Abstract
Primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) are cholestatic liver diseases that have significant clinical impact with debilitating symptoms and mortality. While PBC is predominantly seen in perimenopausal and postmenopausal women, men who are diagnosed with PBC have worse clinical outcomes and all-cause mortality. In contrast, 60% to 70% of patients with PSC are men; the data indicate that female sex may be an independent factor against PSC-related complications. These findings suggest a sex-dependent biological basis for these differences. Estrogen has been implicated in the pathogenesis of intrahepatic cholestasis of pregnancy and may induce cholestasis through a variety of interactions. However, it is unclear why some sexual dimorphic features may provide a protective effect despite known estrogen models that induce cholestasis. This article provides a brief introductory background and discusses the sexual dimorphism in clinical presentation in PSC and PBC. It also explores the role of estrogen signaling in pathogenesis and how it relates to intrahepatic cholestasis of pregnancy. Studies have already targeted certain molecules involved in estrogen signaling, and this review discusses these studies that identify estrogen-related receptor, estrogen receptor-α, estrogen receptor-β, farnesoid X receptor, and mast cells as possible targets, in addition to long noncoding RNA H19–induced cholestasis and sexual dimorphism. It also explores these interactions and their role in the pathogenesis of PBC and PSC.
Primary biliary cholangitis (PBC), previously termed primary biliary cirrhosis,1 and primary sclerosing cholangitis (PSC) are chronic cholestatic liver diseases that primarily target cholangiocytes and can progress to end-stage liver disease over the course of years to decades.2,3 Along with autoimmune hepatitis, PBC and PSC encompass the three major autoimmune pathologies that target the liver and biliary system. The etiopathogenesis of PBC and PSC is unknown, making effective therapeutic targets difficult to identify.2 The differences in sexual predominance in diagnosis and prognosis also add a layer of complexity to PBC and PSC treatment.2 This review article discusses the sexual dimorphism in PBC and PSC,4 how estrogen signaling plays a role in the pathophysiology of these diseases, and how it may be closely related to a similar cholestatic liver disorder, intrahepatic cholestasis of pregnancy (ICP), which could offer clues regarding estrogen-related pathogenesis. It also discusses multiple models that use estrogen-signaling molecules as possible therapeutic targets.
Epidemiology
PBC is a relatively rare disease, with a reported prevalence of 10 to 30 per 100,000 persons in the United States and an incidence of 2 to 3 per 100,000 persons over the last few years.5,6 PBC is believed to predominantly affect middle-aged women of European descent.2 However, recent studies show that there may be more racial and sex diversity than previously thought.7,8 European studies report a sex incidence ratio close to 4:1 female-to-male, rather than the previously reported 8 to 9:1 female-to-male ratio that is widely accepted. Male subjects with PBC likely have a higher overall mortality rate due to unclear etiology, potential underdiagnosis, and a more underlying pathophysiological process.5,7,8
PSC similarly has a sex predisposition, with male subjects being more commonly affected (up to 60%), and an average age of diagnosis close to 40 years.3,9,10 The incidence rate is close to 0 to 2 per 100,000 persons, and prevalence is increasing globally,11 which is likely due to more widespread advancements in both magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography for diagnosis over the last few decades.3,11,12
Pathophysiology
The pathophysiology of PBC and PSC is still incompletely understood. However, cholangiocytes are the key cells involved in both PBC and PSC pathogenesis, and thus it is important to have a proper understanding of their function. Cholangiocytes are epithelial cells that line the bile ducts and are involved in bile formation and regulation of bile flow through the biliary tree.13 These cells play a role in tissue injury and regeneration that helps restore biliary and liver functions during damage or disease.13,14 PBC is secondary to a combination of genetic and environmental risk factors that lead to T cell–mediated destruction of intrahepatic, small bile ducts.2,3,15 PBC-mediated damage to cholangiocyte function leads to decreased bile flow or chronic cholestasis, resulting in prolonged tissue damage and fibrosis that eventually result in cirrhosis after years of cell injury.2,12,13
PSC pathogenesis is also a combination of genetic, environmental, and dietary risk factors.15 Human leukocyte antigen (HLA) genes I and II, specifically HLA-B and HLA-DRB1,16 are particularly high risk at chromosome 6p21. A strong HLA association suggests that the adaptive immune system plays a significant role in pathogenesis. Certain studies theorize an autoimmune response, although the putative antigen in PSC is still unknown. Results of some genome-wide studies suggest that single nucleotide polymorphisms in the chromosome 6 region are linked to PSC. However, data are preliminary, and larger sample sizes are needed.17 Genes in the IL-2 pathway are also likely to contribute to PSC pathogenesis in certain patient populations.3,9
Interestingly, a gut microbiota hypothesis was suggested due to the high co-incidence of ulcerative colitis in patients with PSC. It is proposed that gut microbes may migrate through the portal system to the liver and biliary tract and cause an abnormal cholangiocyte response, including dysregulated proliferation and senescence. The T-cell gut hypothesis suggests that gut microbes stimulate an intestinal T-cell response, which results in T-cell migration to the liver and biliary system, and then eventual immune-mediated cholangiocyte destruction.3 Ongoing research is targeted at fully understanding the pathogenesis of both cholangiopathies.3,10
Clinical Features of PBC and PSC
Up to one-half of patients with PBC are asymptomatic at the time of diagnosis. The most common symptoms by far are fatigue, jaundice, and pruritus, likely secondary to chronic cholestasis. Cholestasis also increases the risk of infectious processes such as cholangitis and biliary obstruction in general. Therefore, patients often present with abdominal pain, which is typically right-sided.1 Up to 75% of patients with PBC are diagnosed with other autoimmune pathologies (eg, Hashimoto thyroiditis, Graves’ disease, Sjogren syndrome), leading to incidental diagnosis of PBC. It is hypothesized that co-diagnosis with other autoimmune disorders could be secondary to epithelial antigen cross-reactivity.15 As with PBC, most patients with PSC have no symptoms at the time of diagnosis but may have chronic elevation in some liver function laboratory test results. The patients who do have symptoms (up to 50%) most commonly present with hepatomegaly and splenomegaly in addition to fatigue and pruritus (closer to 10%).3,9,10 PBC can usually be diagnosed with elevated alkaline phosphatase levels in addition to a positive anti-mitochondrial antibody test result. Although anti-mitochondrial antibody–negative PBC can be seen, it is exceedingly rare, occurring in less than 5% of patients with PBC.3
