Key Points
Question
Does combined prehospital and in-hospital remote ischemic conditioning (RIC) improve functional outcome in patients with acute ischemic stroke or intracerebral hemorrhage?
Findings
In this randomized clinical trial that included 1500 patients with a suspected stroke in the prehospital setting, RIC treatment, compared with a sham control, did not significantly improve functional outcomes at 90 days in patients with a confirmed diagnosis of stroke (odds ratio for a favorable shift on the mRS score, 0.95).
Meaning
Among patients with acute stroke, RIC initiated in the prehospital setting and continued in the hospital did not significantly improve functional outcome at 90 days.
Abstract
Importance
Despite some promising preclinical and clinical data, it remains uncertain whether remote ischemic conditioning (RIC) with transient cycles of limb ischemia and reperfusion is an effective treatment for acute stroke.
Objective
To evaluate the effect of RIC when initiated in the prehospital setting and continued in the hospital on functional outcome in patients with acute stroke.
Design, Setting, and Participants
This was a randomized clinical trial conducted at 4 stroke centers in Denmark that included 1500 patients with prehospital stroke symptoms for less than 4 hours (enrolled March 16, 2018, to November 11, 2022; final follow-up, February 3, 2023).
Intervention
The intervention was delivered using an inflatable cuff on 1 upper extremity (RIC cuff pressure, ≤200 mm Hg [n = 749] and sham cuff pressure, 20 mm Hg [n = 751]). Each treatment application consisted of 5 cycles of 5 minutes of cuff inflation followed by 5 minutes of cuff deflation. Treatment was started in the ambulance and repeated at least once in the hospital and then twice daily for 7 days among a subset of participants.
Main Outcomes and Measures
The primary end point was improvement in functional outcome measured as a shift across the modified Rankin Scale (mRS) score (range, 0 [no symptoms] to 6 [death]) at 90 days in the target population with a final diagnosis of ischemic or hemorrhagic stroke.
Results
Among 1500 patients who were randomized (median age, 71 years; 591 women [41%]), 1433 (96%) completed the trial. Of these, 149 patients (10%) were diagnosed with transient ischemic attack and 382 (27%) with a stroke mimic. In the remaining 902 patients with a target diagnosis of stroke (737 [82%] with ischemic stroke and 165 [18%] with intracerebral hemorrhage), 436 underwent RIC and 466 sham treatment. The median mRS score at 90 days was 2 (IQR, 1-3) in the RIC group and 1 (IQR, 1-3) in the sham group. RIC treatment was not significantly associated with improved functional outcome at 90 days (odds ratio [OR], 0.95; 95% CI, 0.75 to 1.20, P = .67; absolute difference in median mRS score, −1; −1.7 to −0.25). In all randomized patients, there were no significant differences in the number of serious adverse events: 169 patients (23.7%) in the RIC group with 1 or more serious adverse events vs 175 patients (24.3%) in the sham group (OR, 0.97; 95% CI, 0.85 to 1.11; P = .68). Upper extremity pain during treatment and/or skin petechia occurred in 54 (7.2%) in the RIC group and 11 (1.5%) in the sham group.
Conclusions and Relevance
RIC initiated in the prehospital setting and continued in the hospital did not significantly improve functional outcome at 90 days in patients with acute stroke.
Trial Registration
ClinicalTrials.gov Identifier: NCT03481777
A multicenter clinical trial to determine whether combined prehospital and in-hospital remote ischemic conditioning treatment compared with a sham treatment improves functional outcomes in patients with acute ischemic and hemorrhagic stroke.
Introduction
Reperfusion therapy with intravenous thrombolysis and endovascular therapy have improved outcomes in patients with acute ischemic stroke significantly, yet many patients do not regain functional independence after a stroke.1 Consequently, there is a need for adjunctive cerebroprotective therapies with the ability to preserve function and viability of brain cells during ischemia.2 Ideally, such therapies should be made available to a broad range of patients with stroke, including patients living in areas where reperfusion therapy is unavailable.3
Remote ischemic conditioning (RIC) with transient cycles of limb ischemia and reperfusion on the upper extremity exerted distant organ protection in preclinical and clinical studies.4 The exact mechanism is unknown, but both humoral factors and upstream nervous system activations are implicated.4 The early effects of RIC in stroke may include enhanced collateral blood flow in the ischemic area and reduced poststroke inflammation, with the latter being associated with anti-inflammatory macrophage polarization and an increased hematoma reabsorption rate in preclinical intracerebral hemorrhage models.4,5,6 RIC is a simple and noninvasive procedure that has proved to be safe and feasible in numerous smaller clinical trials.7,8,9,10 In a large open-label randomized clinical trial involving patients with acute ischemic stroke, RIC treatment within 48 hours after stroke was associated with significantly improved functional outcome.11 For patients with intracerebral hemorrhage, no safety concerns have been observed, and there have been suggestions of benefit evidenced by an increased hematoma reabsorption rate in preclinical and clinical RIC studies.5,12 This randomized, sham-controlled, multicenter clinical trial was conducted to determine whether combined prehospital and in-hospital RIC treatment improves functional outcomes in patients with acute ischemic and hemorrhagic stroke.
Methods
Study Design
The Remote Ischemic Conditioning in Patients With Acute Stroke Trial (RESIST) was an investigator-initiated, multicenter, randomized, patient and outcome-assessor blinded, sham-controlled clinical trial. The trial was approved by Danish regional research ethics committees (ID: 1-10-72-97-17), Danish Data Protection Agency (ID: 1-16-02-16-18), and Danish Medicines Agency (ID: 2017114177, EUDAMED: CIV-17–11-022324) as an acute study. Consent was waived during the acute phase. Written consent was obtained by a trial guardian, an independent physician not associated with the neurology department, and subsequently by a relative or the patient if competence was regained. The trial was conducted in accordance with good clinical practice guidelines and the Declaration of Helsinki and was monitored by regional Good Clinical Practice units. The trial protocol was written in accordance with SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) and registered at ClinicalTrials.gov. The study protocol is available in Supplement 1 and statistical analysis plan in Supplement 2.
The trial was conducted at 4 stroke centers in Denmark (Aarhus University Hospital, Regional Hospital Gødstrup, Odense University Hospital, and Aalborg University Hospital). Approximately 200 ambulances and 4 helicopters participated, and 1680 paramedics and emergency medical technicians were trained and certified by web-based training.
