Key Points
Question
Is continuous positive airway pressure (CPAP) treatment for obstructive sleep apnea (OSA) associated with a decreased risk of cardiovascular adverse events in participants with both OSA and established cardiovascular disease?
Findings
This systematic review and individual participant data (IPD) meta-analysis of 3 randomized clinical trials including 4186 patients with IPD showed that whereas the IPD meta-analysis based on intention to treat reported no effect of CPAP treatment, the adherent use of CPAP treatment (≥4 hours/day) is associated with a reduced recurrence risk of major adverse cardiac or cerebrovascular events (MACCEs) with a significant hazard ratio of 0.69.
Meaning
Patients with established cardiovascular disease and OSA who used CPAP for 4 or more hours per day had a significantly lower risk of MACCEs than those who used CPAP less than 4 hours per day.
Abstract
Importance
The effect of continuous positive airway pressure (CPAP) on secondary cardiovascular disease prevention is highly debated.
Objective
To assess the effect of CPAP treatment for obstructive sleep apnea (OSA) on the risk of adverse cardiovascular events in randomized clinical trials.
Data Sources
PubMed (MEDLINE), EMBASE, Current Controlled Trials: metaRegister of Controlled Trials, ISRCTN Registry, European Union clinical trials database, CENTRAL (Cochrane Central Register of Controlled Trials), and ClinicalTrials.gov databases were systematically searched through June 22, 2023.
Study Selection
For qualitative and individual participant data (IPD) meta-analysis, randomized clinical trials addressing the therapeutic effect of CPAP on cardiovascular outcomes and mortality in adults with cardiovascular disease and OSA were included.
Data Extraction and Synthesis
Two reviewers independently screened records, evaluated potentially eligible primary studies in full text, extracted data, and cross-checked errors. IPD were requested from authors of the selected studies (SAVE [NCT00738179], ISAACC [NCT01335087], and RICCADSA [NCT00519597]).
Main Outcomes and Measures
One-stage and 2-stage IPD meta-analyses were completed to estimate the effect of CPAP treatment on risk of recurrent major adverse cardiac and cerebrovascular events (MACCEs) using mixed-effect Cox regression models. Additionally, an on-treatment analysis with marginal structural Cox models using inverse probability of treatment weighting was fitted to assess the effect of good adherence to CPAP (≥4 hours per day).
Results
A total of 4186 individual participants were evaluated (82.1% men; mean [SD] body mass index, 28.9 [4.5]; mean [SD] age, 61.2 [8.7] years; mean [SD] apnea-hypopnea index, 31.2 [17] events per hour; 71% with hypertension; 50.1% receiving CPAP [mean {SD} adherence, 3.1 {2.4} hours per day]; 49.9% not receiving CPAP [usual care], mean [SD] follow-up, 3.25 [1.8] years). The main outcome was defined as the first MACCE, which was similar for the CPAP and no CPAP groups (hazard ratio, 1.01 [95% CI, 0.87-1.17]). However, an on-treatment analysis by marginal structural model revealed a reduced risk of MACCEs associated with good adherence to CPAP (hazard ratio, 0.69 [95% CI, 0.52-0.92]).
Conclusions and Relevance
Adherence to CPAP was associated with a reduced MACCE recurrence risk, suggesting that treatment adherence is a key factor in secondary cardiovascular prevention in patients with OSA.
This meta-analysis of 3 randomized clinical trials assesses the effect of continuous positive airway pressure treatment for obstructive sleep apnea on the risk of adverse cardiovascular events.
