Abstract
The use of combination therapy with a biologic agent and immunosuppressant has well-established efficacy and safety and is common practice in the management of inflammatory bowel disease (IBD). Current research has shifted focus toward the use of advanced combination treatment (ACT). This term was coined to describe combination therapy using 2 or more advanced treatments (biologic agents and/or oral small molecule drugs) with the aim of achieving optimal disease control in selected patients. An ACT approach may be particularly beneficial in patients with documented medically refractory IBD and in patients with a poor prognosis, extraintestinal manifestations, or concomitant immune-mediated inflammatory diseases. To date, the body of evidence for ACT strategies in IBD is largely comprised of uncontrolled retrospective case series and cohort studies in highly refractory patients. Recently, results from the VEGA trial have suggested that combination induction therapy with guselkumab and golimumab was more effective in ulcerative colitis than either agent alone. However, questions remain about issues such as related costs, ACT duration, and optimal combinations to adopt. Future randomized controlled trials are likely to evaluate rationally selected combinations of agents. This article summarizes the available literature on ACT, including comparisons with traditional combination therapy and the rheumatology field, and discusses practical recommendations, profiles of IBD patients who should be considered for combination approaches in clinical practice, and remaining knowledge gaps.
Keywords: Advanced combination treatment, dual biologic therapy, Crohn’s disease, ulcerative colitis, inflammatory bowel disease, dual targeted therapy
Multiple new drug classes have recently become available for the treatment of inflammatory bowel disease (IBD), and the drug development pipeline is likely to continue to provide new therapeutic options. However, despite the availability of novel classes of monoclonal antibodies and targeted oral small molecule drugs, a high proportion of patients have an inadequate response or subsequent loss of response to existing advanced therapies. A striking observation is that most advanced therapies report 1-year clinical remission rates of only 30% to 50%,1–4 even when administered under optimal circumstances in patients naive to conventional agents. These rates are substantially lower for patients who have previously failed 1 or more advanced therapies. Thus, a therapeutic ceiling may have been reached with the use of advanced therapies as monotherapies.
Multiple inflammatory pathways are involved in the development and progression of IBD. This is corroborated by the phenotypic heterogeneity in clinical presentations for both ulcerative colitis (UC) and Crohn’s disease (CD). The existence of several cytokine patterns may be a contributing factor explaining why treatment with a single agent is usually not sufficient for durable induction of remission.5 In clinical practice, therapeutic agents effective for luminal disease may not adequately control extraintestinal manifestations (EIMs), perianal fistulizing disease, or concomitant immune-mediated inflammatory diseases (IMIDs) requiring the use of additional agents.6,7
Advanced combination treatment (ACT) is an emerging therapeutic concept that specifies the combination of at least 2 biologic agents or a biologic agent and a small molecule drug with different mechanisms of action.8 The concept is based upon the notion that simultaneously targeting several pathogenic pathways may provide an additive or even synergistic benefit and could be a promising strategy in a wide range of patients who have failed to achieve disease control with monotherapy alone. In several other fields, such as the treatment of HIV, hepatitis C virus, hypercholesterolemia, and epilepsy, the combination of 2 or more drugs with different mechanisms of action has already been used to rationally produce synergistic effects.9–12 However, combination compounds should have similar pharmacokinetic characteristics, avoiding irrational polypharmacy that might lead to antagonistic effects or perhaps supra-additive side effects.
Preliminary evidence from clinical practice consisting of case series and uncontrolled observational studies has described the outcomes of ACT in IBD. Recently, the phase 2a VEGA trial demonstrated that combination induction therapy with guselkumab (Tremfya, Janssen) and golimumab (Simponi, Janssen) was more effective than monotherapy in patients with UC, with no increased safety concerns.13
This article summarizes the available literature on ACT, including comparisons with traditional combination therapy and the rheumatology field, and discusses practical recommendations, profiles of IBD patients who should be considered for combination approaches in clinical practice, and remaining knowledge gaps.
Traditional Combination Therapy in Inflammatory Bowel Disease
The landmark SONIC and UC-SUCCESS trials provided clear evidence for the use of traditional combination therapy consisting of a biologic agent plus azathioprine in patients with IBD. In both trials, combining the anti–tumor necrosis factor (TNF) agent infliximab with azathioprine was superior to monotherapy and associated with higher corticosteroid-free remission and mucosal healing rates in CD and UC.14,15 The reason for the greater efficacy of combination therapy observed in these trials has been the subject of considerable debate. Although it is most plausible that dual therapy was more effective because of the previously described effects on multiple inflammatory pathways, other explanations have been proposed. Specifically, a post hoc analysis indicated that higher infliximab concentrations and lower antidrug antibody rates observed in patients who received concomitant azathioprine may have contributed to the additive effect.16
In PANTS, a prospective cohort study of 1610 patients with active CD that was conducted in the United Kingdom, the addition of a thiopurine or methotrexate was found to have a protective effect on the development of immunogenicity with similar effect sizes for infliximab-treated patients (hazard ratio [HR], 0.39; 95% CI, 0.32-0.46; P<.0001) and adalimumab-treated patients (HR, 0.44; 95% CI, 0.31-0.64; P<.0001).17 Thiopurines were observed to reduce immunogenicity in participants treated with infliximab in a dose-dependent manner. Data from PANTS also suggested that higher remission rates observed at week 54 among patients receiving a concomitant immunosuppressive were independent of drug concentration or antidrug antibody development. This suggests that the addition of an immunosuppressive to anti-TNF therapy may enhance anti-inflammatory effects in distinction to improving pharmacokinetics alone.
The window of opportunity for preventing immunogenicity appears to be during the first months of traditional combination therapy. This observation raises the question of whether continued treatment for more than 6 to 12 months with a concomitant immunosuppressive is necessary. Moreover, observational studies have suggested the potential value of optimizing infliximab exposure by using therapeutic drug monitoring as an alternative strategy to concomitant immunosuppression for the prevention of immunogenicity.18,19 Additional evidence regarding the role of drug discontinuation comes from the SPARE trial.20 This open-label study enrolled patients with CD in sustained corticosteroid-free remission for at least 6 months who had received combination therapy with infliximab and either a thiopurine or methotrexate for at least 8 months. Patients were randomized into 3 arms: continuing combination therapy, discontinuing infliximab, or discontinuing immunosuppressive therapy. Withdrawal of immunosuppression did not appear to increase relapse rates (2-year relapse rate, 10%) in patients continuing infliximab monotherapy, whereas the risk of relapse was 36% at 2 years following withdrawal of infliximab.