Study Population
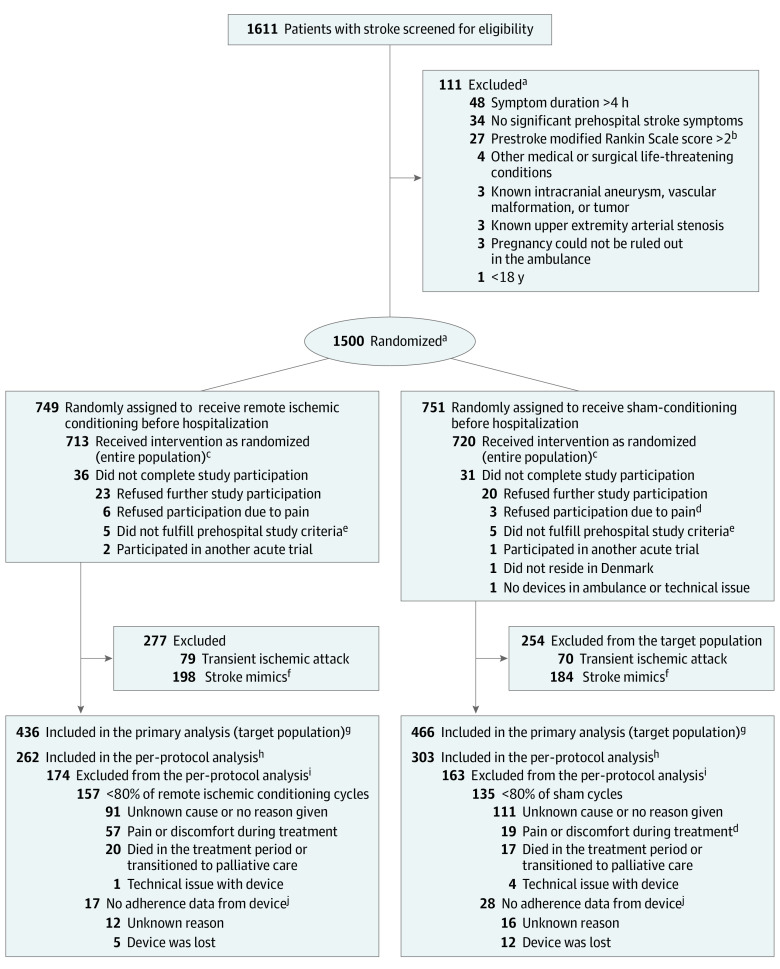
Eligible patients were adult (≥18 years), independent in activities of daily living (modified Rankin Scale [mRS] score ≤2) prior to the suspected stroke event, with a prehospital putative stroke evaluated by the Prehospital Stroke Score (PreSS), presenting within 4 hours of symptom onset. PreSS is a 6-item score evaluating face palsy, arm drift, speech difficulty, eye deviation, and incorrect month or age, and with an additional opportunity to report other neurological symptoms (eg, ataxia, sensory disturbances, and visual field loss). For the purposes of this study, a PreSS score of 1 or greater indicated suspicion of stroke and study eligibility. PreSS was the foundation for the telephone conference between the neurologist and the ambulance personnel (eFigure 1 in Supplement 3).13 Patients were excluded if they had a history of intracranial aneurysm, arteriovenous malformation, cerebral neoplasm, brain abscess or progressive neurodegenerative disease, or severe peripheral arterial disease in the upper extremities or if they had an arteriovenous shunt in the arm that had been selected for cuff placement for RIC (Figure 1). Patients who ultimately received an in-hospital diagnosis of either acute ischemic stroke or intracerebral hemorrhage were defined as the target population (Table 1).
Figure 1. Flow of Patients in the Trial of Remote Ischemic Conditioning in Patients With Acute Ischemic Stroke or Intracerebral Hemorrhage.
aEligibility assessment and randomization was performed by the stroke neurologists on call. Some screened patients had multiple reasons for not meeting study criteria, accounting for more reasons than the number excluded. Patients underwent randomization using a Web-based procedure with a permuted-block design (random blocks of 4, 6, and 8) according to site. Randomization was stratified by the receiving stroke center, age (strata of age: ≥18 to 65, 66 to 80, and >80 years) and prehospital stroke severity (strata of PreSS: 1–2, 3–4, and 5–6 present symptoms/deficits).
bScore range, 0 [no symptoms] to 6 [death].
cThe entire population included all the patients who were randomly assigned to a group and who consented to study participation, disregarding the final diagnosis.
dIn the sham group, 3 patients refused to participate due to pain and 19 patients in the target population experienced pain or discomfort to sham treatment. In the sham group, pain and/or discomfort was typically elicited by agitated arm movement during the 20 mm Hg cuff inflation period.
eIn total, 10 patients were excluded for not fulfilling the prehospital study criteria: 4 patients had symptoms lasting longer than 4 hours, 1 patient presented as a wake-up stroke, 2 patients’ symptoms or history were incorrectly communicated to the stroke neurologist, and 3 patients were not independent in daily activities of living before their stroke.
fPatients could be included without motor deficit, which may partially explain the high stroke mimic rate.
gThe target population included all the patients who were randomly assigned to a group, consented to study participation, and who had a target diagnosis of acute stroke. No patients were lost to follow-up for the primary end point.
hThe per-protocol population included all the patients who had undergone randomization, who had a target diagnosis of acute stroke, and who had acceptable treatment adherence with at least 80% of allocated cycles.
iSome screened patients had multiple reasons for not meeting study criteria, accounting for more reasons than the number excluded.
jThe reasons for “no adherence data from device” besides from lost device, were not registered specifically, but the most frequent causes were that the device was erroneously reset by study personnel before adherence data was downloaded or that the USB-port did not function.
Table 1. Baseline Demographic, Clinical, and Treatment Characteristics of the Target Populationa.
| Characteristics | Remote ischemic conditioning (n = 436) | Sham (n = 466) |
|---|---|---|
| Age, median (IQR), y | 72 (62-79) | 73 (62-80) |
| Sex, No. (%) | ||
| Female | 165 (37.8) | 170 (36.5) |
| Male | 271 (62.2) | 296 (63.5) |
| Comorbidities, No. (%) | ||
| Hypertensionb | 271 (62.2) | 296 (63.5) |
| Prior acute ischemic stroke, No./ total (%) | 72/434 (16.5) | 88/465 (18.8) |
| Atrial fibrillationb | 78 (17.9) | 63 (13.5) |
| Diabetesb | 55 (12.6) | 58 (12.4) |
| Prior transient ischemic attack, No./total (%) | 28/430 (6.4) | 34/464 (7.3) |
| Prehospital stroke score, median (IQR)c | 2 (2-3) | 2 (2-4) |
| Admission NIHSS, median (IQR)d | 5 (2-11) | 5 (2-10) |
| Discharged from stroke unit before 7 d, No. (%) | 354 (81.2) | 379 (81.3) |
| Onset to randomization, median (IQR), min | 55 (34-100) | 56 (33-94) |
| Discharge diagnosis | ||
| Acute ischemic strokee | 349 (80.0) | 388 (83.3) |
| Intracerebral hemorrhage | 87 (20.0) | 78 (16.7) |
| Patients with acute ischemic stroke, No. | 349 | 388 |
| Stroke etiology, No. (%) | ||
| Large artery disease | 62 (17.8) | 85 (21.9) |
| Small vessel disease | 49 (14.0) | 45 (11.6) |
| Cardioembolism | 47 (13.5) | 57 (14.7) |
| Other, rare, multiple, or unknown | 191 (54.7) | 201 (51.8) |
| NIHSS, median (IQR) [No.]d | 4 (2-8) [346] | 5 (2-9) [386] |
| Intravenous thrombolysis, No. (%)f | 223 (67.2) | 239 (65.3) |
| Admission to thrombolysis, median (IQR), ming | 29 (24-36) | 29 (24-38) |
| Thrombectomy, No. (%) | 65 (19.6) | 69 (18.9) |
| Admission to groin puncture, median (IQR), ming | 62 (52-88) | 64 (49-81) |
| Site of occlusion during angiography | ||
| Intracranial internal carotid artery | 13 (18) | 18 (26) |
| Main stem of middle cerebral artery | 27 (43) | 33 (48) |
| Branch from middle cerebral artery | 15 (23) | 6 (9) |
| Basilar artery | 2 (3) | 2 (3) |
| Otherh | 7 (8) | 4 (6) |
| No occlusion at time of endovascular therapy | 1 (2) | 6 (9) |
| mTICI 2b-3 reperfusion, No./total (%)i | 57/64 (88) | 65/69 (94) |
| Reperfusion therapy (intravenous thrombolysis and/or endovascular therapy), No. (%) | 247 (74.4) | 274 (74.6) |
| Patients with intracerebral hemorrhage, No. | 87 | 78 |
| Acute GCS score, median (IQR) [No.]j | 15 (13-15) [86] | 15 (13-15) [77] |
| Deep hematoma localization, No. (%)k | 45 (52) | 35 (45) |
| Platelet inhibitor use, No. (%) | 20 (23) | 17 (22) |
| Oral anticoagulation use, No. (%) | 10 (11) | 11 (14) |
| Hematoma evacuation or external ventricular drainage, No. (%) | 8 (9) | 11 (14) |
Abbreviations: GCS, Glasgow Coma Scale; MRI, magnetic resonance imaging; mTICI, modified treatment in cerebral ischemia; NIHSS, National Institutes of Health Stroke Scale; RIC, remote ischemic conditioning.