Persistent alkaline phosphatase elevations, indicative of cholestasis, for longer than 6 months is considered diagnostic for PSC. Findings can be confirmed with either endoscopic retrograde cholangiopancreatography or magnetic resonance cholangiopancreatography that display the characteristic bile duct stricturing, and a biopsy is not needed for a presumed PSC diagnosis.3,4,9,18,19
Sexual Dimorphism in PBC and PSC
Due to the nature of cholangiopathy epidemiology data, there is interest in the clinical differences and outcomes stratified according to race, age, and—most importantly for the purpose of the current article—sex. In a study by Abdulkarim et al,20 only about 15% of male subjects reported more than one typical PBC symptom (eg, jaundice, pruritus, fatigue). Comparatively, 56% of female subjects reported more than one of those symptoms. However, studies also show that many men did not undergo a diagnostic PBC workup despite typical PBC-like symptoms and liver laboratory abnormalities, attributed mainly to the belief that PBC predominantly affects women. This, along with other factors, likely contributed to the delayed diagnosis of PBC in men (36 months after initial symptoms versus 12 months in women).21 The outcomes of these disparities lead to men having advanced liver disease and sequelae of cirrhosis, including spontaneous bacterial peritonitis, severe jaundice, and acute liver failure by the time of official diagnosis20,22,23 Lleo et al8, 24 reported that male subjects have higher all-cause mortality in Italian and Danish populations, and data from Sayiner et al8, 24 also endorse similar results for a US population. Far fewer data are available that analyze sex differences in PSC.11 Ulcerative colitis is commonly seen with PSC as a concomitant disease process, and Invernizzi et al6, 9, 25 found that it is less commonly seen in women with PSC versus male PSC patients (48% versus 61%). However, data sets from Brazil and Europe show no significant difference between clinical presentation symptoms in male and female subjects.6,9
Despite these findings, female sex is an independent factor against cirrhotic complications with advanced PSC (spontaneous bacterial peritonitis, hepatic encephalopathy, hepatocellular carcinoma), and female subjects also have higher significant transplant-free survival rates compared with men.9,25,26 There are many hypotheses as to why men and postmenopausal women appear to have poorer outcomes with cholangiopathies. One such explanation is that sex hormones, particularly estrogen, may play a key role in cholangiocyte regulation and proliferation, and estrogens likely have a complex dual role, as cholestasis, cholangiopathy, and liver disease still occurs in those who take supplemental estrogen.27
Estrogen Signaling
To understand how estrogen plays a role in cholangiocyte function, it is important to know the synthesis and regulation of estrogen signaling. The hypothalamus secretes gonadotropin-releasing hormone, which subsequently acts on the anterior pituitary to secrete follicle-stimulating hormone and luteinizing hormone. Follicle-stimulating hormone can then stimulate either granulosa or Sertoli cells to synthesize aromatase.27 In women, the granulosa cells are found in the ovaries, which is where aromatase can convert androgens or the intermediates such as dehydroepiandrosterone into estrogen.27,28 Dehydroepiandrosterone is derived from cholesterol and is a widely available steroid hormone found in many organs of the human body, including the liver. There are three main subtypes of estrogen: estrone, estradiol (E2), and estriol. E2 is the most biologically active subtype and main female sex hormone. Estrogen can either be bound to a sex hormone–binding globulin or exist as a free hormone. These forms of estrogen can bind to either estrogen receptor (ER)-α or ER-β in the cell cytoplasm, which can then cross into the nucleus. Once in the nucleus, ERs can modulate and induce transcription. This entire process is regulated from the hypothalamus and anterior pituitary via negative feedback on estrogen through the actions of follicle-stimulating hormone and luteinizing hormone.27,28
E2 is also regulated by the estrogen receptors ER-α and ER-β in addition to a G protein–coupled estrogen receptor called GPER. ER-α and ER-β are both considered to be ligand-activated transcription factors. They share a significant amount of homology, with notable differences being in the N-terminal domain.29 Other proteins can interact with the ligand-bound ER-α and ER-β to influence gene transcription, and the multiprotein complex binds to a specific sequence in the DNA known as the estrogen response element to induce transcription. Although these receptors are normally not expressed at high levels in cholangiocytes, they are elevated in PBC and PSC.30,31 Furthermore, polymorphisms in ER gene expression increase the risk of developing PBC.32 Therefore, models targeting molecules involved in estrogen signaling have been of particular interest over the last decade in hopes of finding a therapeutic target to combat the cholangiopathies.
Estrogen-Related Receptors
Estrogen-related receptors (ERRs) are a member of nuclear hormone super receptors that can bind ERR response elements or estrogen response elements in an estrogen-independent manner. This can lead to regulation of estrogen-induced effects or estrogen-independent effects.33,34 Harada et al35 conducted a study on human biliary epithelial cells (BECs), which when stimulated with estrogen did not result in significant proliferation via ER-α. ERR-α and ERR-γ were both elevated in the human BECs, suggesting that these molecules may play a role in estrogen signaling. ERRs are less numerous in the bile ducts of healthy younger female subjects compared with older postmenopausal women, and the signaling activity is increased in the ducts of older postmenopausal women. Interestingly, ERR-γ levels are also elevated in human bile ducts damaged by bile acid hyperaccumulation in the setting of cholesterol 7 α-hydroxylase (CYP7A1) overexpression.34 Understanding ERR signaling molecules can provide insights into targeted therapies for PBC and PSC.