Advantages of combining anti-TNF agents and immunosuppressive therapy should be weighed against potential safety risks of this approach, including the risk of serious and opportunistic infections. However, in PANTS, combination therapy was not associated with an increased risk of infection in the first year of treatment, even among patients older than 50 years.17 This result aligned with the SONIC and UC-SUCCESS trials, in which the rates of serious adverse events were not significantly different between the combination arm and the infliximab monotherapy arm.14,15 Furthermore, real-world data from long-term surveillance registries (eg, the TREAT registry) have not found an increased risk of infection in patients receiving traditional combination therapy compared with monotherapy.21 Instead, disease activity, corticosteroids, and opiates were identified as key risk factors, highlighting that potential safety concerns associated with immunosuppressive therapy may be offset by improved treatment efficacy and disease control.17 Accordingly, it is reasonable to speculate that any risks attributable to combined therapy may be offset by greater effects on disease activity and reduced exposure to corticosteroids. However, safety concerns associated with longterm immunosuppression (eg, risk of lymphoma) should always be considered. Following induction of remission with combination therapy, maintenance therapy with a biologic agent as monotherapy is recommended, especially in higher-risk populations such as patients older than 65 years.22–24
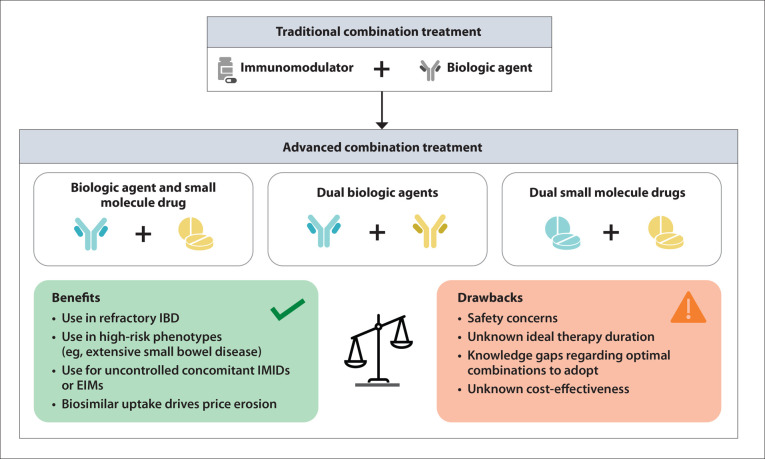
In summary, traditional combination therapy with a biologic agent and concomitant immunosuppressant therapy has well-studied efficacy and safety and is a common strategy for managing CD and UC. More recently, clinical and research interests have shifted toward the potential applications of ACT modalities with a combination of at least 2 biologic agents, or a biologic agent and a small molecule drug, with the aim of achieving optimal disease control (Figure 1).
Figure 1.
From traditional to advanced combination treatment: weighing benefits and drawbacks.
EIM, extraintestinal manifestation; IBD, inflammatory bowel disease; IMID, immune-mediated inflammatory disease.
Lessons From Rheumatology: From Bench to Bedside
The most robust experimental evidence for the use of ACT in IMIDs has emerged from studies in rheumatoid arthritis (RA), with the first preclinical investigations published more than 20 years ago. Combination therapy with an interleukin-1 (IL-1) receptor antagonist (Ra) and PEGylated soluble tumor necrosis factor receptor type I (PEG sTNFRI) was investigated in rat models of RA with established type II collagen–induced arthritis (CIA).25 Dual targeted therapy with IL-1Ra and PEG sTNFRI was associated with greater-than-additive efficacy relative to monotherapy with either agent, providing preliminary support for further clinical investigations in patients with RA. In a preclinical study of anti–IL-1 and anti-TNF therapy in a rat model of autoimmune arthritic disease, the synergistic benefits of dual therapy were particularly evident when suboptimal doses of each agent were given.26 Combination therapy was observed to have substantially superior efficacy over the use of a single class of anticytokine agent, even when combining relatively low doses of anti–IL-1 and anti-TNF agents that did not adequately control inflammation as monotherapy. This raises the possibility that reduced exposure to the individual agents might minimize adverse events.
This line of research led to initiatives to design novel bispecific antibodies that simultaneously target 2 different epitopes.27 These molecules may improve immunogenic response through modulation of different signaling pathways in the same cell or by engaging different cells expressing either antigen. In addition, dual-affinity retargeting (DART) antibodies can be used to simultaneously bind the target receptors in vitro and in intact cells. Veri and colleagues reported construction of DART antibodies that simultaneously bind CD32B and CD79B on the same B cell, resulting in downregulation of B-cell activation, proliferation, and immunoglobulin secretion.28 Treatment with a mouse-specific DART antibody reduced disease activity in a CIA murine model. Other bispecifics have been shown to ameliorate inflammation in murine models, providing further support for the concept of dual targeting for the treatment of RA.29–31
Despite promising preclinical results, safety concerns have been raised by attempts to treat patients with RA using a combination of at least 2 approved biologic disease-modifying antirheumatic drugs (bDMARDs), including infliximab, etanercept, certolizumab pegol (Cimzia, UCB), adalimumab, golimumab, anakinra (Kineret, Sobi), abatacept (Orencia, Bristol Myers Squibb), rituximab, and tocilizumab (Actemra, Genentech). A systematic review identified 5 randomized controlled trials (RCTs) and 1 observational cohort study that evaluated combination therapy in patients with RA using 2 of the following bDMARDs: anti-TNF agents, anakinra, abatacept, rituximab, and tocilizumab.32 On meta-analysis, patients receiving combination therapy had a higher rate of serious adverse events compared with the control group (14.9% vs 6.0%; odds ratio, 2.51; 95% CI, 1.29-4.89; I 2 = 0%), particularly during the first 12 months of treatment and in patients receiving a full dose of both agents. Pooled efficacy data from RCTs showed no clear evidence that receiving combination bDMARD therapy was advantageous. Conversely, the single observational study found that combination therapy with rituximab and etanercept was associated with clinical and biological benefits.33 In this study, patients had persistent, uncontrolled disease activity at enrollment despite previous treatment with up to 6 DMARDs and 3 anti-TNF therapies, denoting a very high-risk study population. After 2 months of rituximab and etanercept combination therapy, all clinical and serologic parameters improved significantly, and no serious infections requiring intravenous therapy or hospitalization were observed.
Of the RCTs included in the aforementioned meta-analysis, 2 evaluated the safety of abatacept, a cytotoxic T lymphocyte–associated antigen-4–immunoglobulin fusion protein, combined with background nonbiologic or biologic DMARDs in patients with active RA.34,35 Although some improvement in physical function and patient-reported disease outcomes was detected, the rate of adverse events and serious adverse events in the combination therapy group was higher than in the background monotherapy groups. On the other hand, 2 RCTs comparing rituximab monotherapy vs combination rituximab with either an anti-TNF agent or tocilizumab found that combination therapy was generally well tolerated; however, no additive efficacy was observed.36,37 Finally, a study by Genovese and colleagues of biologic-naive patients with active RA despite methotrexate therapy concluded that combination treatment with the anti–IL-1 agent anakinra and the anti-TNF agent etanercept was not justified owing to an increased risk of serious infection and neutropenia.38
In summary, the cumulative experience of ACT in rheumatology has been disappointing in that no clear efficacy signal has been observed and an increased risk of infection relative to monotherapy may have been identified for the combinations evaluated. Although these findings provide a cautionary message about safety outcomes, they do not preclude the possibility of future successful ACT regimens. Based upon the aforementioned RA experience, trial investigators recommend that candidate molecules for these regimens should have the following characteristics. First, strong efficacy as monotherapy should be demonstrated. This was not the case with respect to the anakinra studies. Second, component agents should be selected for an optimal safety profile; thus, broad-spectrum immunosuppressives are less attractive for evaluation. Finally, proof of concept should be established in animal models and human translational medicine studies before phase 2 studies are conducted.
Use of Advanced Combination Treatment in Inflammatory Bowel Disease
In patients with IBD, there are at least 3 distinct clinical circumstances in which ACT may be considered: (1) patients with IBD that is refractory to multiple medical therapies, including investigational agents; (2) patients with very high-risk phenotypes such as extensive small bowel disease, and stricturing or fistulizing disease behavior; and (3) patients with severe EIMs or an IMID other than IBD such as psoriasis or ankylosing spondylitis that is inadequately controlled by a single mechanism of action alone. In the first scenario, patients with poorly controlled bowel disease refractory to multiple agents might benefit from combination treatment with agents to which they have previously been exposed and experienced partial or no response, provided there is no contraindication to reintroducing a prior therapy (eg, intolerance or the presence of antidrug antibodies). Alternatively, an add-on approach may be considered when there has been inadequate response (based upon signs, symptoms, and/ or laboratory values) to a current biologic therapy, and a second agent that has not previously been used is added. In the second scenario, for patients with particular high-risk phenotypes, an ACT approach could offer more timely disease control and prevent complications or progression of bowel disease that could develop through inadequate suppression of inflammation with single agents. In the third scenario, the patient profile comprises patients with both intestinal and extraintestinal disease—or IBD and a concomitant IMID—where inhibiting a single mechanism of action has not provided adequate disease control across multiple organ systems. Here, selection of a second advanced therapy would be made with the goal of targeting potential pathways driving the uncontrolled concomitant disease.