Patients with in-hospital diagnoses of acute ischemic stroke or intracranial hemorrhage were defined as the target population.
Known or newly diagnosed.
Score ranges from 0 to 6, with higher scores indicating a greater deficit.
Score ranges from 0 to 42, with higher scores indicating a greater deficit. NIHSS was missing for 3 patients in the RIC group and 2 in the sham group.
Including 22 with transient ischemic attack (ie, resolution of deficits ≤24 hours) with an acute ischemic lesion on MRI in the sham group and 17 in the RIC group.
Only intravenous thrombolysis with alteplase infusion was used during the study period. Numbers are for patients with acute ischemic stroke, excluding patient with symptom remission before scan with an acute ischemic lesion on MRI (n = 39).
Time from admission to thrombolysis was not available for 12 patients, and time from admission to groin puncture was not available for 2 patients.
Other types of vessel occlusion included the anterior cerebral artery, posterior cerebral artery, or the vertebral artery. Numbers are for patients with acute ischemic stroke, excluding patients with symptom remission before scan with an acute ischemic lesion on MRI (n = 39).
Good reperfusion defined as mTICI2b: partial filling of 50% or more of affected territory or mTICI3: complete reperfusion.
Score ranges from 3 (coma, completely unresponsive) to 15 (normal consciousness, responsive), with lower scores indicating reduced levels of consciousness.
Hematoma related to hypertension and commonly located in the basal ganglia, thalamus, or pons.
Randomization
Screening and randomization were performed during the teleconference with the ambulance personnel.14 Patients underwent randomization using a web-based procedure with a permuted-block design (random blocks of 4, 6, and 8) according to site. Eligibility assessment and randomization was performed by the stroke neurologists on call. Patients were assigned in a 1:1 ratio to RIC or sham. Randomization was stratified by the receiving stroke center, age (strata of age: ≥18-65, 66-80, and >80 years) and prehospital stroke severity (strata of PreSS: 1-2, 3-4, and 5-6 present symptoms and deficits) allowing for up to 36 block categories. After the randomization procedure, the treating personnel in the ambulance were informed of the group allocation.
Intervention
RIC devices and sham devices were programmed for 5 cycles, each consisting of 5 minutes of cuff inflation applied to the nonaffected arm followed by 5 minutes with a deflated cuff (a total of 50 minutes for each session). Each application of RIC or sham used these settings. For the RIC device, the default cuff pressure was 200 mm Hg. If the initial systolic blood pressure exceeded 175 mm Hg, the cuff was automatically inflated to 35 mm Hg higher than the systolic blood pressure to ensure complete arterial occlusion (maximum cuff pressure was 285 mm Hg). The sham device only inflated to a pressure of 20 mm Hg.
Treatment was started immediately after randomization in the ambulance or helicopter. At 6 hours after completion of the first RIC or sham treatment protocol, an additional series was performed (postconditioning) for all patients with a target diagnosis. To investigate the optimal dosing of postconditioning, for patients with a stroke diagnosis admitted to Aarhus University Hospital, ischemic postconditioning was continued twice daily (8 am and 8 pm) for 7 days. Ischemic postconditioning was continued at home or a rehabilitation center if the patient had been discharged before day 7.
Concomitant treatment and procedures were performed according to the standard of care at each center in compliance with international guidelines for acute stroke.15 In Denmark, health care services are tax-paid and free of charge. All patients were provided individualized neurorehabilitation if relevant.
RIC and sham devices were developed in collaboration with the Aarhus University faculty of the biomedical engineering department and produced by Seagull Healthcare. There was no industry funding or involvement. No patent application has been filed in relation to this work.
Clinical Assessment
A PreSS assessment was performed in the ambulance and repeated 24 hours later (or at discharge, which ever occurred first) for all randomized patients.13 The National Institutes of Health Stroke Scale (NIHSS) was performed at hospital arrival and after 24 hours in the target population. At the 90-day assessment, 2 independent and blinded assessors performed a functional outcomes assessment (modified Rankin Scale [mRS]) of patients with a target diagnosis of stroke. The assessments were conducted either in person or by telephone. If a disagreement occurred, a third blinded assessor would act as the final assessor. All assessors were certified and used a validated structured mRS questionnaire.16 Serious adverse events during the 90-day study period were adjudicated by a blinded independent clinical event committee consisting of 3 neurologists and 1 cardiologist. Patients and outcome assessors were blinded to treatment group. Treatment adherence was registered on each device, and 80% or more received cycles of the planned number was defined as acceptable in accordance with the protocol.
Outcomes
The primary efficacy end point was a shift toward a favorable outcome across the entire range of the mRS scores (0 [no symptoms] to 6 [dead]) at 90 days in the target population. Early after the trial had commenced (November 29, 2018), the primary outcome measure was changed from a 24-hour difference in PreSS to the difference in functional outcome measured across the entire range of the mRS. The original concern was whether there would be a ceiling effect on the mRS score that would render a score of 0 to 2 among the majority of patients but a more even distribution (on the entire range) could have been achieved on the ordinal use of PreSS. However, a blinded assessment on the distribution of mRS and PreSS scores did not show any difference in distribution between the 2 scales, and the primary end point was changed to mRS (shift analysis).