Introduction
Obstructive sleep apnea (OSA) is a common and chronic disorder characterized by occlusion of the upper airway during sleep that leads to intermittent hypoxia, sleep fragmentation, and intrathoracic pressure changes.1 This disorder has been associated with an increased risk of cardiovascular diseases.2 The criterion-standard treatment for OSA involving continuous positive airway pressure (CPAP) is frequently prescribed in patients with OSA and is effective in reversing the hypoxemia and upper airway obstruction that promote this disorder. The positive effect of CPAP treatment has been demonstrated in reversing the symptoms associated with OSA, mainly daytime sleepiness. Moreover, CPAP treatment has been associated with a reduction in blood pressure in patients with hypertension and OSA, and this effect is greatest in patients with resistant hypertension and patients with higher adherence to CPAP therapy.3 Whereas randomized trials and meta-analyses have shown that CPAP therapy elicits significant reductions in blood pressure in primary cardiovascular prevention,3,4,5 this positive effect has not been demonstrated in secondary prevention of cardiovascular events. Three randomized clinical trials (RCTs)6,7,8 with a large series of participants have explored the impact of CPAP on the prevention of cardiovascular events in OSA. All 3 studies involved exploring the effect of CPAP in nonsleepy patients with OSA and revealed that CPAP did not prevent cardiovascular events in patients with established cardiovascular disease.9
It is increasingly recognized that individual participant data (IPD) meta-analysis is a better basis for modeling treatment effects than aggregated data used in traditional meta-analysis.10 IPD meta-analysis involves the application of standardized analyses across multiple data sets, permitting the analysis of a larger sample size together with sophisticated modeling of moderator effects.11 Applying this type of meta-analysis can provide great precision and power to assess the overall efficacy of CPAP treatment in secondary cardiovascular prevention and, importantly, it allows assessment of the effect of CPAP treatment intervention in clinically important subgroups based on age, sex, severity of OSA, and CPAP adherence. It has been largely argued that good adherence to treatment with CPAP is crucial for cardiovascular benefits.4 The fluctuating nature of adherence to CPAP treatment among patients prevents them from being strictly categorized into accurate adherence groups. Moreover, large follow-up periods can increase the individual variability in this regard, which may confound the relationship between adherence and cardiovascular risk. The hypothesis for the current study was that an appropriate and sustained adherence to CPAP has an impact in the reduction of cardiovascular risk. To this end, the risk of cardiovascular event recurrence was evaluated in a hypothetical scenario in which all patients presented sustained CPAP adherence throughout the follow-up period compared with those who did not adhere to CPAP treatment.
Methods
This systematic review of interventions and IPD meta-analysis follows the current recommendations of the PRISMA-IPD statement.12 The PRISMA-IPD checklist was completed. The registration of this systematic review was prospectively recorded on November 23, 2020, in the PROSPERO database (https://www.crd.york.ac.uk/) (review register: CRD42020214175).
Information Sources
A database search using a highly sensitive search string was conducted by 1 author (G.L.) in September 2022, followed by an update in June 2023. The literature search strategy is available in the eAppendix in Supplement 1. We identified studies from the following databases without language restriction: PubMed (MEDLINE), EMBASE, Current Controlled Trials: metaRegister of Controlled Trials, ISRCTN Registry (https://www.isrctn.com/), European Union clinical trials database (https://euclinicaltrials.eu/), CENTRAL (Cochrane Central Register of Controlled Trials), and ClinicalTrials.gov. Details of these literature search strategies are reported in Figure 1. We also conducted a manual search of the reference lists of all primary studies and abstracts of major respiratory conferences from the American Thoracic Society (2015-2022), the American College of Chest Physicians (2015-2022), the American Academy of Sleep Medicine (2015-2022), and the European Respiratory Society (2015-2022). We also asked the authors of the studies reviewed to identify additional published studies and trials in progress.