Evidence From Case Series and Observational Studies in Inflammatory Bowel Disease
The body of evidence for the use of ACT in patients with IBD is growing. More than 20 case series and reports spanning a range of clinical scenarios have been published,39–61 and several retrospective and uncontrolled observations62–68 have investigated the outcomes of ACT approaches in patients with refractory intestinal disease, or IBD and a concomitant EIM or IMID (Table 1).
Table 1.
Studies on ACT in Patients With IBD
| Authors (year) | Study design | Population | Combination (# of patients) | Safety | Efficacy |
|---|---|---|---|---|---|
| Clark-Snustad et al71 (2020) | Retrospective cohort study | 18 CD patients | Tofacitinib + ustekinumab (10) Tofacitinib + vedolizumab (7) Tofacitinib + certolizumab pegol (1) | No AEs reported | Clinical, endoscopic, and biochemical improvement |
| Dolinger et al69 (2021) | Retrospective cohort study | 16 pediatric IBD patients (9 UC/IBDunspecified, 7 CD) |
Tofacitinib + vedolizumab (9) Vedolizumab + ustekinumab (4) Tofacitinib + ustekinumab (3) | Serious AEs reported in only 1 patient (septic arthritis, deep vein thrombosis) | Corticosteroid-free remission at 6 months |
| Eronen et al68 (2022) | Retrospective cohort study | 16 IBD patients (1 UC, 15 CD) |
Vedolizumab + anti-TNF agent (6) Vedolizumab + ustekinumab (5) Ustekinumab + anti-TNF agent (5) | No serious AEs reported 3 infection complications | Clinical benefit in half of patients |
| Glassner et al62 (2020) | Retrospective cohort study | 50 IBD patients (18 UC, 31 CD, 1 IBD-undetermined) 10 with concomitant IMID Median number of failed biologic agents=2 |
53 ACT regimens: Vedolizumab + ustekinumab (25) Tofacitinib + anti-TNF agent (9) Tofacitinib + vedolizumab (8) Vedolizumab + anti-TNF agent (7) Tofacitinib + ustekinumab (3) Anti-TNF agent + apremilast (1) |
Serious AEs in 12% | Clinical remission (50% vs 14%; P=.0018; Δ36%; 95% CI, 0.13–0.53) and endoscopic remission (34% vs 6%; P=.0039; Δ28%; 95% CI, 0.09–0.47) at follow-up compared with baseline |
| Goessens et al67 (2021) | Retrospective multicenter cohort study | 98 IBD patients (40 UC, 58 CD) 41 with concomitant IMID Median number of failed biologic agents=3 |
104 ACT regimens: Vedolizumab + anti-TNF agent (41) Anti–IL-4/13, -5, -6, -12/23, -17A, or -23 agent + vedolizumab (21) Tofacitinib + vedolizumab (13) Anti-TNF agent + anti–IL-4/13, -5, -6, -12/23, -17A, or -23 agent (11) Tofacitinib + anti-TNF agent (1) Others (17) |
AEs in 42%, mostly related to uncontrolled IBD (10 significant infections, 1 skin cancer) |
Improvement of IBD disease activity in 70% Improvement of IMID/ EIM activity in 81% |
| Goyal et al70 (2020) | Retrospective cohort study | 9 pediatric refractory CD patients (1 with concomitant sacroiliitis) | Vedolizumab + anti-TNF agent (8) Infliximab + anakinra (1) | 1 serious AE (staphylococcal skin infection) | Clinical remission (44.4%) |
| Guillo et al66 (2023) | Ambispective cohort study | 213 IMIDs (91 CD, 54 axial spondyloarthritis, 20 UC, 13 rheumatoid arthritis, 9 psoriatic arthritis, 8 psoriasis, 18 others) 73 with 1 IMID 70 with ≥2 IMIDs |
Vedolizumab + anti-TNF agent (73) Ustekinumab + anti-TNF agent (70) Vedolizumab + ustekinumab (12) |
27 infections reported 3 serious infections leading to discontinuation (Clostridioides difficile colitis, Pseudomonas aeruginosa lung infection, hemophagocytic syndrome related to zoonosis) |
Significant improvement in patient-reported outcomes (50%) Mild-to-moderate improvement (27%) |
| Kwapisz et al63 (2021) | Retrospective cohort study | 15 refractory IBD Patients (1 UC, 14 CD) Median number of failed biologic agents=3.8 |
Vedolizumab + anti-TNF agent (8) Vedolizumab + ustekinumab (5) Ustekinumab + anti-TNF agent (2) |
Infections requiring antibiotics in 27% 3 hospitalizations 3 surgeries 1 discontinuation |
Symptomatic improvement in 73% Reduction of corticosteroid use in 67% Endoscopic or radiographic improvement in 44% |
| Llano et al72 (2021) | Retrospective cohort study | 14 IBD patients (10 UC, 3 CD, 1 indeterminate colitis) |
Tofacitinib + vedolizumab (9) Vedolizumab + ustekinumab (3) Vedolizumab + anti-TNF agent (2) |
No serious AEs (4 infections reported) | Clinical improvement and biochemical response (>50%) |
| Lee et al73 (2022) | Retrospective cohort study | 19 refractory CD patients 18 with prior failure of ≥2 biologic agents |
Tofacitinib + ustekinumab (11) Tofacitinib + vedolizumab (7) Tofacitinib + certolizumab pegol (1) |
AEs in 36.8% of patients (minor infections or CD flares) No serious AEs |
Clinical response (80%) Clinical remission (60%) Endoscopic improvement (54.5%) |
| Privitera et al64 (2020) | Retrospective cohort study | 16 IBD patients (5 UC, 11 CD) 7 with uncontrolled IBD 9 with concomitant IMID |
Vedolizumab + anti-TNF agent (6) Ustekinumab + anti-TNF agent (4) Vedolizumab + ustekinumab (3) Vedolizumab + secukinumab (2) Vedolizumab + apremilast (1) |
AEs in 18.8% 1 discontinuation |
Clinical response in 100% |
| Yang et al65 (2020) | Retrospective cohort study | 22 refractory CD patients Median number of failed biologic agents=4 |
24 ACT regimens: Vedolizumab + anti-TNF agent (13) Vedolizumab + ustekinumab (8) Ustekinumab + adalimumab (2) Ustekinumab + infliximab (1) |
AEs in 13% | Endoscopic improvement in 43% Endoscopic remission in 26% Clinical response in 50% Clinical remission in 41% Significant posttreatment reduction in median SES-CD (from 14 to 6; P<.05) and PRO-2 (from 24.1 to 13.4; P<.05) |
ACT, advanced combination treatment; AE, adverse event; CD, Crohn’s disease; EIM, extraintestinal manifestation; IBD, inflammatory bowel disease; IL, interleukin; IMID, immune-mediated inflammatory disease; PRO-2, patient-reported outcome-2 score; SES-CD, Simplified Endoscopic Score–Crohn’s Disease; TNF, tumor necrosis factor; UC, ulcerative colitis.