For the target population, the secondary efficacy end points were (1) a favorable shift across the entire range of the mRS scores at 90 days in subgroups of patients with acute ischemic stroke, patients with acute ischemic stroke treated with intravenous thrombolysis and/or endovascular therapy, patients with intracerebral hemorrhage, and patients receiving 7 days of RIC treatment; (2) change in PreSS score at 24 hours (range, 0 [normal] to 6 [maximum symptoms/signs of stroke]); (3) major adverse cardiac and cerebral events (MACCEs), which include cardiovascular death, acute myocardial infarction, and stroke, at 90 days; (4) recurrent ischemic events, which includes acute ischemic stroke, transient ischemic attack (TIA), myocardial infarction, or other vascular events, at 90 days; (5) early neurological improvement (≥4-point improvement on NIHSS score during the 24 hours after admission); (6) number of days (length of stay) in the stroke unit; and (7) all-cause mortality at 90 days. The difference in the proportion of patients with a complete remission of symptoms within 24 hours was also assessed among the population that included both individuals with a final diagnosis of stroke or TIA.
For the entire study population, the safety end points were death, change in PreSS score at 24 hours, and the occurrence of serious adverse events.
Dichotomized analyses of mRS scores using different cutoffs (0-1, excellent outcome; 0-2, favorable outcome; 0-3, fair outcome) and cardiovascular mortality at 90 days were not predefined and are presented as post hoc results. The predefined secondary end point of quality of life at 3 months as well as long-term (12 months) registry-based end points and exploratory end points (infarct size and infarct growth for acute ischemic stroke and hematoma reabsorption rate for intracerebral hemorrhage, molecular biomarkers, physical activity levels, and blood pressure profiles) will not be reported herein.
Sample Size Calculation
An effect corresponding to 7% increased odds for a beneficial shift on the mRS was assumed. The assumed effect was small but assessed as clinically relevant by the trial steering committee. Based on previous trial experience with prehospital RIC, we estimated that a sample of 1000 patients with a target diagnosis of acute ischemic stroke or intracerebral hemorrhage would be feasible to include during the study period (among 1500 patients with a prehospital putative stroke).8 Including 1000 patients with a target diagnosis would provide sufficient power (90%) at a significance level of 5% to detect RIC treatment effects of the assumed 7%. We estimated that 85% of patients with confirmed stroke would have acute ischemic stroke and the remaining 15% would have intracerebral hemorrhage.
Statistical Analysis
Primary and secondary efficacy outcomes were assessed in the target population, which included all patients, regardless of treatment adherence, who had undergone prehospital randomization and were later diagnosed with acute stroke. The entire population included all patients who were randomly assigned to a group and who consented to participate in the study, disregarding final diagnosis. The per-protocol population included all the patients who had undergone randomization, who had a target diagnosis of acute stroke, and who had acceptable treatment adherence with having received at least 80% of allocated cycles. Screen failures were defined as patients who were prehospitally screened for inclusion but who did not fulfill all study criteria at the telephone conference.
The primary study end point was performed using the entire range (shift analysis) of the mRS scores (ordinal logistic regression, with a random effect on the block categories as outlined in the Randomization subsection). For the entire population, the 24-hour difference in neurological symptoms (∆PreSS, ordinal logistic regression, shift analysis) was analyzed using the available range. The secondary efficacy outcomes were not corrected for multiple comparisons, so the results cannot be used for hypothesis testing or inference. Forest plots on subgroup interaction only contain point estimates with 95% CIs. Missing data including outcome data were assumed to be missing at random and were handled using multiple imputation-chained equations.17 A secondary per-protocol analysis including only participants who were adherent to their assigned intervention was conducted. In addition, a post hoc sensitivity analysis of the target population with adjustment for sex, age, diagnosis, site, and PreSS score was performed. Time-to-event end points (recurrent ischemic events and MACCEs) were analyzed as hazard rate ratios (HRRs), and data were presented on Kaplan-Meier curves and Aalen-Johansen curves where appropriate. Remaining secondary outcomes, including overall mortality, were analyzed as relative risks (RRs) and difference in means using appropriate regression analysis with a random effect on the block categories groups. An interim analysis for evaluation of the actual event rate after inclusion of 50% of the patients was performed, and the data and safety monitoring board recommended continuing the trial without changes. Interim analysis was assessed using a level of significance of 1%. Neither futility nor efficacy was met at the interim analysis. Analyses were performed with a 2-sided α level of .05. Statistical analyses were performed using STATA version 17.0 (StataCorp).
Results
From March 16, 2018, to November 11, 2022, a total of 1611 patients underwent prehospital screening. Of these, 111 did not meet inclusion criteria (Figure 1). A total of 1500 patients were randomized, 749 were allocated to RIC and 751 to sham. Thirty-six patients (5%) in the RIC group and 31 patients (4%) in the sham group were subsequently excluded, mainly due to withdrawal of consent. The remaining 1433 patients were a median age of 71 years (IQR, 59-78) and 591 (41%) were females. A total of 149 patients (10%) received a discharge diagnosis of TIA, and 382 (27%) were diagnosed with a condition mimicking a stroke (eTable 3 and eTable 10 in Supplement 3). These patients did not meet the target population definition. No patients were lost to follow-up.
A total of 902 patients were included in the target population group, 436 in the RIC group and 466 in the sham group. Of these, 737 patients (82%) had acute ischemic stroke and 165 (18%) had intracerebral hemorrhage. The median time from symptom onset to randomization was 56 minutes (IQR, 34-98), median prehospital systolic blood pressure was 182 mm Hg (IQR, 157-203) and diastolic blood pressure, 92 mm Hg (IQR, 79-106). Baseline demographic, clinical characteristics and reperfusion therapy rates were similar between the groups. The median NIHSS score at the time of the baseline examination was 5 in both groups (Table 1). Of the patients with acute ischemic stroke, 462 (66%) were treated with intravenous thrombolysis and 134 (19%) with endovascular therapy.
Primary Outcome
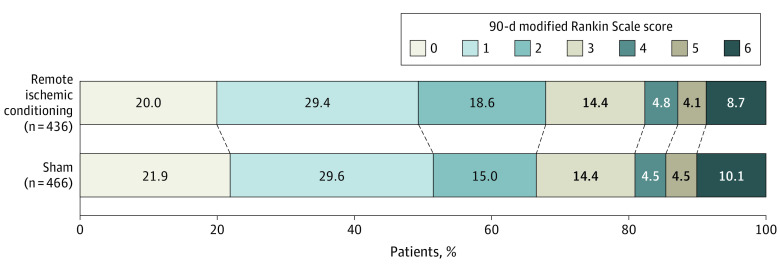
RIC was not associated with a significant shift toward better functional outcome at 90 days in the target population. The median mRS score at 90 days was 2 (IQR, 1-3) in the RIC group and 1 (IQR, 1-3) in the sham group (odds ratio [OR], 0.95; 95% CI, 0.75 to 1.20; P = .67; absolute difference in the median mRS score, −1; 95% CI, −1.7 to −0.25). Between-group distribution of the 90-day mRS is presented in Figure 2. Deviations from the assumption of proportional odds were not observed (Figure 2; eTable 1 in Supplement 3).
Figure 2. Distribution of Modified Rankin Scale Score Between Remote Ischemic Conditioning and Sham in the Target Population Group.