In a different study, Hao et al33 found that sirtuin 6 (Sirt6), a NAD deacetylase molecule, may have a role in decreasing cholestasis and liver fibrosis through interactions with ERR-γ. Sirt6 deacetylation of ERR-γ leads to decreased Cyp7a1 transcription, which results in loss of pro-apoptotic molecule release. In bile duct–ligated mouse model studies, expression of both ERR-γ and Cypa7A1 is increased; however, overexpression of Sirt6 significantly reduces Cyp7A1 expression. Of the multiple ERRs studied, overexpression of ERR-γ alone was specific to the activation of Cyp7A1.
ER-α and ER-β
ER-α may play a significant role in cholestasis-induced liver injury. Along with ER-β, it is one of the main classical ERs. Both ER-α and ER-β are involved in various cellular functions, and the role of these receptors in cancer progression has recently been explored in multiple studies.36 ER-β plays a dual role in cancer: it can suppress colorectal cancer development,36,37 but is also oncogenic in prostate cancer.36,38
Thus far, ER-α has not been shown to have oncogenic suppression activity, which casts doubt on its seminal role in cholangiopathy pathogenesis. Cao et al30 hypothesized that ER-α was likely involved in the pro-inflammatory and mononuclear cell recruitment in PBC. As part of the study, liver biopsy results of eight postmenopausal PBC patients were compared with those of control subjects, and ER-α was found to be expressed at significantly higher levels in the PBC group. Human intrahepatic BEC lines treated with the ER-α agonist 4,4,4-(4-propyl-[1H] pyrazole-1,3,5-triyl)-tris-pheno had increased expression of pyruvate dehydrogenate complex E2, an autoantigen of PBC. The 4,4,4-(4-propyl-[1H] pyrazole-1,3,5-triyl)-tris-pheno treatment led to activation of pro-inflammatory cytokines IL-6, IL-8, and tumor necrosis factor–α in human intrahepatic BECs. The pathogenesis of these pro-inflammatory cytokines is believed to be due to increased leukocyte/neutrophil activation and recruitment (possibly through induction via enzymes c-Jun N-terminal kinase, p38, and STAT3 phosphorylation), which can result in apoptosis and injury in human intrahepatic BECs. While preliminary results showed that ER-α likely has a significant role in the pathogenesis of PBC, studies with larger sample sizes are needed before attempting targeted therapy.
Role of Estrogen and Progesterone in ICP
ICP is normally seen in the second or third trimester of pregnancy. Incidence of ICP in the United States is relatively rare, with rates ranging from 0.32% to 5.6% of the population depending on ethnicity.39 It is commonly associated with pruritus and bile acid elevation in mothers, in addition to major fetal complications such as stillbirths, preterm birth, neonatal respiratory distress, and more.40,41 Although the pathophysiology of ICP remains incompletely understood, it is likely through a combination of genetic, environmental, and hormonal (including estrogen and progesterone) interactions.42 Interestingly, women with chronic liver diseases prior to pregnancy such as hepatitis B, hepatitis C, or gallstone disease seem to be at increased risk of developing ICP.42, 43, 44
Due to the nature of this disorder and the patient population affected, the hormonal aspect and estrogen/progesterone interactions in the pathophysiology of ICP are of interest. Because of the timing of symptom onset (later in pregnancy), estrogen and progesterone are believed to be heavily involved in pathophysiology. Bile salt exporter (BSEP), which is involved in the secretion of bile acids, is inversely related to levels of E2; this possibly leads to estrogen-induced repression of BSEP, which can subsequently result in cholestasis.45 Similarly, progesterone metabolites may inhibit farnesoid X receptor (FXR)-mediated BSEP activation leading to cholestasis.46 For estrogen, a common cholestatic pathway in these pregnant patients is E2 activation of FXR/ER-α interactions, which leads to BSEP down-regulation.42 However, a study by Song et al45 found that although BSEP levels were reduced by E2 in wild-type mice, this was not the case in ERα−/− mice, suggesting that ER-α is also likely a key component in the pathogenesis of ICP. Chen et al47 found that ER-α has ligand-dependent and ligand-independent repressive activity on BSEP. From what is currently known of ER-α, and its involvement in signaling/pro-inflammatory cytokine recruitment in PBC, this is an interesting relationship. Estrogen, progesterone, their respective receptors, and FXR, all likely play a large role in ICP, and further understanding of these molecular interactions will help in shedding light on hormone-related pathogenesis in other cholangiopathies.
Estrogen-Induced Cholestasis and Discrepancies
Estrogen is a key player in bile acid synthesis and regulation and is often thought to be the precipitant of cholestasis. There are several studies suggesting possible mechanisms, and one study implies estrogen-induced dysregulation of bile acid transporters48 similar to ICP disease pathogenesis discussed in Role of Estrogen and Progesterone in ICP. Other studies have found that estrogen can lead to oxidative stress and resulting cholestasis through decreased levels of glutathione and steroid cyanide in the liver, resulting in lipid peroxidation and free radical production.49 Aberrations in hepatic cell membranes, particularly increased tight junction permeability caused by estrogen, may even lead to impaired bile acid secretion and cell injury.48,50
These pathologic mechanisms have all been linked to estrogen-inducing cholestasis. However, there seems to be a discrepancy with these models and the hypothetical “protective” effects of estrogen in the PBC and PSC disease states. One possible explanation may be the enzyme cytochrome P450 3A4, which is up-regulated by FXR as a defense against cholestasis but has been shown to be less effective with higher bile acid levels.51 Cytochrome P450 3A4 levels are higher in women compared with men and decrease significantly during menopause.52,53 Although the exact role that cytochrome P450 3A4 plays in PBC and PBC is unclear, it may be one of the factors responsible for dimorphism and discrepancy seen in these cholangiopathies.