Yang and colleagues provided data on the use of ACT in a high-risk group of patients with medically refractory CD.65 Of note, the majority of patients had undergone prior surgical resections, had stricturing or fistulizing disease, and failed a median of 4 biologic agents. The most common combinations included vedolizumab (Entyvio, Takeda) and ustekinumab (Stelara, Janssen). The majority of ACT regimens (79%) included at least 1 biologic agent that had previously induced initial response with secondary nonresponse (recycling strategy), and 29% of ACT trials utilized a compound that had not been previously administered. Almost 50% of patients treated with dual therapy had clinical and endoscopic improvements (41% achieved clinical remission, 43% endoscopic improvement). Compared with baseline, significant posttreatment reductions were reported for median Simplified Endoscopic Score (SES)-CD (from 14 to 6; P<.05) and patient-reported outcome–2 score (from 24.1 to 13.4; P<.05).
A retrospective cohort study examined data from 50 patients in the United States with medically refractory IBD (31 with CD, 18 with UC, 1 with IBD-undetermined) who received ACT from 2015 to 2019.62 Ten patients were affected by a concomitant IMID. Vedolizumab and ustekinumab were most frequently combined (47.2%). Twenty patients received a combination of the small molecule Janus kinase (JAK) inhibitor therapy tofacitinib (Xeljanz, Pfizer), with either an anti-TNF agent (16.9%), vedolizumab (15.1%), or ustekinumab (5.7%). The combination of vedolizumab and an anti-TNF agent was administered in 13.3%. At follow-up, increased rates of clinical remission (50% vs 14%; P=.0018; ∆36%; 95% CI, 0.13-0.53) and endoscopic remission (34% vs 6%; P=.0039; ∆28%; 95% CI, 0.09-0.47) were found when compared with baseline. With respect to safety outcomes, 23 adverse events were reported. Eight of these were serious infections that, according to the authors, may have been observed in the course of CD and were not necessarily related to combination treatment (eg, abdominal wall abscesses, peristomal cellulitis, peripherally inserted central catheter line infections).
More recently, the results of a large ambispective French cohort study of 143 patients with IMIDs treated with ACT were published.66 This study provided examples of ACT use in cases of either highly refractory disease after the failure of multiple treatment lines or concomitant uncontrolled IMIDs. Patients with CD comprised the majority of the study population (63.6%), followed by patients with axial spondyloarthritis (37.7%), UC (14%), RA (9.1%), psoriatic arthritis (6.3%), and psoriasis (5.6%). Nearly half of the patients had 2 IMIDs. The 3 most frequent combinations were an anti-TNF agent and vedolizumab (30%), an anti-TNF agent and ustekinumab (28.7%), and vedolizumab and ustekinumab (8%). Corroborating previous results, this study found that ACT appeared to be effective in achieving significant (50%) and mild-to-moderate (27%) improvement in patientreported outcomes at the end of follow-up. The authors also noted that ACT in patients with 2 diseases resulted in a numerically higher rate of significant improvement compared with patients with a single disease. Overall, 27 infections occurred during the study. Seven of these occurred in patients receiving an immunosuppressant, either azathioprine or methotrexate, in addition to ACT. Most infections were considered to be mild to moderate; however, there were 9 cases of serious infections, of which 3 were associated with methotrexate use.
Few studies have looked specifically at the effect of combination therapy in pediatric patients.51,69,70 Although the available evidence is scarce, combination therapy (including 2 biologic agents or 1 biologic agent and tofacitinib) appears to be a promising strategy in younger populations, who have limited therapeutic options, and few serious adverse events have been reported.
Regarding the use of oral small molecule drugs, emerging evidence suggests that the combination of tofacitinib with a biologic agent, mainly vedolizumab or ustekinumab, induces clinical response and endoscopic improvements without triggering any new safety signals in patients with refractory active disease.71–73
A large amount of observational data has accumulated supporting a number of ACT regimens; however, these studies have been initiated by investigators and have lacked adequate controls and randomized designs.
Clinical Trials in Inflammatory Bowel Disease
The first RCT evaluating dual biologic drugs in IBD was conducted by Sands and colleagues in 2007 in which 79 patients with active CD (Crohn’s Disease Activity Index score ≥150) receiving infliximab treatment were randomized to receive either an anti-integrin agent, natalizumab (Tysabri, Biogen), or placebo.74 It should be noted that the primary objective was to evaluate safety and tolerability because regulatory authorities were concerned about the potential toxicity of overlapping the agents in clinical practice; therefore, the study was not statistically powered to evaluate any potential efficacy differences among the treatment regimens. The authors found that the proportion of patients experiencing adverse events was similar between the combination and monotherapy groups (27% vs 30%, respectively). A higher proportion of patients in the combination group achieved clinical remission over the course of the study compared with the infliximab monotherapy arm (46% vs 41%, albeit P=not significant).
The VEGA study was a phase 2 induction trial that evaluated the combination of an anti–IL-23 agent (guselkumab) and an anti-TNF agent (golimumab) in 214 patients who had moderately to severely active UC bio-naive to anti-TNF agents and who had a history of inadequate response or failure to conventional therapy.13 Results supported both the efficacy and safety of ACT with guselkumab and golimumab in this population. A greater proportion of patients receiving combination therapy achieved the primary outcome of clinical response after 12 weeks (59/71; 83.1%) compared with monotherapy with either guselkumab (53/71; 74.6%) or golimumab (44/72; 61.1%). The comparison with golimumab monotherapy met the perceived criterion for significance. Clinical remission rates were higher for the combination group compared with either monotherapy. Furthermore, mucosal healing, a composite outcome defined as endoscopic improvement and histologic remission, was achieved in approximately twice as many patients treated with ACT (40.8%) compared with guselkumab (26.8%) or golimumab (15.3%) monotherapy. A favorable safety profile was reported, and only 1 patient developed a serious infection of influenza and sepsis of the 72 patients treated with ACT. Interestingly, a large change relative to baseline in genes associated with the T helper 17 axis, inflammation, and epithelial homeostasis was observed in the combination arm; the number of genes upregulated at week 12 was 633, 495, and 4776 for golimumab monotherapy, guselkumab monotherapy, and combination therapy, respectively, and the number of genes downregulated at week 12 was 709, 613, and 4867 in the 3 groups, respectively.75 Genes modulated by antiTNF and anti–IL-23 agents are suggestive of more robust suppression of inflammatory pathways, with decreased T helper 17 activity and increased epithelial modulation. Genes modulated by anti-TNF and anti–IL-23 agents are suggestive of more robust suppression of inflammatory pathways, with decreased T helper 17 activity and increased epithelial normalization.
Based upon these promising results, two phase 2 RCTs are currently investigating combination therapy with guselkumab and golimumab in moderately to severely active UC (DUET-UC; NCT05242484) and CD (DUET-CD; NCT05242471). In contrast to the VEGA trial, these are dose-ranging studies exploring 3 different doses of combination therapy (high, mid, and low) with either monotherapy or placebo for both induction and maintenance of remission.
The potential advantages of a vedolizumab-based triple combination therapy are being evaluated in the EXPLORER trial (NCT02764762) in patients with a diagnosis of CD established within 24 months of study entry. Participants were selected for increased risk of complications using a scoring system and had documented endoscopic disease activity (SES-CD score ≥7, or ≥4 if isolated ileal disease). In this open-label, phase 4 trial, all participants received vedolizumab infusions (at weeks 0, 2, 6, 14, and 22), adalimumab subcutaneous injections (every 2 weeks until week 26), and oral methotrexate (15 mg to week 34). After coinduction, all participants received vedolizumab monotherapy for a follow-up period of 102 weeks. An interim analysis showed that the primary outcome of endoscopic remission (SES-CD score 0-2) at 26 weeks was reached in 34.5% of patients and that more than 50% of patients were in clinical remission at this time point.76 Over the 26-week period, more than one-third of patients (36%) developed an infection; however, only 2 cases (perirectal abscess, gastroenteritis) were considered serious, and it was not apparent that any increase in infectious complications was evident beyond the expected incidence in this patient population.