Differences in the scores on the modified Rankin Scale (mRS) between groups at 90 days are shown. Each stratum is presented in percentage. The raw distribution of scores is shown. Scores range from 0 to 6 (0, no symptoms; 1, symptoms without clinically significant disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; and 6, death).
Secondary Outcomes
Among the 737 patients with acute ischemic stroke, RIC treatment was not significantly associated with improvement in functional outcome among the 521 patients treated with reperfusion therapies (odds ratio [OR], 1.08; 95% CI, 0.79-1.49; P = .63) or among the 216 patients who did not receive reperfusion therapies (OR, 0.88; 95% CI, 0.54-1.42; P = .60; Table 2 and eTable 5 eFigures 2, 3, and 6 in Supplement 3).
Table 2. Primary and Secondary Outcomes.
| Remote ischemic conditioning (n = 436) | Sham (n = 466) | Absolute difference (95% CI) | Relative difference (95% CI) | P value | |
|---|---|---|---|---|---|
| Primary efficacy outcome | |||||
| mRS score at 90 d, median (IQR)a | 2 (1 to 3) | 1 (1 to 3) | −1 (−1.7 to −0.25)b | OR, 0.95 (0.75-1.20) | .67 |
| Selected secondary efficacy outcomes | |||||
| ≥4-Point NIHSS score improvement during 24 h, No./total (%)c | 108/425 (25) | 131/451 (29) | −3.7% (−9.5 to 2.2)d | OR, 0.85 (0.64-1.13) | .27 |
| Difference in PreSS during 24 h, median (IQR) [No.]c | −1 (−2 to 0) [429] | −1 (−2 to 0) [452] | −0.36 (−1.46 to 0.74)b | OR, 0.86 (0.68-1.09) | .21 |
| Complete remission of symptoms within 24 h, No./total (%)e | 96/515 (18.6) | 90/536 (16.9) | 1.7 (−2 to 5.4)d | OR, 1.10 (0.89-1.36) | .39 |
| Subgroup analyses | |||||
| Acute ischemic stroke patients, No. | 349 | 388 | |||
| mRS score at 90 d, median (IQR)a | 1 (1 to 2) | 1 (0 to 3) | 0 (−0.27 to 0.27)b | OR, 0.99 (0.76-1.29) | .95 |
| Treated with intravenous thrombolysis, No. | 233 | 240 | |||
| mRS score at 90 d, median (IQR)a | 1 (1 to 3) | 1 (1 to 3) | 0 (−0.39 to 0.39)b | OR, 1.20 (0.86-1.68) | .29 |
| Treated with endovascular therapy, No. | 65 | 69 | |||
| mRS score at 90 d, median (IQR)a | 2 (1 to 3) | 2 (1 to 3) | 0 (−2.4 to 2.4)b | OR, 1.17 (0.62-2.2) | .62 |
| Treated with reperfusion therapy, No.f | 247 | 274 | |||
| mRS score at 90 d, median (IQR)a | 1 (1 to 3) | 1 (1 to 3) | 0 (−0.38 to 0.38)b | OR, 1.08 (0.79-1.49) | .63 |
| Not treated with reperfusion therapy, No.f | 102 | 114 | |||
| mRS score at 90 d, median (IQR)a | 2 (1 to 3) | 1 (1 to 3) | −1 (2.2 to 0.22)b | OR, 0.88 (0.54-1.42) | .60 |
| Intracerebral hemorrhage patients, No. | 87 | 78 | |||
| mRS score at 90 d, median (IQR)a | 3 (2 to 5) | 3 (2 to 5) | 0 (−1.2 to 1.2)b | OR, 1.15 (0.65-2.04) | .60 |
| Safety outcomesg | 713 | 720 | |||
| ≥1 Serious adverse event, No. (%) | 169 (23.7) | 175 (24.3) | −0.7 (−3.9 to 2.5)d | RR, 0.97 (0.85-1.11) | .68 |
| 24-h change in PreSS, median (IQR) [No.]e | −1 (−2 to 0) [693] | −1 (−2 to 0) [705] | 0 (−0.12 to 0.12)b | OR, 0.99 (0.82-1.20) | .95 |
| 90-d all-cause mortality, No. (%) | 41 (5.9) | 47 (6.7) | −0.9 (−3.3 to 1.6)d | RR, 0.87 (0.58-1.31) | .51 |
| Post hoc analysis | 436 | 466 | |||
| mRS score 0-2 at 90 d, No. (%) | 296 (68) | 310 (66) | 1.1 (−5.5 to 7.6)d | OR, 1.06 (0.74-1.52) | .75 |
| Cardiovascular 90-d mortality, No. (%) | 32 (7.3) | 31 (6.7) | 0.9 (−2.8 to 4.7)d | HRR, 1.15 (0.70-1.89) | .58 |
Abbreviations: HRR, hazard rate ratio; mRS, modified Rankin Scale, NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; PreSS, prehospital stroke scale; RD, risk difference; RR, relative risk.
mRS at day 90 was assessed for all patients and imputation was not relevant. Ordinal logistic regression using the entire range of mRS. An OR of more than 1 favors RIC.
Difference in medians (95% CI) estimated using quantile regression analysis.
Missing NIHSS and PreSS data were imputed for the logistic regression analysis.
Absolute difference in risk (in percent) (95% CI).
Including individuals with stroke (as in the target population) and additionally those with a final diagnosis of transient ischemic attack (70 in the sham group and 79 in the RIC group).
Treatment with intravenous thrombolysis and/or endovascular therapy.
The entire population included all the patients who were randomly assigned to a group and who consented to study participation, disregarding the final diagnosis.
Among the 165 patients with intracerebral hemorrhage, RIC was also not significantly associated with a shift toward improved functional outcome (OR, 1.15; 95% CI, 0.65-2.04; P = .60) and 90-day mortality was 24% in both groups (eTable 5 in Supplement 3). In the target population, no significant differences on secondary efficacy end points were found, including a 4-point or more improvement on the NIHSS score that occurred in 108 (25%) in the RIC group and 131 (29%) in the sham group (OR, 0.85; 95% CI, 0.64-1.13; P = .27) and a 24-hour change in PreSS of −1 point (IQR, −2 to 0) in the RIC group and −1 point (IQR, −2 to 0) in the sham group (OR for a favorable shift in PreSS score distribution, 0.86; 95% CI, 0.68-1.09; P = .21). In the per-protocol analysis of primary and secondary efficacy outcomes, results similar to the primary analysis on the target population were found (eTable 4 in Supplement 3). The distribution of mRS according to duration of received postconditioning treatment is presented in eFigures 4 and 5 in Supplement 3.
In the target population, RIC treatment did not differ significantly from the sham treatment at 90 days with 57 patients (13.1%) in the RIC treatment group vs 62 (13.3%) in the sham group experiencing MACCEs (HRR, 0.98; 95% CI, 0.68-1.41), 25 (5.7%) vs 32 (6.9%) experiencing recurrent ischemic events (HRR, 0.83; 95% CI, 0.49-1.39), and 37 (8.5%) vs 46 (9.9%) experiencing all-cause mortality (RR, 0.88; 95% CI, 0.59-1.33; Figure 3).
