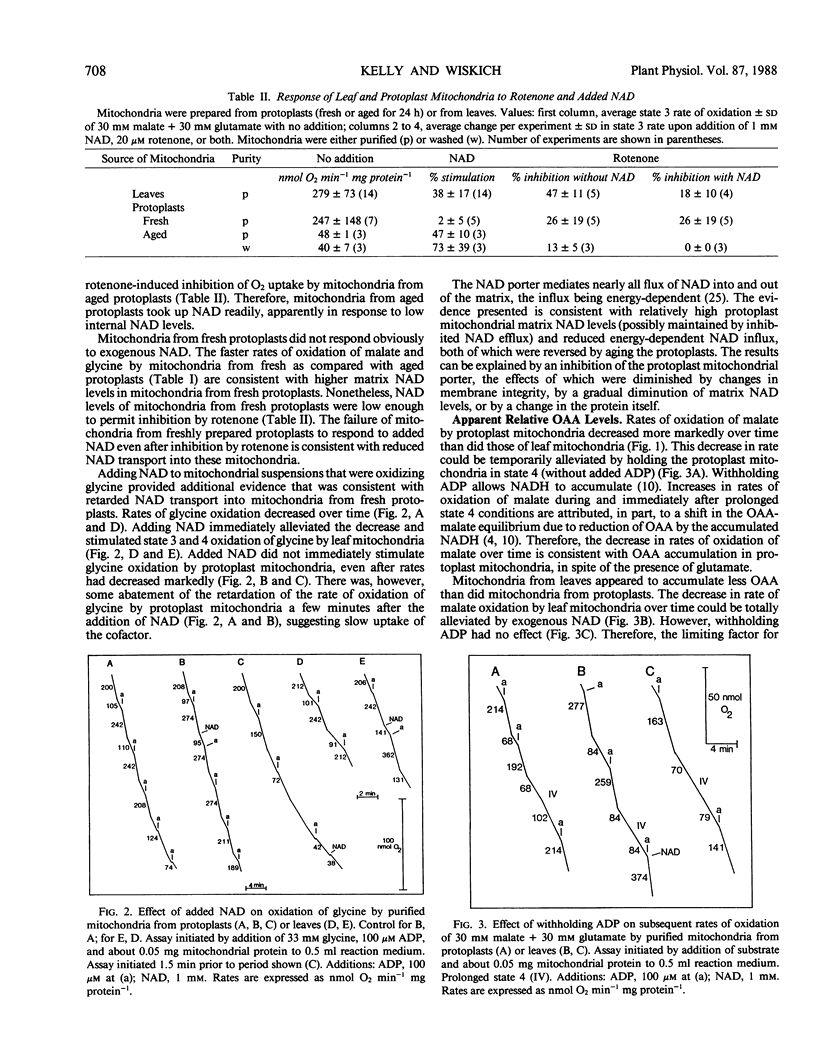
Mitochondria isolated from mesophyll protoplasts differed from mitochondria isolated directly from leaves of Avena sativa in that protoplast mitochondria (a) had a lower overall respiratory capacity, (b) were less able to use low concentrations of exogenous NADH, (c) did not respond rapidly or strongly to added NAD, (d) appeared to accumulate more oxaloacetate, and (e) oxidized both succinate and tetramethyl-p-phenylene-diamine (an electron donor for cytochrome oxidase) more slowly than did leaf mitochondria. It is concluded that cytochrome oxidase activity was inhibited, the external NADH dehydrogenase had a reduced affinity for NADH, succinate oxidation was inhibited, NAD and oxaloacetate porters were probably inhibited, and accessibility to respiratory paths may have been reduced in protoplast mitochondria. The results also suggest that there was a reduced affinity of a succinate porter for this substrate in oat mitochondria. In addition, all oat mitochondria required salicylhydroxamic acid (SHAM) as well as cyanide to block malate and succinate oxidation. Malate oxidation that did not appear to saturate the cytochrome pathway was sensitive to SHAM in the absence of cyanide, suggesting that the oat mitochondria studied had concomitant alternative and subsaturating cytochrome oxidase pathway activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell D. T., Koeppe D. E., Miller R. J. The effects of drought stress on respiration of isolated corn mitochondria. Plant Physiol. 1971 Oct;48(4):413–415. doi: 10.1104/pp.48.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. Effect of phthalonic acid on respiration and metabolite transport in higher plant mitochondria. Arch Biochem Biophys. 1981 Oct 1;211(1):100–107. doi: 10.1016/0003-9861(81)90434-3. [DOI] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Polyamines and plant stress: activation of putrescine biosynthesis by osmotic shock. Science. 1982 Sep 24;217(4566):1259–1261. doi: 10.1126/science.217.4566.1259. [DOI] [PubMed] [Google Scholar]
- Hampp R., Goller M., Füllgraf H. Determination of compartmented metabolite pools by a combination of rapid fractionation of oat mesophyll protoplasts and enzymic cycling. Plant Physiol. 1984 Aug;75(4):1017–1021. doi: 10.1104/pp.75.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp R., Mehrle W., Zimmermann U. Assay of photosynthetic oxygen evolution from single protoplasts. Plant Physiol. 1986 Jul;81(3):854–858. doi: 10.1104/pp.81.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly B. M. Light-Stimulated Changes in the Acidity of Suspensions of Oat Protoplasts: Dependence upon Photosynthesis. Plant Physiol. 1983 Jun;72(2):351–355. doi: 10.1104/pp.72.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly B. M. Role of o(2) and mitochondrial respiration in a photosynthetic stimulation of oat protoplast acidification of a surrounding medium. Plant Physiol. 1983 Jun;72(2):356–361. doi: 10.1104/pp.72.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger M., Day D. A., Douce R. Transport of NAD in Percoll-Purified Potato Tuber Mitochondria: Inhibition of NAD Influx and Efflux by N-4-Azido-2-nitrophenyl-4-aminobutyryl-3'-NAD. Plant Physiol. 1985 Jun;78(2):405–410. doi: 10.1104/pp.78.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B. Use of lipophilic cations to measure the membrane potential of oat leaf protoplasts. Plant Physiol. 1978 Dec;62(6):927–929. doi: 10.1104/pp.62.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooy C. D., Lin W. Amino Acid transport in protoplasts isolated from soybean leaves. Plant Physiol. 1986 May;81(1):8–11. doi: 10.1104/pp.81.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]


