Abstract
This study examines the association of the receipt of wild-type BNT162b2 vaccine with medically attended COVID-19 outcomes among children younger than 5 years in the US.
Wild-type COVID-19 mRNA vaccines were recommended for children aged 6 months through 4 years on June 18, 2022.1 However, COVID-19 vaccine uptake in this age group is low, with less than 5% completing a primary vaccine series as of May 24, 2023.2 Only 1 study has described the association between COVID-19 vaccination and disease outcomes among children younger than 5 years, but it did not include children younger than 3 years or evaluate medically attended outcomes.3 This study estimated the association between receipt of wild-type BNT162b2 vaccine (Pfizer) and medically attended COVID-19 outcomes among children younger than 5 years.
Methods
Similar to previous studies,4 a test-negative case-control design compared the risk of vaccination between COVID-19 cases and test-negative controls. We included patients aged 6 months through 4 years at Kaiser Permanente Southern California (KPSC) who were diagnosed with acute respiratory infection (ARI; eTable in Supplement 1) and tested for SARS-CoV-2 using polymerase chain reaction (PCR) testing in the emergency department (ED) or urgent care (UC) or in-person outpatient setting from July 23, 2022, through May 19, 2023. Cases were defined as those with a positive SARS-CoV-2 PCR test result and controls had negative test results and no evidence of a test result positive for SARS-CoV-2 in the past 90 days (Supplement 1). Vaccination status was determined based on receipt of vaccine 14 days or more before COVID-19 exposure. BNT162b2 is given as a 3-dose series (3 μg/dose) in this age group, with dose 2 given 3 to 8 weeks after dose 1 and dose 3 given at least 8 weeks after dose 2. Patients who did not follow the dosing schedule, received only 1 or more than 3 doses of BNT162b2, or received any other COVID-19 vaccine were excluded. Odds ratios (ORs) and 95% CIs were calculated from logistic regression models that included date of encounter, age, sex, race and ethnicity, pediatric risk score, and history of SARS-CoV-2 infection (separately for each encounter type to approximate outcome severity and separately by number of doses; Supplement 1). The 95% CIs were calculated using the Wald method. Analyses were performed using SAS, version 8.2 (SAS Institute). This study was approved by the KPSC institutional review board, which waived requirement for informed consent.
Results
Of 176 773 ED, UC, or outpatient ARI encounters in patients aged 6 months to 4 years, 24 261 met inclusion criteria (11 615 [48%] in the ED, 7074 [29%] in UC, and 5572 [23%] outpatient) during the study period. Overall, 2337 (10%) had positive test results for SARS-CoV-2 and 1457 (6%) were vaccinated. A total of 76 of 2337 cases (3.3%) and 1381 of 21 924 controls (6.3%) were vaccinated with 2 or 3 doses of BNT162b2 (Table). Among those who received only 2 doses, the median (range) number of days since dose 2 was 59 (14-277), since dose 3 was 74 (14-244), and between doses 2 and 3 was 61 (56-163).
Table. Study Population Characteristics (N = 24 261).
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| Wild-type BNT162b2 vaccination status | Case (n = 2337) | Control (n = 21 924) | Total (N = 24 261) | |||
| Unvaccinated (n = 22 804) | Received 2 doses (n = 826) | Received 3 doses (n = 631) | ||||