Figure 3. Aalen-Johansen Cumulative Incidence Curves of Mortality, MACCE, and Recurrent Ischemic Events.
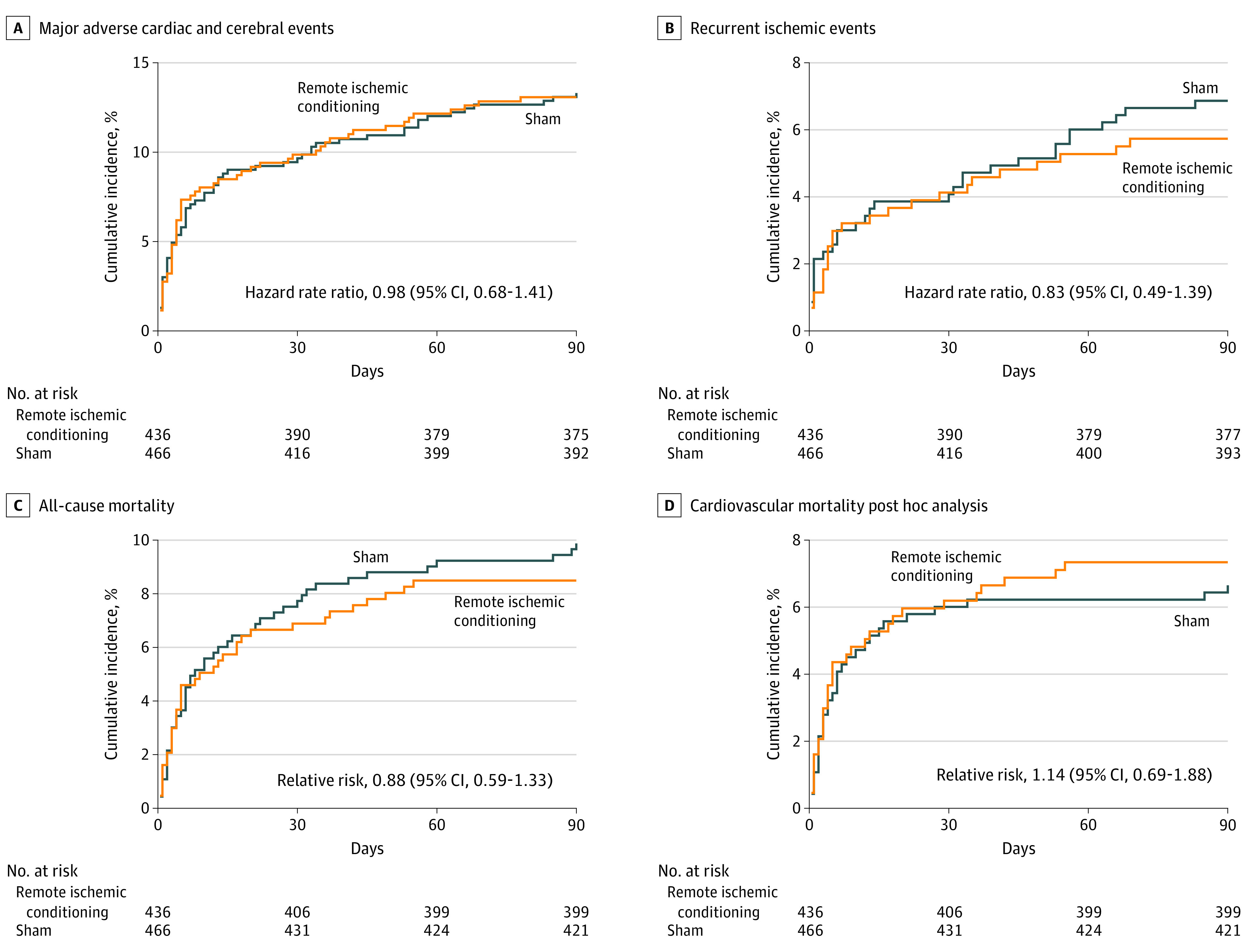
A, Ninety days major adverse cardiac and cerebral events (MACCE) (cardiovascular death, acute myocardial infarction, stroke) in the target population. B, Ninety days recurrent ischemic events (acute ischemic stroke, transient ischemic attack, myocardial infarction, or other vascular events) in the target population. C, Ninety days all-cause mortality in the target population. D, Ninety days cardiovascular mortality in the target population (post hoc).
For all analysis the median observation times were 90 days (IQR, 90-90) in both groups. The reference is the sham treatment.
Among participants with a final diagnosis of stroke or TIA, RIC was not significantly associated with remission of symptoms within 24 hours with 96 patients (18.6%) in the RIC group vs 90 patients (16.9%) in the sham group achieving complete remission (OR, 1.10; 95% CI, 0.89-1.36; P = .39; Table 2).
Post Hoc Analyses
In the target population, a favorable functional outcome (mRS 0 to 2) was found in 296 (67.9%) in the RIC group vs 310 (66.5%) in the sham group (eTable 1 in Supplement 3). At 90 days, 32 patients (7.3%) in the RIC group and 31 (6.7%) in sham group experienced cardiovascular mortality (RR, 1.14; 95% CI, 0.69-1.88; P = .61; Figure 3). In a sensitivity analysis on primary and secondary end points adjusted for sex, age, diagnosis, site of inclusion, and PreSS score, results similar to the primary analysis on the target population were found (eTable 6 in Supplement 3)
Safety Outcomes and Adverse Events
In the overall population of 1500 patients (prior to exclusions), a total of 660 serious adverse events were registered: 335 in the RIC group vs 325 in the sham group. In the RIC group, 169 patients (23.7%) experienced at least 1 serious adverse event vs 175 patients (24.3%) in the sham group (RR, 0.97; 95% CI, 0.85-1.11; P = .68; Table 2). Upper extremity pain during treatment and/or skin petechia occurred in 54 patients (7.2%) in the RIC group and 11 patients (1.5%) in the sham group. A detailed list of all registered adverse events is available in eTables 8 and 9 in Supplement 3.
Among the entire population of 1433 patients, 41 patients (5.8%) of 713 in the RIC group vs 47 patients (6.5%) of 720 in the sham group died within 90 days (RR, 0.87; 95% CI, 0.58-1.31; P = .51; Table 2).
Adherence
In the target population, the overall median adherence to the assigned treatment was 90% (IQR, 65%-100%), without significant between-group difference for the hyperacute (prehospital) and 6-hour treatment. Adherence to the extended 7-day treatment (postconditioning) was lower in the RIC group than in the sham group with a median of 89% (IQR, 51%-100%) vs 90% (IQR, 71%-100%; P = .03; eTable 7 in Supplement 3). A total of 565 patients (62.6%) in the target population had an acceptable treatment adherence of 80% or more, 262 (60.1%) in the RIC group and 303 (65.0%) in the sham group (P = .09). Adherence data from the devices of 45 patients (5.0%) were not available (Figure 1; eTable 7 in Supplement 3). A total of 714 target population patients (79%) were admitted to the Aarhus site and received postconditioning for 7 days. eTable 11 in Supplement 3 shows patient characteristics stratified by duration of postconditioning treatment.