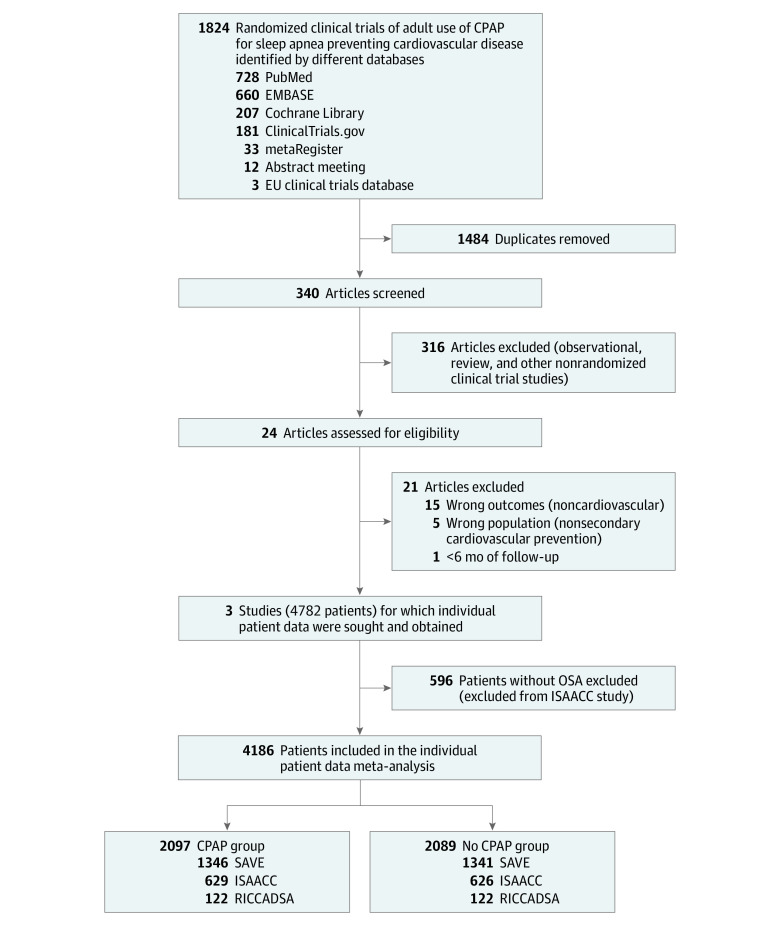
Figure 1. Selection of Randomized Trials Evaluating the Effect of the Use of CPAP on Major Cardiovascular and Cerebrovascular Adverse Events.
CPAP indicates continuous positive airway pressure; EU, European Union; ISAACC, Impact of Sleep Apnea Syndrome in the Evolution of Acute Coronary Syndrome7; OSA, obstructive sleep apnea; RICCADSA, Randomized Intervention With CPAP in Coronary Artery Disease and Sleep Apnea8; and SAVE, Sleep Apnea cardioVascular Endpoints.6
Eligibility Criteria and Selection of Studies
Two authors (G.L. and J.D.) evaluated the identified references and added them to the Covidence software (Veritas Health Innovation Ltd). A highly specific hand search was performed for title screening and, in a separate step, the abstracts from the literature search were independently reviewed by the same authors. The eligible studies were exhaustively reviewed and evaluated for eligibility according to our prespecified inclusion and exclusion criteria described above. Any discrepancies were resolved by discussion (M.S.dlT., G.L., J.D., Y.P., D.M., F.B.). Following this, a table defining the characteristics of the excluded studies was created. We included publications with adult patients (aged ≥18 years) with confirmed moderate to severe OSA defined by current recommendations from the American Academy of Sleep Medicine.13 We included studies using an oxygen desaturation index of 12 events or more per hour or an apnea-hypopnea index of 15 events or more per hour as the diagnosis of OSA. OSA diagnoses were achieved by either polysomnography or a home sleep apnea test. We included articles involving patients with medical indications for CPAP therapy based on the current recommendation of the American Academy of Sleep Medicine.13 Regarding CPAP intervention, we included trials using any CPAP device regardless of the manufacturer. We included studies involving CPAP therapy for more than 6 months; as a comparison, we included sham CPAP or optimal medical management. Studies with a follow-up period shorter than 6 months and studies in which patients with predominantly central sleep apnea/Cheyne-Stokes respiration were enrolled were excluded. We also excluded review articles, letters to the editor, or RCTs with other interventions. The following information from the included studies was extracted: study characteristics (title, authors, year of publication, journal, country, volume [issue]), inclusion criteria, exclusion criteria, setting, definition and diagnosis of OSA, baseline comorbidity, cardiovascular outcomes, general characteristics of participants (including sample size, age, and sex), length of follow-up period, follow-up manner, incidence of cardiovascular events, and analytical methods (statistical models).