FXR and Estrogen
Along with ursodeoxycholic acid, obeticholic acid is one of the few approved therapies for PBC.2 Obeticholic acid is a bile acid analogue that leads to FXR activation (a nuclear receptor that is activated by bile acids and can regulate bile acid synthesis2,54,55). However, its use has been limited due to hepatotoxic side effects recorded in patients with cholestatic liver disease.2 FXR has a key role in the pathogenesis of cholestasis, and thus the role of estrogen and related signaling molecules can be key in forming a therapeutic model. Liu et al55 hypothesized that FXR was likely a regulator of estrogen metabolism in the liver. In the study, elevated levels of estrogen were seen in patients with higher levels of bile acids, particularly in patients with cholestasis. Interestingly, in animal bile duct–ligated mouse models, estrogen levels were similarly elevated, and FXR deletion led to lower E2 levels, suggesting a correlation.
SULT1E1 (sulfotransferase family 1E member 1) is an enzyme that deactivates estrogen through sulfatase activity. SULT1E1 and steroid sulfatase interactions are being studied as therapeutic approaches to combat estrogen-associated tumors.56 Lower expression of SULT1E1 and higher levels of steroid sulfatase are seen in PBC.31 This enzyme reportedly has an inverse relationship with FXR activation in mice models.55 Overall, FXR-induced SULT1E1 inactivation results in higher levels of bile acids associated with E2. Communication between estrogen signaling and FXR likely leads to bile acid regulation; however, additional studies are needed to determine the exact role of estrogen.
Long Noncoding RNA H19 and Estrogen in Cholestasis
Noncoding RNAs are a type of RNA that do not transcribe proteins but rather have a regulatory function in a multitude of molecular processes.57 Long noncoding RNA H19 (H19) is normally expressed preterm in fetuses; however, it reportedly is expressed at higher levels in chronic liver diseases.58 Li et al59 found that in cholestatic multidrug resistance 2 knockout (Mdr2−/−) mice, H19 was induced at much higher levels in female, but not male, mice compared with their wild-type counterparts. Interestingly, estrogen interaction with H19 enhanced liver damage in female Mdr2−/− mice compared with their male counterparts, suggesting that estrogen/H19 leads to sexual dimorphic features of cholestatic liver injury. Estrogen activated extracellular signal-regulated kinase 1/2 via ER-α, which subsequently increased H19-dependent regulation of bile acid synthesis and subsequent damage in female Mdr2−/− mice.59 The same study also showed that knocking down H19 decreased cholestasis in the female Mdr2−/− mice. Beyond chronic liver disease models, H19 also had higher levels of activity in ER-positive MCF7 (a breast cancer cell line), and estrogen had a dose-dependent inductive effect on H19.60 Clearly, there is a relationship between estrogen and H19, and further investigation may reveal more information on the sexual dimorphism in cholangiopathies.
Mast Cells and Estrogen in Cholangiopathies
Mast cells are part of the innate immune system and are activated by IgE cross-linking via IgE receptors after antigen stimulation, usually allergens. However, E2 may also play a role in the physiological process.61,62 Mast cells in the uterus fluctuate during the estrous cycles of mice, suggesting a link between estrogen and mast cell activation.63 Once activated, mast cells can release multiple molecules, including histamine, which plays a key role in the body’s inflammatory process.61 Jing et al64 studied the effects of estrogen and the number of mast cells and histamine in rat mammary glands. Their results showed that the number of mast cells increased in a dose-dependent fashion when estrogen was introduced to a rat model. Oophorectomy resulted in fewer mast cells in rat mammary glands. Most importantly, ERs were found to be located surrounding the nucleus of mast cells in the animal models, suggesting that estrogen signaling played a role in mast cell activity.44
Mast cells play a significant role in cholestatic liver disease and are found in liver biopsy specimens of rodent models of PBC and PSC patients.65 A recent review highlights the significant findings regarding mast cells and liver disease.66 The relationship between mast cells and PSC is regulated by interactions between histidine decarboxylase, histamine receptor, and stem cell factor/transforming growth factor–β1. Wild-type mice treated with histamine had an increase in PSC phenotypes and histamine-induced hepatic damage. Histamine antagonists decreased proliferation of mast cells and PSC phenotypes in Mdr2−/− mice, which are a model of PSC.
Although the complete pathophysiological pathway is still not fully understood, histamine has been shown to enhance transforming growth factor–β1 (a known growth factor involved in PSC progression) signaling in mice, and thus there is likely crosstalk between these molecules that leads to pathogenesis. Liu et al67 studied the effect of histamine on ovarian cells via ER-α and ER-β and found that histamine (derived primarily from mast cells) up-regulated ER-α and ER-β expression, which could result in ovarian cancer. Knowing that both ER-α and ER-β are elevated in the ducts of patients with confirmed PBC or PSC and that mast cells infiltrate portal areas, there is likely a crosstalk between estrogen signaling and mast cells/histamine. Understanding these interactions will prove to be beneficial in future models that target estrogen signaling.
Conclusions
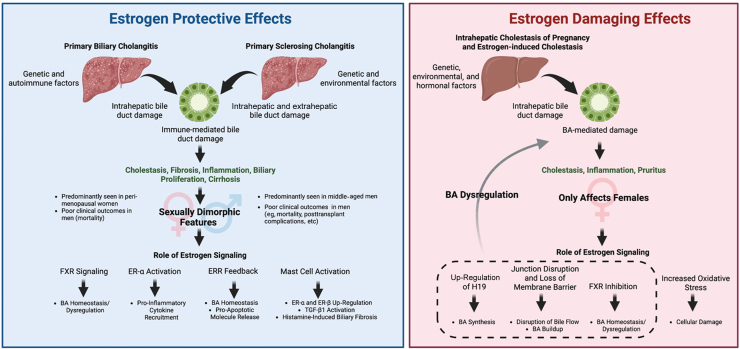
This review focused on sexual dimorphism in PBC and PSC epidemiology, clinical features, mortality, and how understanding the role of estrogen signaling (both in promoting and inhibiting damage) may help provide proper therapies to combat PBC, PSC, and ICP. Although we discussed studies that model the roles of ER-α, ER-β, FXR, ERRs, and mast cells, a sizable gap remains in understanding all these complex interactions. Epidemiologic data/mortality rates and the models discussed here indicate protective functions of estrogen in PBC and PSC. However, it is in contrast with the physiological patterns seen in ICP, estrogen-induced cholestasis, and H19 signaling in cholestasis (Figure 1). It is possible that in the pathogenesis of PBC and PSC, the complex interactions between estrogen, ER, and other signaling molecules/proteins may lead to these discrepancies. Estrogen/ER may have different pathophysiological effects in patients depending on environmental, autoimmune, and genetic factors, which may make some individuals susceptible to these cholangiopathies. Overall, there are many promising avenues to explore the role of estrogen in these complex pathologies, and additional insights gained can lead to human studies and eventually to therapies.