Discussion
This randomized, sham-controlled, double-blind trial did not confirm the primary hypothesis that combined prehospital and in-hospital treatment with RIC in patients with acute stroke improves functional outcome at 90 days. There were no significant differences in secondary efficacy outcomes, mortality, or serious adverse events between the groups.
In an initial open-label prehospital pilot study involving 443 patients with acute ischemic stroke treated with thrombolysis, prehospital RIC (unilateral upper extremity) treatment was feasible and without notable differences in adverse events.8 No differences on penumbral salvage measured by perfusion magnetic resonance imaging (MRI) were observed. However, voxel-wise analysis after adjustment for baseline perfusion and diffusion lesion severity suggested that RIC reduced the risk of tissue infarction.
The subsequent nonsham controlled Remote Ischemic Conditioning in Acute Brain Infarction (RESCUE-BRAIN) trial,7 which evaluated unilateral lower extremity RIC among 188 patients, and the sham-controlled Remote Ischaemic Conditioning After Stroke Trial 2 (RECAST-2),9 which evaluated unilateral upper extremity RIC among 60 patients, both applied RIC within 6 hours of onset. In the sham-controlled Repeated Remote Ischemic Postconditioning After an Ischemic Stroke (REPOST),18 which evaluated unilateral upper extre mity RIC among 88 patients, the RIC treatment was started within 24 hours of onset. RIC in these 4 trials was not significantly associated with improved functional outcome in patients with stroke. In the most recent randomized, nonsham-controlled Remote Ischemic Conditioning for Acute Moderate Ischemic Stroke (RICAMIS) trial,11 bilateral upper extremity RIC was initiated within 48 hours of symptom onset among 1893 patients with moderate to severe ischemic stroke (NIHSS score, 6-16), who were not treated with reperfusion therapy. The median NIHSS score was 7, the mean time from onset to randomization was 25 hours, and RIC treatment was continued for 10 to 14 days. Compared with usual care, RIC treatment was associated with a significantly higher proportion of patients with excellent functional outcome: 67.4% in the RIC group vs 62.0% in the sham group achieved an mRS score of 0 to 1 (OR, 1.27; 95% CI, 1.05-1.54). The effect was dose-dependent over time, and an increased number of days with RIC was associated with improved functional outcome.19 The previous large RICA trial,20 using a sham-controlled design, could not demonstrate a significant effect of daily RIC treatment on the primary end point of time to first recurrent ischemic stroke. However, in patients with better adherence (defined as adhering >50% of days), daily RIC did significantly reduce recurrent stroke.
Several potential factors may explain the diverging results between RICAMIS and the present study. Unilateral compared with bilateral upper extremity application of RIC may be insufficient, and some clinical trials using bilateral treatment have found signals of efficacy.11,20 The timing and duration of RIC treatment also differed. In the present study, RIC or sham was reapplied after 6 hours in all patients followed by twice daily application for 7 days in almost 80% of the patients, and the outcomes were comparable between acute RIC (prehospital RIC and at 6 hours) and the extended 7 days of RIC treatment. The majority of patients with acute ischemic stroke in this study received reperfusion therapy (75%) vs none in the RICAMIS study. The number of patients with a good functional outcome (mRS score, 0 to 2) in the RICAMIS trial was higher than those in this study and likely reflects later randomization in the RICAMIS trial and differences in study eligibility criteria. Furthermore, the median NIHSS score was 5 in this study (vs 7 in the RICAMIS trial), which may suggest a more limited amount of salvageable brain tissue at risk of infarction and thereby a more limited potential for a demonstratable treatment effect. RIC had no significant beneficial effect in patients with acute ischemic stroke among 278 patients with an NIHSS score greater than 5 at admission, but the study may not have been sufficiently powered to detect a difference in subgroup analyses. The aim of hyperacute administration of the study intervention was achieved, with half of the patients being treated within the first 60 minutes after the onset of stroke, and these results did not demonstrate a significant benefit from RIC when applied in the acute phases of reperfused and nonreperfused acute ischemic stroke or intracerebral hemorrhage.
Limitations
This study has several limitations. First, the proportion of patients with TIA was higher (10% vs 4%) than anticipated, thus decreasing the number of patients in the target population (902 vs the planned 1000 patients). Given the width of the CIs around the effect estimates, a small yet potentially clinically important difference may not have been detected. Second, the stroke severities at baseline were mild to moderate (median NIHSS score, 5) in this study, and the majority of patients with acute ischemic stroke received reperfusion therapies (74.5%), factors that risk introducing a ceiling effect to any potential treatment benefit of RIC. Third, a heterogeneous group of patients with both ischemic and hemorrhagic stroke was included and may affect the generalizability and comparison with other trials. Fourth, only 62.6% had an acceptable treatment adherence and 79% of patients were admitted at only 1 of the 4 stroke centers involved in the study. Still, the results of the per-protocol analysis were in line with the results of the target population analysis. Randomization was stratified by site, but unmeasured differences between the sites may still exist. Fifth, demographics on race and ethnicity were not collected in this study, which may limit the generalizability of the findings. Sixth, PreSS used as a safety end point was designed and validated as a prehospital triage tool and not as a clinical end point measure.13 For that reason, the occurrence of serious adverse events was included as a safety end point (Supplement 3). Seventh, this study was conducted as an acute study, and a small proportion of patients (3.4%) or their relatives declined written consent or withdrew their consent. Nevertheless, retention of the remaining patients in the target population through the final follow-up visit was complete (100%).
Conclusions
RIC initiated in the prehospital setting and continued in the hospital did not significantly improve functional outcome at 90 days among patients with acute stroke.