Furthermore, the oral small molecule BI 706321 is currently being investigated as add-on treatment to biologic therapy in patients with CD receiving ustekinumab induction treatment (NCT04978493). Motivated by mechanistic hypotheses regarding potential additive or synergistic effects rather than opportunist combination based upon availability, agents targeting immune pathways (eg, vedolizumab) have been combined with strategies targeting the microbiome (eg, diet, fecal microbiota transplantation) in ongoing trials (NCT04231110, NCT03309865).
Practical Recommendations for Advanced Combination Treatment in Clinical Practice
There are specific clinical presentations in which ACT may be realistically considered after careful assessment of the patient’s needs and potential safety issues. Key recommendations for clinical practice are summarized in Table 2.
Table 2.
Key Recommendations for the Use of ACT in Clinical Practice
| Who | Patients with IBD refractory to multiple medical therapies Patients with very high-risk phenotypes Patients with a concomitant EIM/IMID |
| When | The risk of doing nothing (eg, uncontrolled disease) is higher than the risk of adding a combination molecule |
| Where | Centers with clinical expertise and multidisciplinary teams |
| Why | Differential and combination mechanisms of action with dual targeted treatments Lack of available options for inducing and maintaining remission and response |
| How |
|
| Preference for agents with the most favorable safety profiles (eg, vedolizumab, ustekinumab), especially in frail or elderly patients | |
| Preference for an anti-TNF agent in CD, especially in ileal CD or with bowel damage | |
| Preference for vedolizumab in UC patients | |
| Preference for an anti-TNF agent or ustekinumab (or anti–IL-23 blocker when approved) or a JAK inhibitor in patients with concomitant EIM or IMID | |
| Unknown areas | Most appropriate combinations to administer Treatment duration Cost-effectiveness of combination regimens |
ACT, advanced combination treatment; CD, Crohn’s disease; EIM, extraintestinal manifestation; IBD, inflammatory bowel disease; IL, interleukin; IMID, immune-mediated inflammatory disease; JAK, Janus kinase; TNF, tumor necrosis factor; UC, ulcerative colitis.
To date, the most common ACT regimens investigated in patients with IBD have been based upon an antiTNF agent and vedolizumab, followed by ustekinumab with vedolizumab.77 For combinations of an oral small molecule drug with a biologic agent, therapy with tofacitinib and either vedolizumab or ustekinumab has most frequently been evaluated.
Given that the evidence available regarding the efficacy and safety of these ACT regimens is derived mainly from uncontrolled observational studies,62–68 we highlight the recommendations below regarding cautious use in clinical practice.
This approach should be reserved for patients who have disease refractory to medical therapy, very high-risk phenotypes, or a concomitant EIM or IMID that cannot be controlled by a single agent. The practice of ACT is off-label and carries potential risks of serious infections and unknown longer-term complications. The risks of untreated disease should be weighed against the potential risks of ACT after careful discussion with the patient. Disease phenotype, comorbidities, prior drug failures, and drug pharmacodynamics are key considerations for the selection of appropriate combinations. Furthermore, targeting an alternative concomitant untreated inflammatory pathway should take into account agents with multiple cross-talk interactions that have previously caused immunogenicity. Choosing agents that are orthogonal to one another or further apart in the cross-talk maps may increase the chance of improved efficacy. Modulation of multiple pathways simultaneously through anti-TNF agents, JAK inhibitors, or anti–IL-12/23 agents should be favored. The VEGA study is a clear example of rationally combining an anti-TNF agent and anti–IL-23 agent based upon preclinical mechanistic data as well as in-silico modeling, showing that distinct and complementary modes of action can increase the amplitude of response overall.75
Finally, preference should be given to agents with the most favorable safety profiles, such as vedolizumab or ustekinumab.78,79 Data on the use of ACT in rheumatology have raised concerns about an increased risk of adverse or unknown effects that must be discussed with patients before shared decision-making. However, extrapolating these observations to IBD should be done with caution. Combination therapy in rheumatology literature included agents that are not approved or have been shown ineffective in IBD, including anakinra, abatacept, rituximab, and tocilizumab. Additionally, patients with IBD are typically younger with less comorbidity and are therefore less prone to developing serious infections.32
Combinations that include an anti-TNF agent and ustekinumab represent valid options in CD, whereas the use of vedolizumab should be considered in patients with UC. The presence of bowel damage in CD (eg, fistula, strictures) emphasizes the need to first evaluate anti-TNF agents. When choosing the second compound, both recycling strategies and simultaneous coinductions appear feasible.
Knowledge Gaps and Limitations
Despite increasing evidence on the use of ACT in the setting of IBD, key questions regarding this new approach remain unanswered and require further research to address (Table 2). For instance, the use of dual biologic therapy is off-label and experimental, and the most appropriate combinations for different indications within IBD have not yet been established.80 Additionally, whether ACT should be continued indefinitely or used as an induction strategy with monotherapy maintenance therapy as a potential next step needs to be defined. Until more data become available, limiting the use of combination therapy to a short period of time, rather than extended or indefinite use, may be appropriate given the risk of adverse events reported in prior experiences. A recent systematic review and meta-analysis included 30 studies with 279 patients with IBD for a median follow-up of 32 weeks (interquartile range, 24-52 weeks).77 The findings showed not only that ACT may be a viable therapeutic strategy in highly selected populations, but also that rates of adverse events, infections, and malignancy were similar to those reported on anti-TNF monotherapy (pooled rate of adverse events, 31.4%; 95% CI, 12.9%-53.7%).81 In line with current strategies for de-escalation of therapy in IBD, a switch from combination therapy to monotherapy with a targeted agent should be considered after remission has been achieved.
The costs associated with administering ACT regimens are an important potential limitation for their use. However, the uptake of biosimilars under switching initiatives in clinical practice provides a favorable outlook. The introduction of biosimilars has already resulted in substantial cost savings, and as more biosimilars and biobetters (modified versions of a specific approved biologic agent) enter the IBD economic landscape, it is possible that further price erosion of available therapeutic options will occur.82–84 Although the argument that dual biologic agents will be more costly than immunosuppressive combinations is valid, improved efficacy and possible savings associated with increased use of biosimilars may counterbalance these expenses for select difficult-to-treat, high-risk IBD patient populations, who are at high risk for other major cost drivers such as hospitalization and surgery. However, the potential for greater efficacy with ACT owing to better incremental cost-effectiveness ratios despite higher drug acquisition costs needs to be assessed by rigorous pharmacoeconomic analyses.
Conclusion
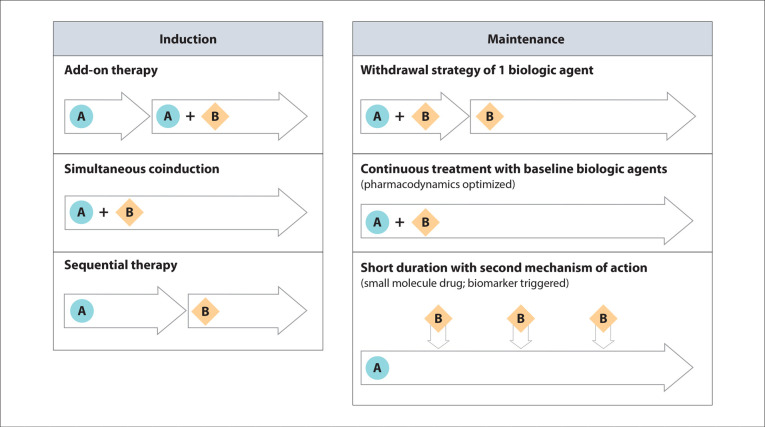
Given that a therapeutic ceiling may have been reached in IBD with limited remission rates through the use of single agents and that multiple pathways drive immune-mediated inflammatory processes, shifting focus toward the rational combination of well-established therapies is an important strategy for realizing optimal disease control. Combination with 2 targeted advanced therapies has been shown to induce a greater reduction in inflammation and improvement in epithelial homeostasis compared with monotherapy, suggesting differential and complementary mechanisms of action with a dual approach. Although less investigated in real-world settings, ACT with a small molecule drug and a biologic agent appears to be a promising option as well. Future well-controlled and adequately powered clinical trials exploring different strategies in both induction and maintenance phases are eagerly awaited to address remaining knowledge gaps (Figure 2). Potential designs for induction might include several approaches (eg, add-on therapy, simultaneous treatment, and sequential treatment). There is a growing interest in combination therapies leveraging ACT for early disease control during the induction phase, followed by monotherapy with likely a gut-selective and safe compound or with a short course of a recycled small molecule drug.