Figure 1.
Primary biliary cholangitis, primary sclerosing cholangitis, and intrahepatic cholestasis of pregnancy are believed to be driven by a combination of genetic, environmental, hormonal, and autoimmune risk factors that lead to immune-mediated biliary epithelial cell damage, resulting in increased cholestasis, biliary proliferation, fibrosis, inflammation, and eventually cirrhosis. Sex-dependent differences in clinical presentation/outcomes may be attributed to estrogen-signaling aberrations, particularly the estrogen-induced mast cell activation, estrogen receptor (ER)-α activation, farnesoid X receptor (FXR) modulation by estrogen, or estrogen-related receptor (ERR) regulation (left panel). Estrogen-damaging effects can also be predominantly found in female patients and may be regulated by long noncoding RNA H19 (H19), bile acid (BA) synthesis, junction disruption and loss of membrane barrier, and FXR inhibition. Furthermore, oxidative stress may increase induction of cellular damage (right panel). Figure made using BioRender.com (Toronto, ON, Canada).
Footnotes
Stress Responses and Cellular Crosstalk in the Pathogenesis of Liver Disease Theme Issue
Supported by Career Development Award-2 (1IK2BX005306) (L.K.) from the US Department of Veterans Affairs, Biomedical Laboratory Research and Development Service, and Strategic Research Initiative funds, Indiana University (H.F.).
Disclosures: None declared.
This article is part of a review series focused on the role of cellular stress in driving molecular crosstalk between hepatic cells that may contribute to the development, progression, or pathogenesis of liver diseases.
Contributor Information
Lindsey Kennedy, Email: linkenn@iu.edu.
Heather Francis, Email: heafranc@iu.edu.
Author Contributions
A.I. performed literature search, wrote and edited the manuscript, and prepared figures; and L.K. and H.F. edited the manuscript and acquired funding. All authors approved the final version of the manuscript.
References
- 1.Purohit T., Cappell M.S. Primary biliary cirrhosis: pathophysiology, clinical presentation and therapy. World J Hepatol. 2015;7:926–941. doi: 10.4254/wjh.v7.i7.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park J.-W., Kim J.-H., Kim S.-E., Jung J.H., Jang M.-K., Park S.-H., Lee M.-S., Kim H.-S., Suk K.T., Kim D.J. Primary biliary cholangitis and primary sclerosing cholangitis: current knowledge of pathogenesis and therapeutics. Biomedicines. 2022;10:1288. doi: 10.3390/biomedicines10061288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarcognato S., Sacchi D., Grillo F., Cazzagon N., Fabris L., Cadamuro M., Cataldo I., Covelli C., Mangia A., Guido M. Autoimmune biliary diseases: primary biliary cholangitis and primary sclerosing cholangitis. Pathologica. 2021;113:170–184. doi: 10.32074/1591-951X-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchioni Beery R.M., Vaziri H., Forouhar F. Primary biliary cirrhosis and primary sclerosing cholangitis: a review featuring a women’s health perspective. J Clin Transl Hepatol. 2014;2:266–284. doi: 10.14218/JCTH.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu M., Zhou Y., Haller I.V., Romanelli R.J., VanWormer J.J., Rodriguez C.V., Anderson H., Boscarino J.A., Schmidt M.A., Daida Y.G., Sahota A., Vincent J., Bowlus C.L., Lindor K., Zhang T., Trudeau S., Li J., Rupp L.B., Gordon S.C. Increasing prevalence of primary biliary cholangitis and reduced mortality with treatment. Clin Gastroenterol Hepatol. 2018;16:1342–1350. doi: 10.1016/j.cgh.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 6.Nardelli M.J., Bittencourt P.L., Cançado G.G.L., Faria L.C., Villela-Nogueira C.A., Rotman V., Silva de Abreu E., Maria Farage Osório F., Evangelista A.S., Sampaio Costa Mendes L., Ferraz de Campos Mazo D., Hyppolito E.B., de Souza Martins A., Codes L., Signorelli I.V., Perez Medina Gomide G., Agoglia L., Alexandra Pontes Ivantes C., Ferreira de Almeida E.B.V., Coral G.P., Eulira Fontes Rezende R., Lucia Gomes Ferraz M., Raquel Benedita Terrabuio D., Luiz Rachid Cançado E., Couto C.A. Clinical features and outcomes of primary sclerosing cholangitis in the highly admixed Brazilian population. Chin J Gastroenterol Hepatol. 2021;2021 doi: 10.1155/2021/7746401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adejumo A.C., Akhtar D.H., Dennis B.B., Cholankeril G., Alayo Q., Ogundipe O.A., Kim D., Ahmed A. Gender and racial differences in hospitalizations for primary biliary cholangitis in the USA. Dig Dis Sci. 2021;66:1461–1476. doi: 10.1007/s10620-020-06402-3. [DOI] [PubMed] [Google Scholar]
- 8.Lleo A., Jepsen P., Morenghi E., Carbone M., Moroni L., Battezzati P.M., Podda M., Mackay I.R., Gershwin M.E., Invernizzi P. Evolving trends in female to male incidence and male mortality of primary biliary cholangitis. Sci Rep. 2016;6 doi: 10.1038/srep25906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carbone M., Kodra Y., Rocchetti A., Manno V., Minelli G., Gerussi A., Ronca V., Malinverno F., Cristoferi L., Floreani A., Invernizzi P., Conti S., Taruscio D. Primary sclerosing cholangitis: burden of disease and mortality using data from the National Rare Diseases Registry in Italy. Int J Environ Res Publ Health. 2020;17:3095. doi: 10.3390/ijerph17093095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazaridis K.N., LaRusso N.F. Primary sclerosing cholangitis. N Engl J Med. 2016;375:1161–1170. doi: 10.1056/NEJMra1506330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta T.I., Weissman S., Fung B.M., Sotiriadis J., Lindor K.D., Tabibian J.H. Global incidence, prevalence and features of primary sclerosing cholangitis: a systematic review and meta-analysis. Liver Int. 2021;41:2418–2426. doi: 10.1111/liv.15007. [DOI] [PubMed] [Google Scholar]