Trial Protocol
Statistical Analysis Plan
eMethods 1. Trial background
eMethods 2. Device description
eResults. Primary Endpoint Analysis without Imputation of Missing Data
eTable 1. Primary Endpoint—Post hoc Sensitivity Analyses
eTable 2. Safety Analysis—Serious Adverse Events at 90 days
eTable 3. Baseline characteristics of the all included (safety population)
eTable 4. Primary and Secondary Efficacy Outcomes – Per-Protocol Population
eTable 5. Additional Efficacy and Safety Outcomes – Target population
eTable 6. Sensitivity analysis on primary and secondary endpoints adjusted for sex, age, diagnosis, site of inclusion, and prehospital PreSS
eTable 7. Device treatment adherence
eTable 8. Events assessed and adjudicated by the clinical event committee
eTable 9. All registered events as assessed by the investigators
eTable 10. Category of diagnosis in the stroke mimics group
eTable 11. Baseline demographic, clinical and treatment characteristics of the target population stratified by duration of postconditioning
eTable 12. Pairwise consistency and agreement measure represented by relative and absolute intra- class agreement
eFigure 1. Investigational device (RIC and Sham devices) photo
eFigure 2. Subgroup Analysis – Pre-Specified Subgroups (forest plot)
eFigure 3. Subgroup Analysis – Pre-Specified Subgroups on demographics (forest plot)
eFigure 4. Stacked bar chart on the distribution of mRS score stratified by duration of postconditioning treatment
eFigure 5. Stacked bar chart on the distribution of mRS score stratified by duration of postconditioning treatment
eFigure 6. Stacked bar chart on the distribution of mRS score in patients with acute ischemic stroke
Data Sharing Statement
References
- 1.Norrving B, Barrick J, Davalos A, et al. Action plan for stroke in Europe 2018-2030. Eur Stroke J. 2018;3(4):309-336. doi: 10.1177/2396987318808719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyden PD. Cerebroprotection for acute ischemic stroke: looking ahead. Stroke. 2021;52(9):3033-3044. doi: 10.1161/STROKEAHA.121.032241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyden P, Buchan A, Boltze J, Fisher M; STAIR XI Consortium* . Top priorities for cerebroprotective studies—a paradigm shift: report from STAIR XI. Stroke. 2021;52(9):3063-3071. doi: 10.1161/STROKEAHA.121.034947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess DC, Blauenfeldt RA, Andersen G, et al. Remote ischaemic conditioning—a new paradigm of self-protection in the brain. Nat Rev Neurol. 2015;11(12):698-710. doi: 10.1038/nrneurol.2015.223 [DOI] [PubMed] [Google Scholar]
- 5.Vaibhav K, Braun M, Khan MB, et al. Remote ischemic post-conditioning promotes hematoma resolution via AMPK-dependent immune regulation. J Exp Med. 2018;215(10):2636-2654. doi: 10.1084/jem.20171905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma J, Ma Y, Dong B, Bandet MV, Shuaib A, Winship IR. Prevention of the collapse of pial collaterals by remote ischemic perconditioning during acute ischemic stroke. J Cereb Blood Flow Metab. 2017;37(8):3001-3014. doi: 10.1177/0271678X16680636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pico F, Lapergue B, Ferrigno M, et al. Effect of in-hospital remote ischemic perconditioning on brain infarction growth and clinical outcomes in patients with acute ischemic stroke: the RESCUE BRAIN randomized clinical trial. JAMA Neurol. 2020;77(6):725-734. doi: 10.1001/jamaneurol.2020.0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hougaard KD, Hjort N, Zeidler D, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke. 2014;45(1):159-167. doi: 10.1161/STROKEAHA.113.001346 [DOI] [PubMed] [Google Scholar]
- 9.England TJ, Hedstrom A, O’Sullivan SE, et al. Remote ischemic conditioning after stroke trial 2: a phase IIb randomized controlled trial in hyperacute stroke. J Am Heart Assoc. 2019;8(23):e013572. doi: 10.1161/JAHA.119.013572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.England TJ, Hedstrom A, O’Sullivan S, et al. RECAST (Remote Ischemic Conditioning After Stroke Trial): a pilot randomized placebo controlled phase II trial in acute ischemic stroke. Stroke. 2017;48(5):1412-1415. doi: 10.1161/STROKEAHA.116.016429 [DOI] [PubMed] [Google Scholar]
- 11.Chen HS, Cui Y, Li XQ, et al. ; RICAMIS Investigators . Effect of remote ischemic conditioning vs usual care on neurologic function in patients with acute moderate ischemic stroke: the RICAMIS randomized clinical trial. JAMA. 2022;328(7):627-636. doi: 10.1001/jama.2022.13123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao W, Jiang F, Li S, et al. Safety and efficacy of remote ischemic conditioning for the treatment of intracerebral hemorrhage: a proof-of-concept randomized controlled trial. Int J Stroke. 2022;17(4l):425-433. doi: 10.1177/17474930211006580 [DOI] [PubMed] [Google Scholar]
- 13.Gude MF, Blauenfeldt RA, Behrndtz AB, et al. The prehospital stroke score and telephone conference: a prospective validation. Acta Neurol Scand. 2022;145(5):541-550. doi: 10.1111/ane.13580 [DOI] [PubMed] [Google Scholar]
- 14.Blauenfeldt RA, Hjort N, Gude MFMF, et al. A multicentre, randomised, sham-controlled trial on Remote Ischemic Conditioning in Patients With Acute Stroke (RESIST)—rationale and study design. Eur Stroke J. 2020;5(1):94-101. doi: 10.1177/2396987319884408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 Update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 16.Bruno A, Akinwuntan AE, Lin C, et al. Simplified modified Rankin Scale questionnaire: reproducibility over the telephone and validation with quality of life. Stroke. 2011;42(8):2276-2279. doi: 10.1161/STROKEAHA.111.613273 [DOI] [PubMed] [Google Scholar]
- 17.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 18.Landman TRJ, Schoon Y, Warlé MC, Meijer FJA, Leeuw FE, Thijssen DHJ. The effect of Repeated Remote Ischemic Postconditioning After an Ischemic Stroke (REPOST): a randomized controlled trial. Int J Stroke. 2023;18(3):296-303. doi: 10.1177/17474930221104710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui Y, Chen H-S. Remote ischemic conditioning vs usual care and neurologic function in acute moderate ischemic stroke—reply. JAMA. 2022;328(23):2363. doi: 10.1001/jama.2022.18829 [DOI] [PubMed] [Google Scholar]
- 20.Hou C, Lan J, Lin Y, et al. ; RICA investigators . Chronic remote ischaemic conditioning in patients with symptomatic intracranial atherosclerotic stenosis (the RICA trial): a multicentre, randomised, double-blind sham-controlled trial in China. Lancet Neurol. 2022;21(12):1089-1098. doi: 10.1016/S1474-4422(22)00335-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods 1. Trial background
eMethods 2. Device description
eResults. Primary Endpoint Analysis without Imputation of Missing Data
eTable 1. Primary Endpoint—Post hoc Sensitivity Analyses
eTable 2. Safety Analysis—Serious Adverse Events at 90 days
eTable 3. Baseline characteristics of the all included (safety population)
eTable 4. Primary and Secondary Efficacy Outcomes – Per-Protocol Population
eTable 5. Additional Efficacy and Safety Outcomes – Target population
eTable 6. Sensitivity analysis on primary and secondary endpoints adjusted for sex, age, diagnosis, site of inclusion, and prehospital PreSS
eTable 7. Device treatment adherence
eTable 8. Events assessed and adjudicated by the clinical event committee
eTable 9. All registered events as assessed by the investigators
eTable 10. Category of diagnosis in the stroke mimics group
eTable 11. Baseline demographic, clinical and treatment characteristics of the target population stratified by duration of postconditioning
eTable 12. Pairwise consistency and agreement measure represented by relative and absolute intra- class agreement
eFigure 1. Investigational device (RIC and Sham devices) photo
eFigure 2. Subgroup Analysis – Pre-Specified Subgroups (forest plot)
eFigure 3. Subgroup Analysis – Pre-Specified Subgroups on demographics (forest plot)
eFigure 4. Stacked bar chart on the distribution of mRS score stratified by duration of postconditioning treatment
eFigure 5. Stacked bar chart on the distribution of mRS score stratified by duration of postconditioning treatment
eFigure 6. Stacked bar chart on the distribution of mRS score in patients with acute ischemic stroke
Data Sharing Statement