Figure 2.
Potential advanced combination treatment trial designs. A represents the first drug and B represents the second drug in the combination.
References
- Hanauer SB, Feagan BG, Lichtenstein GR et al. ACCENT I Study Group. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- Colombel JF, Sandborn WJ, Rutgeerts P et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132(1):52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- Rosario M, Dirks NL, Gastonguay MR et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther. 2015;42(2):188–202. doi: 10.1111/apt.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagan BG, Sandborn WJ, Gasink C et al. UNITI–IM-UNITI Study Group. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375(20):1946–1960. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- Schett G, McInnes IB, Neurath MF. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N Engl J Med. 2021;385(7):628–639. doi: 10.1056/NEJMra1909094. [DOI] [PubMed] [Google Scholar]
- Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal manifestations of inflammatory bowel disease: current concepts, treatment, and implications for disease management. Gastroenterology. 2021;161(4):1118–1132. doi: 10.1053/j.gastro.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burisch J, Jess T, Egeberg A. Incidence of immune-mediated inflammatory diseases among patients with inflammatory bowel diseases in Denmark. Clin Gastroenterol Hepatol. 2019;17(13):2704–2712.e3. doi: 10.1016/j.cgh.2019.03.040. [DOI] [PubMed] [Google Scholar]
- Danese S, Solitano V, Jairath V, Peyrin-Biroulet L. The future of drug development for inflammatory bowel disease: the need to ACT (advanced combination treatment). Gut. 2022;71(12):2380–2387. doi: 10.1136/gutjnl-2022-327025. [DOI] [PubMed] [Google Scholar]
- Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166(9):637–648. doi: 10.7326/M16-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray M, Logan R, Sterne JA et al. HIV-CAUSAL Collaboration. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24(1):123–137. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudzune KA, Monroe AK, Sharma R, Ranasinghe PD, Chelladurai Y, Robinson KA. Effectiveness of combination therapy with statin and another lipid-modifying agent compared with intensified statin monotherapy: a systematic review. Ann Intern Med. 2014;160(7):468–476. doi: 10.7326/M13-2526. [DOI] [PubMed] [Google Scholar]
- Margolis JM, Chu BC, Wang ZJ, Copher R, Cavazos JE. Effectiveness of antiepileptic drug combination therapy for partial-onset seizures based on mechanisms of action. JAMA Neurol. 2014;71(8):985–993. doi: 10.1001/jamaneurol.2014.808. [DOI] [PubMed] [Google Scholar]
- Feagan BG, Sands BE, Sandborn WJ et al. VEGA Study Group. Gusel kumab plus golimumab combination therapy versus guselkumab or golimumab monotherapy in patients with ulcerative colitis (VEGA): a randomised, double-blind, controlled, phase 2, proof-of-concept trial. Lancet Gastroenterol Hepatol. 2023;8(4):307–320. doi: 10.1016/S2468-1253(22)00427-7. [DOI] [PubMed] [Google Scholar]
- Colombel JF, Sandborn WJ, Reinisch W et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- Panaccione R, Ghosh S, Middleton S et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146(2):392–400.e3. doi: 10.1053/j.gastro.2013.10.052. [DOI] [PubMed] [Google Scholar]
- Colombel JF, Adedokun OJ, Gasink C et al. Combination therapy with infliximab and azathioprine improves infliximab pharmacokinetic features and efficacy: a post hoc analysis. Clin Gastroenterol Hepatol. 2019;17(8):1525–1532.e1. doi: 10.1016/j.cgh.2018.09.033. [DOI] [PubMed] [Google Scholar]
- Kennedy NA, Heap GA, Green HD et al. UK Inflammatory Bowel Disease Pharmacogenetics Study Group. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4(5):341–353. doi: 10.1016/S2468-1253(19)30012-3. [DOI] [PubMed] [Google Scholar]
- Reinisch W, Colombel JF, Sandborn WJ et al. Factors associated with shortand long-term outcomes of therapy for Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13(3):539–547.e2. doi: 10.1016/j.cgh.2014.09.031. [DOI] [PubMed] [Google Scholar]
- Van Assche G, Magdelaine-Beuzelin C, D’Haens G et al. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology. 2008;134(7):1861–1868. doi: 10.1053/j.gastro.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Louis E, Resche-Rigon M, Laharie D et al. GETAID and the SPARE-Biocycle research group. Withdrawal of infliximab or concomitant immunosuppressant therapy in patients with Crohn’s disease on combination therapy (SPARE): a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2023;8(3):215–227. doi: 10.1016/S2468-1253(22)00385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein GR, Feagan BG, Cohen RD et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4(5):621–630. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Torres J, Bonovas S, Doherty G et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14(1):4–22. doi: 10.1093/ecco-jcc/jjz180. [DOI] [PubMed] [Google Scholar]
- Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018;155(2):337–346.e10. doi: 10.1053/j.gastro.2018.04.012. [DOI] [PubMed] [Google Scholar]
- Lemaitre M, Kirchgesner J, Rudnichi A et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318(17):1679–1686. doi: 10.1001/jama.2017.16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendele AM, Chlipala ES, Scherrer J et al. Combination benefit of treatment with the cytokine inhibitors interleukin-1 receptor antagonist and PEGylated soluble tumor necrosis factor receptor type I in animal models of rheumatoid arthritis. Arthritis Rheum. 2000;43(12):2648–2659. doi: 10.1002/1529-0131(200012)43:12<2648::AID-ANR4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Feige U, Hu YL, Gasser J, Campagnuolo G, Munyakazi L, Bolon B. Anti-interleukin-1 and anti-tumor necrosis factor-αsynergistically inhibit adjuvant arthritis in Lewis rats. Cell Mol Life Sci. 2000;57(10):1457–1470. doi: 10.1007/PL00000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrijn AF, Janmaat ML, Reichert JM, Parren PWHI. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18(8):585–608. doi: 10.1038/s41573-019-0028-1. [DOI] [PubMed] [Google Scholar]
- Veri MC, Burke S, Huang L et al. Therapeutic control of B cell activation via recruitment of Fcgamma receptor IIb (CD32B) inhibitory function with a novel bispecific antibody scaffold. Arthritis Rheum. 2010;62(7):1933–1943. doi: 10.1002/art.27477. [DOI] [PubMed] [Google Scholar]
- Kanakaraj P, Puffer BA, Yao XT et al. Simultaneous targeting of TNF and Ang2 with a novel bispecific antibody enhances efficacy in an in vivo model of arthritis. MAbs. 2012;4(5):600–613. doi: 10.4161/mabs.21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Kan F, Ye X et al. A bispecific antibody against IL-1βand IL-17A is beneficial for experimental rheumatoid arthritis. Int Immunopharmacol. 2012;14(4):770–778. doi: 10.1016/j.intimp.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Liu M, Xie M, Jiang S et al. A novel bispecific antibody targeting tumor necrosis factor αand ED-B fibronectin effectively inhibits the progression of established collagen-induce arthritis. J Biotechnol. 2014;186:1–12. doi: 10.1016/j.jbiotec.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Boleto G, Kanagaratnam L, Dramé M, Salmon JH. Safety of combination therapy with two bDMARDs in patients with rheumatoid arthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2019;49(1):35–42. doi: 10.1016/j.semarthrit.2018.12.003. [DOI] [PubMed] [Google Scholar]
- Blank N, Max R, Schiller M, Briem S, Lorenz HM. Safety of combination therapy with rituximab and etanercept for patients with rheumatoid arthritis. Rheumatology (Oxford). 2009;48(4):440–441. doi: 10.1093/rheumatology/ken491. [DOI] [PubMed] [Google Scholar]
- Weinblatt M, Combe B, Covucci A, Aranda R, Becker JC, Keystone E. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease-modifying antirheumatic drugs: a one-year randomized, placebo-controlled study. Arthritis Rheum. 2006;54(9):2807–2816. doi: 10.1002/art.22070. [DOI] [PubMed] [Google Scholar]
- Weinblatt M, Schiff M, Goldman A et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann Rheum Dis. 2007;66(2):228–234. doi: 10.1136/ard.2006.055111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MW, Shergy WJ, Kaine JL, Sweetser MT, Gilder K, Linnik MD. Evaluation of the safety of rituximab in combination with a tumor necrosis factor inhibitor and methotrexate in patients with active rheumatoid arthritis: results from a randomized controlled trial. Arthritis Rheum. 2011;63(3):622–632. doi: 10.1002/art.30194. [DOI] [PubMed] [Google Scholar]
- https://clinicaltrials. gov/ct2/show/results/NCT00845832 ClinicalTrials.gov. A study of combination treatment with MabThera (rituximab) and RoActemra (tocilizumab) versus RoActemra in patients with rheumatoid arthritis with an incomplete response to methotrexate. Identifier: NCT00845832. Accessed January 14, 2023.