Data Extraction and Assessment of Risk of Bias
Two review authors (G.L. and J.D.) independently extracted outcome data from eligible studies, which included demography, baseline characteristics, inclusion criteria, and main outcomes (see the Outcomes section); 1 review author (G.L.) transferred the trial-level data into Review Manager (RevMan, Version 5.3; The Cochrane Collaboration). The risk of bias assessment was independently assessed by the same authors, using the criteria outlines in the Cochrane handbook for systematic reviews of interventions for RCTs.14 This tool includes the following domains: (1) random sequence (selection bias); (2) allocation concealment (selection bias); (3) blinding of participants and personnel (performance bias); (4) blinding of outcome assessment (detection bias); (5) incomplete outcome data (attrition bias); and (6) selective reporting (reporting bias). Any disagreement between reviewers was resolved by discussion (G.L., J.D., E.G.L., I.D.B., R.W., C.E.). Each trial was approved by an ethics committee according to the respective country’s legislation, and all patients were informed of the research at the time of inclusion and provided written consent.
To obtain individual patient data, the principal investigators of the included RCTs were contacted to provide the data in anonymized electronic data sets. Protocols and data consistency were evaluated with each principal investigator and data manager. Database dictionaries were reviewed before the harmonization of the study databases.
Outcomes
The primary outcome was a composite of the first major adverse cardiac or cerebrovascular event (MACCE) or death from cardiovascular causes, myocardial infarction, stroke, revascularization procedure, hospital admission for heart failure, hospital admission for unstable angina, or hospital admission for transient ischemic attack. Secondary cardiovascular end points included the individual components of the primary composite end point.
Statistical Analysis
Baseline characteristics are described using IPD. Means and SDs for quantitative data and percentages for qualitative data are reported.
One-stage and 2-stage IPD meta-analyses10,15,16 were used to evaluate the effect of CPAP treatment on recurrent cardiovascular events according to the intention-to-treat principle. The 1-stage IPD meta-analysis was based on a mixed-effects Cox regression model for the primary end point with randomized group as a fixed effect and trial as a random effect. On the other hand, in the 2-stage IPD meta-analysis, we first fitted a Cox regression model separately per clinical trial, and then data were combined using a random-effect meta-analysis method.17 The percentage of variability attributable to heterogeneity between clinical trials was assessed with the I2 statistic. Hazard ratios (HRs) and corresponding 95% CIs were reported and summarized using forest plots. Furthermore, components of the primary end point were also analyzed using both 1-stage and 2-stage IPD methods.
The primary end point was additionally evaluated in subgroups according to the severity of OSA, tertiles of age, sex, hypertension, blood pressure medication, and tertiles of mean blood pressure. All subgroup analyses were performed in a 1-stage IPD meta-analysis due to low number of events when classified by subgroups.
An on-treatment analysis was performed to assess the effect of CPAP adherence considering intrapatient changes over time using marginal structural models. Patients randomized to the CPAP group and CPAP adherence (dichotomized using the cutoff of 4 hours per day) at each time period was used as the exposure measure. Marginal structural models using inverse probability of treatment weighting were performed to adjust for the baseline and time-varying confounding factors to compare cardiovascular risk between CPAP adherence groups (eMethods and eFigure 1 in Supplement 1).
All Cox models passed the proportional hazard test.18 All analyses were run on R version 4.2 (R Core Team, 2022).19 Package “coxme” for the 1-stage IPD and packages “survival” and “metafor” were used for the 2-stage IPD meta-analysis. Package “ipw” was also used for the inverse probability of treatment weighted analysis. The P value indicative of statistical significance was set at .05.
Results
Literature Search
From a total of 1824 references, 24 studies were identified for potential inclusion after our literature search, 21 of which were excluded based on the preestablished criteria. A summary with the reasons for exclusion is shown in eTable 1 in Supplement 1. Finally, 3 RCTs were included for qualitative and IPD analysis. The summary of the literature search following the PRISMA flowchart is presented in Figure 1. All the studies were parallel RCTs, and the mean age of patients ranged between 60 and 66 years; the population included in each study was mostly male (range, 81%-84%). The mean follow-up ranged between 34 and 52 months. The Epworth Sleepiness Scale score ranged between 5.3 and 7.4 points, and all sleep studies used home or hospital sleep apnea testing to establish eligibility.