- 12.Boonstra K., Beuers U., Ponsioen C.Y. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56:1181–1188. doi: 10.1016/j.jhep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Banales J.M., Huebert R.C., Karlsen T., Strazzabosco M., LaRusso N.F., Gores G.J. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol. 2019;16:269–281. doi: 10.1038/s41575-019-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalopoulos G.K. The regenerative altruism of hepatocytes and cholangiocytes. Cell Stem Cell. 2018;23:11–12. doi: 10.1016/j.stem.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Mago S., Wu G.Y. Primary sclerosing cholangitis and primary biliary cirrhosis overlap syndrome: a review. J Clin Transl Hepatol. 2020;8:336–346. doi: 10.14218/JCTH.2020.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Næss S., Lie B.A., Melum E., Olsson M., Hov J.R., Croucher P.J.P., Hampe J., Thorsby E., Bergquist A., Traherne J.A., Schrumpf E., Boberg K.M., Schreiber S., Franke A., Karlsen T.H. Refinement of the MHC risk map in a Scandinavian primary sclerosing cholangitis population. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsen T.H., Folseraas T., Thorburn D., Vesterhus M. Primary sclerosing cholangitis—a comprehensive review. J Hepatol. 2017;67:1298–1323. doi: 10.1016/j.jhep.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Rabiee A., Silveira M.G. Primary sclerosing cholangitis. Transl Gastroenterol Hepatol. 2021;6:29. doi: 10.21037/tgh-20-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trivedi P.J., Hirschfield G.M. Recent advances in clinical practice: epidemiology of autoimmune liver diseases. Gut. 2021;70:1989–2003. doi: 10.1136/gutjnl-2020-322362. [DOI] [PubMed] [Google Scholar]
- 20.Abdulkarim M., Zenouzi R., Sebode M., Schulz L., Quaas A., Lohse A.W., Schramm C., Weiler-Normann C. Sex differences in clinical presentation and prognosis in patients with primary biliary cholangitis. Scand J Gastroenterol. 2019;54:1391–1396. doi: 10.1080/00365521.2019.1683226. [DOI] [PubMed] [Google Scholar]
- 21.Podda M., Selmi C., Lleo A., Moroni L., Invernizzi P. The limitations and hidden gems of the epidemiology of primary biliary cirrhosis. J Autoimmun. 2013;46:81–87. doi: 10.1016/j.jaut.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Gatselis N.K., Zachou K., Lygoura V., Azariadis K., Arvaniti P., Spyrou E., Papadamou G., Koukoulis G.K., Dalekos G.N., Rigopoulou E.I. Geoepidemiology, clinical manifestations and outcome of primary biliary cholangitis in Greece. Eur J Intern Med. 2017;42:81–88. doi: 10.1016/j.ejim.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Marschall H.-U., Henriksson I., Lindberg S., Söderdahl F., Thuresson M., Wahlin S., Ludvigsson J.F. Incidence, prevalence, and outcome of primary biliary cholangitis in a nationwide Swedish population-based cohort. Sci Rep. 2019;9 doi: 10.1038/s41598-019-47890-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sayiner M., Golabi P., Stepanova M., Younossi I., Nader F., Racila A., Younossi Z.M. Primary biliary cholangitis in Medicare population: the impact on mortality and resource use. Hepatology. 2019;69:237–244. doi: 10.1002/hep.30174. [DOI] [PubMed] [Google Scholar]
- 25.Invernizzi F., Cilla M., Trapani S., Guarino M., Cossiga V., Gambato M., Morelli M.C., Morisco F., Burra P., Floreani A. Special Interest Group Gender in Hepatology of the Italian Association for the Study of the Liver (AISF): Gender and autoimmune liver diseases: relevant aspects in clinical practice. J Pers Med. 2022;12:925. doi: 10.3390/jpm12060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John B.V., Aitcheson G., Schwartz K.B., Khakoo N.S., Dahman B., Deng Y., Goldberg D., Martin P., Taddei T.H., Levy C., Kaplan D.E. Male sex is associated with higher rates of liver-related mortality in primary biliary cholangitis and cirrhosis. Hepatology. 2021;74:879–891. doi: 10.1002/hep.31776. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton K.J., Hewitt S.C., Arao Y., Korach K.S. Estrogen hormone biology. Curr Top Dev Biol. 2017;125:109–146. doi: 10.1016/bs.ctdb.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar A., Banerjee A., Singh D., Thakur G., Kasarpalkar N., Gavali S., Gadkar S., Madan T., Mahale S.D., Balasinor N.H., Sachdeva G. Estradiol: a steroid with multiple facets. Horm Metab Res. 2018;50:359–374. doi: 10.1055/s-0044-100920. [DOI] [PubMed] [Google Scholar]
- 29.Eyster K.M. The estrogen receptors: an overview from different perspectives. Methods Mol Biol. 2016;1366:1–10. doi: 10.1007/978-1-4939-3127-9_1. [DOI] [PubMed] [Google Scholar]