- Genovese MC, Cohen S, Moreland L et al. 20000223 Study Group. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50(5):1412–1419. doi: 10.1002/art.20221. [DOI] [PubMed] [Google Scholar]
- Bass J, Goyal A. P012 Successful use of combination biologic therapy in medically refractory pediatric Crohn’s disease and sacroiliitis. Am J Gastroenterol. 2019;114(1):S3–S4. [Google Scholar]
- Bethge J, Meffert S, Ellrichmann M, Conrad C, Nikolaus S, Schreiber S. Combination therapy with vedolizumab and etanercept in a patient with pouchitis and spondylarthritis. BMJ Open Gastroenterol. 2017;4(1):e000127. doi: 10.1136/bmjgast-2016-000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscaglia G, Piazzolla M, Cocomazzi F et al. Landmarks for dual biological therapy in inflammatory bowel disease: lesson from two case reports of vedolizumab in combination with ustekinumab. Eur J Gastroenterol Hepatol. 2020;32(12):1579–1582. doi: 10.1097/MEG.0000000000001919. [DOI] [PubMed] [Google Scholar]
- Buer LCT, Høivik ML, Warren DJ, Medhus AW, Moum BA. Combining anti-TNF-αand vedolizumab in the treatment of inflammatory bowel disease: a case series. Inflamm Bowel Dis. 2018;24(5):997–1004. doi: 10.1093/ibd/izx110. [DOI] [PubMed] [Google Scholar]
- Cline A, Pichardo RO. Successful treatment of hidradenitis suppurativa in the setting of Crohn disease with combination adalimumab and ustekinumab. Dermatol Online J. 2019;25(9):13030/qt0hw2w4nr. [PubMed] [Google Scholar]
- Elmoursi A, Barrett TA, Perry C. Double biologic therapy for refractory stricturing Crohn’s disease: a successful case of deep remission with ustekinumab and vedolizumab. Inflamm Bowel Dis. 2020;26(7):e62–e63. doi: 10.1093/ibd/izaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Rath T, Geppert CI et al. Long-term combination therapy with anti-TNF plus vedolizumab induces and maintains remission in therapy-refractory ulcerative colitis. Am J Gastroenterol. 2017;112(10):1621–1623. doi: 10.1038/ajg.2017.242. [DOI] [PubMed] [Google Scholar]
- Fumery M, Yzet C, Brazier F. Letter: combination of biologics in inflammatory bowel diseases. Aliment Pharmacol Ther. 2020;52(3):566–567. doi: 10.1111/apt.15891. [DOI] [PubMed] [Google Scholar]
- Hirten R, Longman RS, Bosworth BP, Steinlauf A, Scherl E. Vedolizumab and infliximab combination therapy in the treatment of Crohn’s disease. Am J Gastroenterol. 2015;110(12):1737–1738. doi: 10.1038/ajg.2015.355. [DOI] [PubMed] [Google Scholar]
- Huff-Hardy K, Bedair M, Vazquez R, Burstein E. Efficacy of combination vedolizumab and ustekinumab for refractory Crohn’s disease. Inflamm Bowel Dis. 2017;23(10):E49. doi: 10.1097/MIB.0000000000001232. [DOI] [PubMed] [Google Scholar]
- Liu EY, Loomes DE. Ustekinumab and vedolizumab dual biologic therapy in the treatment of Crohn’s disease. Case Rep Med. 2017;2017:5264216. doi: 10.1155/2017/5264216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao EJ, Lewin S, Terdiman JP, Beck K. Safety of dual biological therapy in Crohn’s disease: a case series of vedolizumab in combination with other biologics. BMJ Open Gastroenterol. 2018;5(1):e000243. doi: 10.1136/bmjgast-2018-000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbjørn C, Rove JB, Jahnsen J. Combination of biological agents in moderate to severe pediatric inflammatory bowel disease: a case series and review of the literature. Paediatr Drugs. 2020;22(4):409–416. doi: 10.1007/s40272-020-00396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaccione N, Novak K, Seow C et al. P717 The use of combination biologic therapy in inflammatory bowel disease: a single tertiary-centre experience. J Crohns Colitis. 2019;13(suppl 1):S480–S480. [Google Scholar]
- Afzali ACM. Combination of biologic agents in the management of severe refractory Crohn’s disease: a case report of concomitant treatment with vedolizumab and adalimumab. Am J Gastroenterol. 2016;111:S823–S824. [Google Scholar]
- Kim J, Hou SJ, Nandi N. Combination ustekinumab and vedolizumab for vulvar and Crohn’s colitis highlights the advantages and limitations of systemic and mucosal specific biologic therapy. Am J Gastroenterol. 2017. 112 suppl 1S1090-S1091. Abstract 1971 [Google Scholar]
- Kuehbacher T, Abu Hashem R, Langel N, Schreiber S, Drvarov O. P544 Combination therapy of vedolizumab and a TNF antagonist in IBD patients with severe chronic active, therapy refractory disease course. J Crohns Colitis. 2017;11(suppl 1):S357–S357. [Google Scholar]
- Le Berre C, Loeuille D, Peyrin-Biroulet L. Combination therapy with vedolizumab and tofacitinib in a patient with ulcerative colitis and spondyloarthropathy. Clin Gastroenterol Hepatol. 2019;17(4):794–796. doi: 10.1016/j.cgh.2018.08.017. [DOI] [PubMed] [Google Scholar]
- Lee JA, Magavi PR, Konijeti GG. Treatment of ulcerative colitis and seronegative inflammatory spondyloarthritis with vedolizumab and tofacitinib. Inflamm Bowel Dis. 2020;26(11):e146. doi: 10.1093/ibd/izaa158. [DOI] [PubMed] [Google Scholar]
- Richard N, Hazel EM, Haroon N, Inman RD. Simultaneous inhibition of α4/ β7 integrin and tumour necrosis factor-αin concomitant spondyloarthritis and inflammatory bowel disease. Ann Rheum Dis. 2018;77(12):e86. doi: 10.1136/annrheumdis-2017-212819. [DOI] [PubMed] [Google Scholar]
- Roblin X, Paul S, Ben-Horin S. Co-treatment with golimumab and vedolizumab to treat severe UC and associated spondyloarthropathy. J Crohns Colitis. 2018;12(3):379–380. doi: 10.1093/ecco-jcc/jjx142. [DOI] [PubMed] [Google Scholar]
- Yzet C, Dupas JL, Fumery M. Ustekinumab and anti-TNF combination therapy in patients with inflammatory bowel disease. Am J Gastroenterol. 2016;111(5):748–749. doi: 10.1038/ajg.2016.66. [DOI] [PubMed] [Google Scholar]
- Wang W, Cleveland NK, Ollech J, Rubin DT. Use of tofacitinib for the treatment of arthritis associated with ulcerative colitis. ACG Case Rep J. 2019;6(9):e00226. doi: 10.14309/crj.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassner K, Oglat A, Duran A et al. The use of combination biological or small molecule therapy in inflammatory bowel disease: a retrospective cohort study. J Dig Dis. 2020;21(5):264–271. doi: 10.1111/1751-2980.12867. [DOI] [PubMed] [Google Scholar]