Studies and Patient Characteristics
Data from a total of 4186 individual patients were obtained from 3 randomized trials.6,7,8 None of the 3 studies analyzed included blinding processes to assess the effect of the intervention. A summary of the characteristics of the included trials is shown in Table 1 and eTable 2 in Supplement 1. IDP integrity analysis showed that there was consistency among trials, and no values outside the range were found. The IPD meta-analysis included 2097 patients (50.1%) randomized to receive CPAP treatment and 2089 (49.9%) randomized to no CPAP. In the SAVE (Sleep Apnea Cardiovascular Endpoints) and ISAACC (Impact of Sleep Apnea Syndrome in the Evolution of Acute Coronary Syndrome) trials, both groups received dietary education to reduce overweight, standard care of cardiovascular risk factors (control of hypertension, dyslipidemia, diabetes, and smoking cessation), and sleep hygiene counseling (avoidance of evening alcohol, sedatives, and sleep deprivation). The mean (SD) follow-up time was 39 (21.6) months. The baseline characteristics of the study groups are detailed in Table 2.
Table 1. Trial Designs, Patient Characteristics, and Definitions Used in Meta-Analysesa.
| SAVE Study (McEvoy et al,6 2016) | ISAACC Study (Sánchez-de-la-Torre et al,7 2020) | RICCADSA Study (Peker et al,8 2016) | |
|---|---|---|---|
| Patients | 2687 (CPAP = 1346; usual care = 1341) | 1255 (CPAP = 629; usual care = 626) | 244 (CPAP = 122; no CPAP = 122) |
| Condition or disease |
|
|
|
| Setting | 89 Centers in 7 countries (Australia, Brazil, China, India, New Zealand, Spain, and United States) | 15 Hospitals across Spain | Two sites in Skaraborg County, West Sweden |
| Recruiting period | 2008-2013 | 2011-2018 | 2005-2010 |
| Design | International, multicenter, randomized, parallel-group, open-label trial with blinded end point assessment | Multicenter, open-label, parallel, prospective, randomized clinical trial | Single-center (2 sites), prospective, open, randomized, parallel, interventional, superiority trial |
| OSA diagnosis |
|
|
|
|
|
|
|
|
|
|
|
| Primary outcome | A composite of the cardiovascular end points of cardiovascular death, nonfatal acute myocardial infarction, nonfatal stroke, hospital admission for heart failure, and new hospitalization for unstable angina or transient ischemic attack | Rate of cardiovascular events: cardiovascular death, nonfatal acute myocardial infarction, nonfatal stroke, hospital admission for heart failure, and new hospitalization for unstable angina or transient ischemic attack | The combined rate of cardiovascular mortality, stroke, myocardial infraction, and the need for new revascularization |
| Major exclusions |
|
|
|
| Follow-up, mean (IQR), mo | 37.8 (25.9-55.2) | 31.3 (13.7-55.0) | 52.1 (35.6-70.8) |
| Baseline characteristics | |||
| Age, mean (SD), y | 61.2 (7.8) | 60.3 (10.2) | 66 (8.4) |
| Sex, No. (%) | |||
| Men | 2174 (81) | 1058 (84) | 205 (84) |
| Women | 513 (19) | 197 (16) | 39 (16) |
| BMI, median (IQR) | 28.1 (25.7-30.9) | 29.0 (26.4-31.9) | 28.4 (26.1-30.2) |
| Hypertension, % | 78 | 56 | 64 |
| Apnea-hypopnea index, median (IQR), events/hd | 25.0 (16.0-40.0) | 31.3 (21.0-46.0) | 24.4 (18.4-35.9) |
| Epworth Sleepiness Scale score, median (IQR)b | 7.0 (5.0-10.0) | 5.0 (3.0-7.0) | 6.0 (4.0-7.0) |
| CPAP use, median (IQR), hours/d | 3.3 (1.3-5.1) | 2.2 (0.03-5.1) | 2.4 (0.2-4.8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CPAP, continuous positive airway pressure; OSA, obstructive sleep apnea; TIA, transient ischemic attack.
See eTable 2 in Supplement 1 for additional trial characteristics and eFigure 2 in Supplement 1 for the risk of bias assessment.
Epworth Sleepiness Scale: questionnaire routinely used to assess daytime sleepiness. Total score can range from 0 to 24. A score higher than 10 is associated with sleepiness.