- 30.Cao H., Zhu B., Qu Y., Zhang W. Abnormal expression of ER[alpha] in cholangiocytes of patients with primary biliary cholangitis mediated intrahepatic bile duct inflammation. Front Immunol. 2019;10:2815. doi: 10.3389/fimmu.2019.02815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kilanczyk E., Ruminkiewicz D., Banales J.M., Milkiewicz P., Milkiewicz M. DHEA protects human cholangiocytes and hepatocytes against apoptosis and oxidative stress. Cells. 2022;11:1038. doi: 10.3390/cells11061038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L., Zhang H., Jiang Y.-F., Jin Q.-L., Zhang P., Li X., Gao P.-J., Niu J.-Q. Association of estrogen receptor gene polymorphisms and primary biliary cirrhosis in a Chinese population: a case-control study. Chin Med J (Engl) 2015;128:3008–3014. doi: 10.4103/0366-6999.168964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao L., Bang I.H., Wang J., Mao Y., Yang J.D., Na S.-Y., Seo J.K., Choi H.-S., Bae E.J., Park B.-H. ERR[gamma] suppression by Sirt6 alleviates cholestatic liver injury and fibrosis. JCI Insight. 2020;5 doi: 10.1172/jci.insight.137566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y., Kim D.-K., Lee J.-M., Park S.B., Jeong W.-I., Kim S.H., Lee I.-K., Lee C.-H., Chiang J.Y.L., Choi H.-S. Orphan nuclear receptor oestrogen-related receptor [gamma] (ERR[gamma]) plays a key role in hepatic cannabinoid receptor type 1-mediated induction of CYP7A1 gene expression. Biochem J. 2015;470:181–193. doi: 10.1042/BJ20141494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harada K., Yuko K., Sato Y., Ikeda H., Nakanuma Y. Significance of oestrogen-related receptor gamma on biliary epithelial cells in the pathogenesis of primary biliary cirrhosis. J Clin Pathol. 2014;67:566–572. doi: 10.1136/jclinpath-2013-201735. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y., Ma H., Yao J. ER[alpha], a key target for cancer therapy: a review. OncoTargets Ther. 2020;13:2183–2191. doi: 10.2147/OTT.S236532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anestis A., Sarantis P., Theocharis S., Zoi I., Tryfonopoulos D., Korogiannos A., Koumarianou A., Xingi E., Thomaidou D., Kontos M., Papavassiliou A.G., Karamouzis M.V. Estrogen receptor beta increases sensitivity to enzalutamide in androgen receptor-positive triple-negative breast cancer. J Cancer Res Clin Oncol. 2019;145:1221–1233. doi: 10.1007/s00432-019-02872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lombardi A.P.G., Pisolato R., Vicente C.M., Lazari M.F.M., Lucas T.F.G., Porto C.S. Estrogen receptor beta (ER[beta]) mediates expression of [beta]-catenin and proliferation in prostate cancer cell line PC-3. Mol Cell Endocrinol. 2016;430:12–24. doi: 10.1016/j.mce.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Smith D.D., Rood K.M. Intrahepatic cholestasis of pregnancy. Clin Obstet Gynecol. 2020;63:134–151. doi: 10.1097/GRF.0000000000000495. [DOI] [PubMed] [Google Scholar]
- 40.Jurk S.M., Kremer A.E., Schleussner E. Intrahepatic cholestasis of pregnancy. Geburtshilfe Frauenheilkd. 2021;81:940–947. doi: 10.1055/a-1522-5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer K.R., Xiaohua L., Mol B.W. Management of intrahepatic cholestasis in pregnancy. Lancet. 2019;393:853–854. doi: 10.1016/S0140-6736(18)32323-7. [DOI] [PubMed] [Google Scholar]
- 42.Xiao J., Li Z., Song Y., Sun Y., Shi H., Chen D., Zhang Y. Molecular pathogenesis of intrahepatic cholestasis of pregnancy. Can J Gastroenterol Hepatol. 2021;2021 doi: 10.1155/2021/6679322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marschall H.-U., Wikström Shemer E., Ludvigsson J.F., Stephansson O. Intrahepatic cholestasis of pregnancy and associated hepatobiliary disease: a population-based cohort study. Hepatology. 2013;58:1385–1391. doi: 10.1002/hep.26444. [DOI] [PubMed] [Google Scholar]
- 44.Wu K., Yin B., Li S., Zhu X., Zhu B. Prevalence, risk factors and adverse perinatal outcomes for Chinese women with intrahepatic cholestasis of pregnancy: a large cross-sectional retrospective study. Ann Med. 2022;54:2966–2974. doi: 10.1080/07853890.2022.2136400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song X., Vasilenko A., Chen Y., Valanejad L., Verma R., Yan B., Deng R. Transcriptional dynamics of bile salt export pump during pregnancy: mechanisms and implications in intrahepatic cholestasis of pregnancy. Hepatology. 2014;60:1993–2007. doi: 10.1002/hep.27171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abu-Hayyeh S., Papacleovoulou G., Lövgren-Sandblom A., Tahir M., Oduwole O., Jamaludin N.A., Ravat S., Nikolova V., Chambers J., Selden C., Rees M., Marschall H.-U., Parker M.G., Williamson C. Intrahepatic cholestasis of pregnancy levels of sulfated progesterone metabolites inhibit farnesoid X receptor resulting in a cholestatic phenotype. Hepatology. 2013;57:716–726. doi: 10.1002/hep.26055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y., Vasilenko A., Song X., Valanejad L., Verma R., You S., Yan B., Shiffka S., Hargreaves L., Nadolny C., Deng R. Estrogen and estrogen receptor-[alpha]-mediated transrepression of bile salt export pump. Mol Endocrinol. 2015;29:613–626. doi: 10.1210/me.2015-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zu Y., Yang J., Zhang C., Liu D. The pathological mechanisms of estrogen-induced cholestasis: current perspectives. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.761255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu L., Liu X., Li X., Yuan Z., Yang H., Zhang L., Jiang Z. Protective effects of SRT1720 via the HNF1[alpha]/FXR signalling pathway and anti-inflammatory mechanisms in mice with estrogen-induced cholestatic liver injury. Toxicol Lett. 2016;264:1–11. doi: 10.1016/j.toxlet.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 50.Mottino A.D., Hoffman T., Crocenzi F.A., Sánchez Pozzi E.J., Roma M.G., Vore M. Disruption of function and localization of tight junctional structures and Mrp2 in sustained estradiol-17[beta]-D-glucuronide-induced cholestasis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G391–402. doi: 10.1152/ajpgi.00496.2006. [DOI] [PubMed] [Google Scholar]