- Kwapisz L, Raffals LE, Bruining DH et al. Combination biologic therapy in inflammatory bowel disease: experience from a tertiary care center. Clin Gastroenterol Hepatol. 2021;19(3):616–617. doi: 10.1016/j.cgh.2020.02.017. [DOI] [PubMed] [Google Scholar]
- Privitera G, Onali S, Pugliese D et al. Dual targeted therapy: a possible option for the management of refractory inflammatory bowel disease. J Crohns Colitis. 2020;15(2):335–339. doi: 10.1093/ecco-jcc/jjaa149. [DOI] [PubMed] [Google Scholar]
- Yang E, Panaccione N, Whitmire N et al. Efficacy and safety of simultaneous treatment with two biologic medications in refractory Crohn’s disease. Aliment Pharmacol Ther. 2020;51(11):1031–1038. doi: 10.1111/apt.15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillo L, Flachaire B, Avouac J et al. COMBIO Study Group. Efficacy and safety of combination targeted therapies in immune-mediated inflammatory disease: the COMBIO study. Dig Liver Dis. 2023;55(1):61–68. doi: 10.1016/j.dld.2022.07.012. [DOI] [PubMed] [Google Scholar]
- Goessens L, Colombel JF, Outtier A et al. European COMBIO study group. Safety and efficacy of combining biologics or small molecules for inflammatory bowel disease or immune-mediated inflammatory diseases: a European retrospective observational study. United European Gastroenterol J. 2021;9(10):1136–1147. doi: 10.1002/ueg2.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eronen H, Kolehmainen S, Koffert J et al. Combining biological therapies in patients with inflammatory bowel disease: a Finnish multi-centre study. Scand J Gastroenterol. 2022;57(8):936–941. doi: 10.1080/00365521.2022.2045350. [DOI] [PubMed] [Google Scholar]
- Dolinger MT, Spencer EA, Lai J, Dunkin D, Dubinsky MC. Dual biologic and small molecule therapy for the treatment of refractory pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2021;27(8):1210–1214. doi: 10.1093/ibd/izaa277. [DOI] [PubMed] [Google Scholar]
- Goyal A, Bass J. P090 Safety and efficacy of combining biologicals in children with inflammatory bowel disease. Gastroenterology. 2020;158(3):S122–S123. [Google Scholar]
- Clark-Snustad KD, Singla A, Harper J, Barahimi M, Lee SD. Tu1896 Tofacitinib is safe and effective as monotherapy or in combination with biologic therapy in patients with Crohn’s disease. Gastroenterology. 2020. 158 6S-1208 [Google Scholar]
- Llano EM, Shrestha S, Burstein E, Boktor M, Fudman DI. Favorable outcomes combining vedolizumab with other biologics or tofacitinib for treatment of inflammatory bowel disease. Crohns Colitis 360. 2021;3(3):otab030. doi: 10.1093/crocol/otab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SD, Singla A, Harper J et al. Safety and efficacy of tofacitinib in combination with biologic therapy for refractory Crohn’s disease. Inflamm Bowel Dis. 2022;28(2):309–313. doi: 10.1093/ibd/izab176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands BE, Kozarek R, Spainhour J et al. Safety and tolerability of concurrent natalizumab treatment for patients with Crohn’s disease not in remission while receiving infliximab. Inflamm Bowel Dis. 2007;13(1):2–11. doi: 10.1002/ibd.20014. [DOI] [PubMed] [Google Scholar]
- Desai P, Branigan P, Richards D, Vetter M, Cua D, Freeman T. Differential and combinatorial mechanism of action of golimumab and guselkumab in ulcerative colitis induction therapy: Il-23 blockade drives restoration of normal epithelium and mucosal healing. United European Gastroenterol J. 2022;10(supplement 8) [Google Scholar]
- Colombel JF, Ungaro RC, Sands BE. et al. 885: Triple combination therapy with vedolizumab, adalimumab, and methotrexate in patients with high-risk Crohn’s disease: interim analysis from the open-label, phase 4 Explorer trial. Gastroenterology. 2022. 162 7S-215 [Google Scholar]
- Ahmed W, Galati J, Kumar A et al. Dual biologic or small molecule therapy for treatment of inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20(3):e361–e379. doi: 10.1016/j.cgh.2021.03.034. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Dignass A, Peyrin-Biroulet L et al. Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol. 2018;53(9):1048–1064. doi: 10.1007/s00535-018-1480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn WJ, Rebuck R, Wang Y et al. Five-year efficacy and safety of ustekinumab treatment in Crohn’s disease: the IM-UNITI trial. Clin Gastroenterol Hepatol. 2022;20(3):578–590.e4. doi: 10.1016/j.cgh.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solitano V, Facciorusso A, Jess T et al. Comparative risk of serious infections with biologic agents and oral small molecules in inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2023;21(4):907–921.e2. doi: 10.1016/j.cgh.2022.07.032. [DOI] [PubMed] [Google Scholar]
- Lichtenstein GR, Feagan BG, Cohen RD et al. Infliximab for Crohn’s disease: more than 13 years of real-world experience. Inflamm Bowel Dis. 2018;24(3):490–501. doi: 10.1093/ibd/izx072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanters TA, Stevanovic J, Huys I, Vulto AG, Simoens S. Adoption of biosimilar infliximab for rheumatoid arthritis, ankylosing spondylitis, and inflammatory bowel diseases in the EU5: a budget impact analysis using a Delphi panel. Front Pharmacol. 2017;8:322. doi: 10.3389/fphar.2017.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TB, Bartels D, Sædder EA et al. The Danish model for the quick and safe implementation of infliximab and etanercept biosimilars. Eur J Clin Pharmacol. 2020;76(1):35–40. doi: 10.1007/s00228-019-02765-3. [DOI] [PubMed] [Google Scholar]
- D’Amico F, Solitano V, Aletaha D et al. Biobetters in patients with immune-mediated inflammatory disorders: an international Delphi consensus. Autoimmun Rev. 2021;20(7):102849. doi: 10.1016/j.autrev.2021.102849. [DOI] [PubMed] [Google Scholar]