Oxygen desaturation index: mean number of desaturation episodes occurring per hour, where desaturation episodes are defined as a decrease in the mean oxygen saturation of ≥4% (over the last 120 seconds) that lasts for at least 10 seconds.
Apnea-hypopnea index: apnea was defined as an absence of airflow lasting ≥10 seconds. Hypopnea was defined as a reduction in airflow lasting ≥10 seconds, associated with oxygen desaturation. The apnea-hypopnea index was defined as the number of episodes of apnea and hypopnea per hour of recording.
Table 2. Baseline Characteristics of the Study Groupsa.
| No. (%) | ||
|---|---|---|
| CPAP (n = 2097) | No CPAP (n = 2089) | |
| Age, mean (SD), y | 61.1 (8.62) | 61.4 (8.77) |
| Sex | ||
| Male | 1720 (82.0) | 1717 (82.2) |
| Female | 377 (18.0) | 372 (17.8) |
| BMI, median (IQR) | 28.4 (25.9-31.2) | 28.4 (26.0-31.1) |
| Lifestyle factors | ||
| Smoking status | ||
| Never | 781 (37.3) | 811 (38.9) |
| Former | 774 (37.0) | 773 (37.1) |
| Current | 539 (25.7) | 502 (24.1) |
| Current alcohol consumption | 513 (26.1) | 497 (25.6) |
| Obstructive sleep apnea measures | ||
| Apnea-hypopnea index, median (IQR)b | 27.0 (18.0-41.0) | 27.0 (18.0-42.0) |
| Oxygen desaturation index, median (IQR)c | 24.0 (16.0-37.0) | 24.0 (16.0-38.0) |
| Mean Sao2, mean (SD) | 92.9 (5.45) | 92.9 (4.81) |
| Minimum Sao2, mean (SD) | 78.8 (6.84) | 78.6 (8.01) |
| Proportion of time with Sao2 <90%, median (IQR), % | 7.39 (2.19-19.3) | 6.76 (2.20-19.2) |
| Epworth Sleepiness Scale score, median (IQR)d | 6.00 (4.00-9.00) | 6.00 (4.00-9.00) |
| Medical historye | ||
| Hypertension | 1491 (71.2) | 1475 (70.7) |
| Any transient ischemic attack | 617 (29.5) | 626 (30.0) |
| Diabetes mellitus | 611 (29.2) | 575 (27.6) |
| Medicationf | ||
| Antihypertensive drugs | 1484 (70.9) | 1501 (71.9) |
| Antiplatelet and antithrombotic drugs | 1297 (62.0) | 1287 (61.7) |
| Lipid-lowering drug | 1118 (53.4) | 1141 (54.7) |
| Antidiabetics oral medication | 443 (21.1) | 431 (20.6) |
| Insulin | 130 (6.20) | 132 (6.32) |
Abbreviations: CPAP, continuous positive airway pressure; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); Sao2, arterial oxygen saturation.
See eTable 2 in Supplement for additional trial characteristics and to eFigure 2 in Supplement for the risk of bias assessment.
Apnea-hypopnea index (apnea was defined as an absence of airflow lasting ≥10 seconds). Hypopnea was defined as a reduction in airflow lasting ≥10 seconds, associated with oxygen desaturation. The apnea-hypopnea index was defined as the number of episodes of apnea and hypopnea per hour of recording.
Oxygen desaturation index: mean number of desaturation episodes occurring per hour, where desaturation episodes are defined as a decrease in the mean oxygen saturation of ≥4% (over the last 120 seconds) that lasts for at least 10 seconds.
Epworth Sleepiness Scale: questionnaire routinely used to assess daytime sleepiness. Total score can range from 0 to 24. A score higher than 10 is associated with sleepiness.
Medical history data were obtained from the patients by evaluating the information in patient report, performing diagnostic tests, and specific forms and self-administered questionaries.
The categories of medications were established by the clinicians participating in the study.
Risk of Bias Within Studies
We found a low risk of bias in several domains: all studies were randomized, reported adequate randomization concealment, and outcome assessment was blind.