| Unvaccinated | 2261 (96.7) | 20 543 (93.7) | 22 804 (9.4) | |||
| Received 2 vaccine doses | 41 (1.8) | 785 (3.6) | 826 (3.4) | |||
| Received 3 vaccine doses | 35 (1.5) | 596 (2.7) | 631 (2.6) | |||
| Case | 2261 (9.9) | 41 (5.0) | 35 (5.5) | 2337 (9.6) | ||
| Control | 20 543 (90.1) | 785 (95.0) | 596 (94.5) | 21 924 (90.4) | ||
| Age at time of encounter, y | ||||||
| <1 | 4488 (19.7) | 82 (9.9) | 17 (2.7) | 907 (38.8) | 3680 (16.8) | 4587 (18.9) |
| 1 | 4835 (21.2) | 154 (18.6) | 123 (19.5) | 559 (23.9) | 4553 (20.8) | 5112 (21.1) |
| 2 | 4505 (19.8) | 140 (16.9) | 180 (28.5) | 314 (13.4) | 4511 (20.6) | 4825 (19.9) |
| 3 | 4504 (19.8) | 195 (23.6) | 149 (23.6) | 284 (12.2) | 4564 (20.8) | 4848 (20.0) |
| 4 | 4472 (19.6) | 255 (30.9) | 162 (25.7) | 273 (11.7) | 4616 (21.1) | 4889 (20.2) |
| Sex | ||||||
| Female | 10 466 (45.9) | 397 (48.1) | 298 (47.2) | 1103 (47.2) | 10 058 (45.9) | 11 161 (46.0) |
| Male | 12 338 (54.1) | 429 (51.9) | 333 (52.8) | 1234 (52.8) | 11 866 (54.1) | 13 100 (54.0) |
| Self-reported race and ethnicity | ||||||
| African American or Black | 2035 (8.9) | 36 (4.4) | 34 (5.4) | 169 (7.2) | 1936 (8.8) | 2105 (8.7) |
| Asian or Pacific Islander | 2073 (9.1) | 191 (23.1) | 150 (23.8) | 268 (11.5) | 2146 (9.8) | 2414 (10.0) |
| Hispanic or Latinx | 12 929 (56.7) | 368 (44.6) | 239 (37.9) | 1325 (56.7) | 12 211 (55.7) | 13 536 (55.8) |
| White | 4464 (19.6) | 183 (22.2) | 184 (29.2) | 442 (18.9) | 4389 (20) | 4831 (19.9) |
| Other or unknowna | 1303 (5.7) | 48 (5.8) | 24 (3.8) | 133 (5.7) | 1242 (5.7) | 1375 (5.7) |
| Encounter type | ||||||
| Emergency department | 11 040 (48.4) | 357 (43.2) | 218 (34.5) | 1272 (54.4) | 10 343 (47.2) | 11 615 (47.9) |
| Urgent care | 6624 (29) | 254 (30.8) | 196 (31.1) | 457 (19.6) | 6617 (30.2) | 7074 (29.2) |
| In-person outpatient | 5140 (22.5) | 215 (26.0) | 217 (34.4) | 608 (26.0) | 4964 (22.6) | 5572 (23.0) |
| Pediatric risk scoreb | ||||||
| 0 | 10 672 (46.8) | 378 (45.8) | 293 (46.4) | 1121 (48) | 10 222 (46.6) | 11 343 (46.8) |
| 1 | 5160 (22.6) | 197 (23.8) | 159 (25.2) | 395 (16.9) | 5121 (23.4) | 5516 (22.7) |
| 2 | 4016 (17.6) | 133 (16.1) | 95 (15.1) | 491 (21.0) | 3753 (17.1) | 4244 (17.5) |
| 3 | 1444 (6.3) | 49 (5.9) | 34 (5.4) | 170 (7.3) | 1357 (6.2) | 1527 (6.3) |
| ≥4 | 1512 (6.6) | 69 (8.4) | 50 (7.9) | 160 (6.8) | 1471 (6.7) | 1631 (6.7) |
| Previous positive PCR test result for SARS-CoV-2 | ||||||
| 0 | 18 664 (81.8) | 656 (79.4) | 513 (81.3) | 2156 (92.3) | 17 677 (80.6) | 19 833 (81.7) |
| 1 | 4140 (18.2) | 170 (20.6) | 118 (18.7) | 181 (7.7) | 4247 (19.4) | 4428 (18.3) |
| Received flu vaccine in year prior to encounter | ||||||
| 0 | 12 142 (53.2) | 180 (21.8) | 81 (12.8) | 1166 (49.9) | 11 237 (51.3) | 12 403 (51.1) |
| 1 | 10 662 (46.8) | 646 (78.2) | 550 (87.2) | 1171 (50.1) | 10 687 (48.7) | 11 858 (48.9) |
Abbreviations: BNT162b2, Pfizer-BioNTech COVID-19 vaccine bivalent; PCR, polymerase chain reaction.
Race and ethnicity were reported by the participant’s parent or guardian and recorded in the health record. “Other” includes individuals who self-identified as American Indian or “multiple” or “other” race and ethnicity categories. Race and ethnicity were included as a confounder due to associations with vaccination and COVID-19 health care–seeking behavior and outcomes.
A summary measure of disease burden in which a higher score indicates higher comorbidity.