- 51.Chen J., Zhao K.-N., Chen C. The role of CYP3A4 in the biotransformation of bile acids and therapeutic implication for cholestasis. Ann Transl Med. 2014;2:7. doi: 10.3978/j.issn.2305-5839.2013.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phelps T., Snyder E., Rodriguez E., Child H., Harvey P. The influence of biological sex and sex hormones on bile acid synthesis and cholesterol homeostasis. Biol Sex Differ. 2019;10:52. doi: 10.1186/s13293-019-0265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoon S., Jeong S., Jung E., Kim K.S., Jeon I., Lee Y., Cho J.-Y., Oh W.-Y., Chung J.-Y. Effect of CYP3A4 metabolism on sex differences in the pharmacokinetics and pharmacodynamics of zolpidem. Sci Rep. 2021;11 doi: 10.1038/s41598-021-98689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheung A.C., Lammers W.J., Murillo Perez C.F., van Buuren H.R., Gulamhusein A., Trivedi P.J., Lazaridis K.N., Ponsioen C.Y., Floreani A., Hirschfield G.M., Corpechot C., Mayo M.J., Invernizzi P., Battezzati P.M., Parés A., Nevens F., Thorburn D., Mason A.L., Carbone M., Kowdley K.V., Bruns T., Dalekos G.N., Gatselis N.K., Verhelst X., Lindor K.D., Lleo A., Poupon R., Janssen H.L.A., Hansen B.E., Global PBC Study Group. Effects of age and sex of response to ursodeoxycholic acid and transplant-free survival in patients with primary biliary cholangitis. Clin Gastroenterol Hepatol. 2019;17:2076–2084.e2. doi: 10.1016/j.cgh.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 55.Liu X., Xue R., Yang C., Gu J., Chen S., Zhang S. Cholestasis-induced bile acid elevates estrogen level via farnesoid X receptor-mediated suppression of the estrogen sulfotransferase SULT1E1. J Biol Chem. 2018;293:12759–12769. doi: 10.1074/jbc.RA118.001789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Secky L., Svoboda M., Klameth L., Bajna E., Hamilton G., Zeillinger R., Jäger W., Thalhammer T. The sulfatase pathway for estrogen formation: targets for the treatment and diagnosis of hormone-associated tumors. J Drug Deliv. 2013;2013 doi: 10.1155/2013/957605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Z., Zhang T., Han S., Kusumanchi P., Huda N., Jiang Y., Liangpunsakul S. Long noncoding RNA H19—a new player in the pathogenesis of liver diseases. Transl Res. 2021;230:139–150. doi: 10.1016/j.trsl.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang Y., Huang Y., Cai S., Song Y., Boyer J.L., Zhang K., Gao L., Zhao J., Huang W., Liang G., Liangpunsakul S., Wang L. H19 is expressed in hybrid hepatocyte nuclear factor 4[alpha](+) periportal hepatocytes but not cytokeratin 19(+) cholangiocytes in cholestatic livers. Hepatol Commun. 2018;2:1356–1368. doi: 10.1002/hep4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X., Liu R., Yang J., Sun L., Zhang L., Jiang Z., Puri P., Gurley E.C., Lai G., Tang Y., Huang Z., Pandak W.M., Hylemon P.B., Zhou H. The role of long noncoding RNA H19 in gender disparity of cholestatic liver injury in multidrug resistance 2 gene knockout mice. Hepatology. 2017;66:869–884. doi: 10.1002/hep.29145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun H., Wang G., Peng Y., Zeng Y., Zhu Q.-N., Li T.-L., Cai J.-Q., Zhou H.-H., Zhu Y.-S. H19 lncRNA mediates 17[beta]-estradiol-induced cell proliferation in MCF-7 breast cancer cells. Oncol Rep. 2015;33:3045–3052. doi: 10.3892/or.2015.3899. [DOI] [PubMed] [Google Scholar]
- 61.Gibbs B.F., Levi-Schaffer F. H(4) receptors in mast cells and basophils: a new therapeutic target for allergy? Front Biosci (Landmark Ed) 2012;17:430–437. doi: 10.2741/3936. [DOI] [PubMed] [Google Scholar]
- 62.Zierau O., Zenclussen A.C., Jensen F. Role of female sex hormones, estradiol and progesterone, in mast cell behavior. Front Immunol. 2012;3:169. doi: 10.3389/fimmu.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woidacki K., Popovic M., Metz M., Schumacher A., Linzke N., Teles A., Poirier F., Fest S., Jensen F., Rabinovich G.A., Maurer M., Zenclussen A.C. Mast cells rescue implantation defects caused by c-kit deficiency. Cell Death Dis. 2013;4:e462. doi: 10.1038/cddis.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jing H., Wang Z., Chen Y. Effect of oestradiol on mast cell number and histamine level in the mammary glands of rat. Anat Histol Embryol. 2012;41:170–176. doi: 10.1111/j.1439-0264.2011.01120.x. [DOI] [PubMed] [Google Scholar]
- 65.Kundu D., Kennedy L., Meadows V., Baiocchi L., Alpini G., Francis H. The dynamic interplay between mast cells, aging/cellular senescence, and liver disease. Gene Expr. 2020;20:77–88. doi: 10.3727/105221620X15960509906371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pham L., Kennedy L., Baiocchi L., Meadows V., Ekser B., Kundu D., Zhou T., Sato K., Glaser S., Ceci L., Alpini G., Francis H. Mast cells in liver disease progression: an update on current studies and implications. Hepatology. 2022;75:213–218. doi: 10.1002/hep.32121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu M., Zhang Y., Xu Q., Liu G., Sun N., Che H., He T. Apigenin inhibits the histamine-induced proliferation of ovarian cancer cells by downregulating ER[alpha]/ER[beta] expression. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.682917. [DOI] [PMC free article] [PubMed] [Google Scholar]