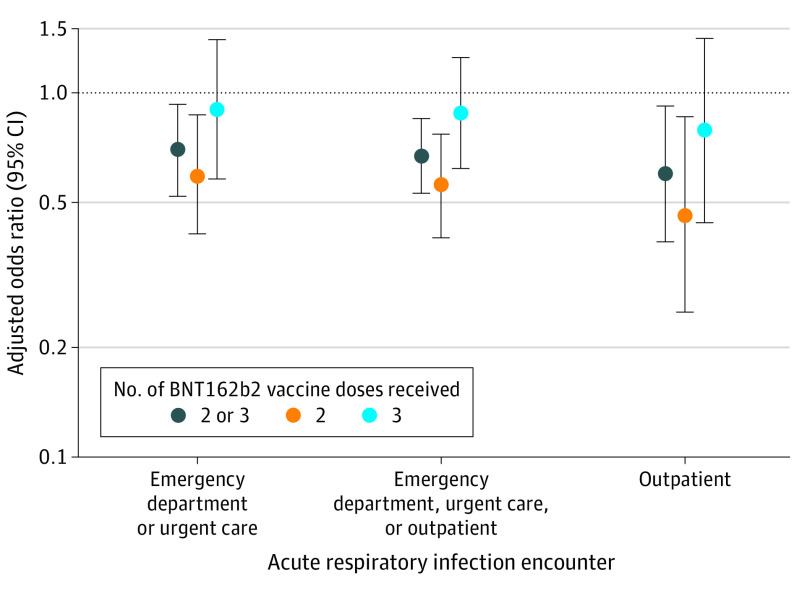
Compared with unvaccinated children, the adjusted OR for children who received 2 or 3 doses of BNT162b2 was 0.70 (95% CI, 0.52-0.93) for a COVID-19–related ED or UC encounter, 0.60 (95% CI, 0.39-0.92) for outpatient visits, and 0.67 (95% CI, 0.53-0.85) for either outcome (Figure). The risk of a positive test result for SARS-CoV-2 during ED or UC and outpatient encounters was 0.56 (95% CI, 0.40-0.77) for those who received 2 doses vs 0.88 (95% CI, 0.62-1.25) for those who received 3 doses.
Figure. Risk of Outcome by Number of Vaccine Doses Among Children Aged 6 Months to 5 Years.
Models adjusted for date of encounter, age, sex, self-reported race and ethnicity, pediatric risk score, and prior SARS-CoV-2 infection. The reference group (dotted line) is unvaccinated participants.
Discussion
Receiving at least 2 doses of wild-type BNT162b2 vaccine was associated with a reduced risk of COVID-19 ED or UC and outpatient visits in children younger than 5 years. The risk of SARS-CoV-2 encounters appeared lower for those with 2 vs 3 doses of BNT162b2, albeit with wide CIs, which is likely due to more immune-evasive Omicron sublineages (eg, BQ.1-related and XBB-related strains) becoming dominant by the time young children received their third dose5 and longer median time since dose 3 compared with dose 2. The OR of 0.67 is equivalent to vaccine effectiveness (1 minus the OR) of 33%, which is lower than the approximately 70% efficacy against symptomatic COVID-19 seen in a clinical trial when BA.2 sublineages were predominant.6 Study limitations include the potential for unmeasured confounding, potential misclassification of prior infection or whether ARI encounters were truly related to COVID-19, and the restriction to only 1 mRNA product. Updated vaccines will likely be needed to maintain protection against contemporary Omicron strains in young children.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Karen Lasser, MD, and Kristin Walter, MD, Senior Editors.
eMethods and eTable
Data sharing statement
References
- 1.Fleming-Dutra KE, Wallace M, Moulia DL, et al. Interim recommendations of the Advisory Committee on Immunization Practices for Use of Moderna and Pfizer-BioNTech COVID-19 Vaccines in Children Aged 6 Months-5 Years: United States, June 2022. MMWR Morb Mortal Wkly Rep. 2022;71(26):859-868. doi: 10.15585/mmwr.mm7126e2 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . COVID-19 vaccination coverage and vaccine confidence among children: COVIDVaxView. Accessed May 25, 2023. https://www.cdc.gov/vaccines/imz-managers/coverage/covidvaxview/interactive/children.html
- 3.Fleming-Dutra KE, Ciesla AA, Roper LE, et al. Preliminary estimates of effectiveness of monovalent mRNA vaccines in preventing symptomatic SARS-CoV-2 infection among children aged 3-5 years: increasing community access to testing program, United States, July 2022-February 2023. MMWR Morb Mortal Wkly Rep. 2023;72(7):177-182. doi: 10.15585/mmwr.mm7207a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tartof SY, Frankland TB, Slezak JM, et al. Effectiveness associated with BNT162b2 vaccine against emergency department and urgent care encounters for Delta and Omicron SARS-CoV-2 infection among adolescents aged 12 to 17 years. JAMA Netw Open. 2022;5(8):e2225162. doi: 10.1001/jamanetworkopen.2022.25162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . COVID data tracker: variant proportions. Accessed May 20, 2023. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
- 6.Muñoz FM, Sher LD, Sabharwal C, et al. ; C4591007 Clinical Trial Group . Evaluation of BNT162b2 Covid-19 vaccine in children younger than 5 years of age. N Engl J Med. 2023;388(7):621-634. doi: 10.1056/NEJMoa2211031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods and eTable
Data sharing statement