However, no placebos as comparators were reported in the trials (performance bias); and none of the 3 studies analyzed included blinding processes to assess the effect of the intervention. A summary of risk of bias according to the Cochrane Risk of Bias tool is shown in eFigure 2 in Supplement 1.
End Point Analysis
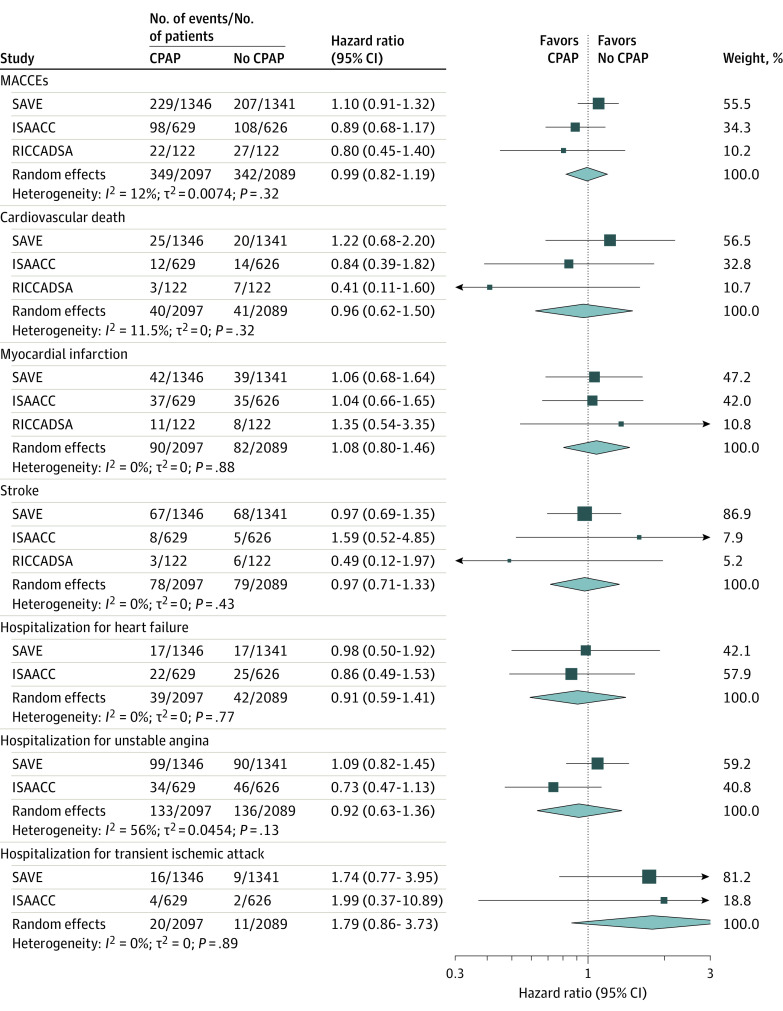
Among the 4186 patients, a total of 691 MACCEs (16.5%) were observed, distributed by groups as follows: 349 in the CPAP group and 342 in the no CPAP group. The intention-to-treat analysis using 1-stage analyses did not find statistically significant differences in the MACCEs between groups (1-stage HR of CPAP, 1.01 [95% CI, 0.87-1.17]; P = .94) (Figure 2A). We evaluated the evolution of CPAP adherence over the follow-up. CPAP adherence significantly decreased over the first 12 months and was subsequently maintained over the rest of the follow-up period (Figure 2B). A 2-stage analysis corroborated the result from the 1-stage analysis, and showed a nonstatistically significant I2 of 12% between studies (Figure 3). Additionally, the analysis for the individual components of the composite that defined the MACCEs revealed no significant effect of CPAP treatment (Figure 3; eTable 3 in Supplement 1) in 1-stage and 2-stage analyses. Moreover, in the subgroup analysis for the study subgroups based on OSA severity (apnea-hypopnea index), age, sex, hypertension status, blood pressure medication, and mean blood pressure at baseline, no statistically significant effects of CPAP randomization were identified (eFigure 3 in Supplement 1).
Figure 2. Cumulative Incidence of MACCEs and CPAP Adherence Over Time in the Individual Participant Data Meta-Analysis.
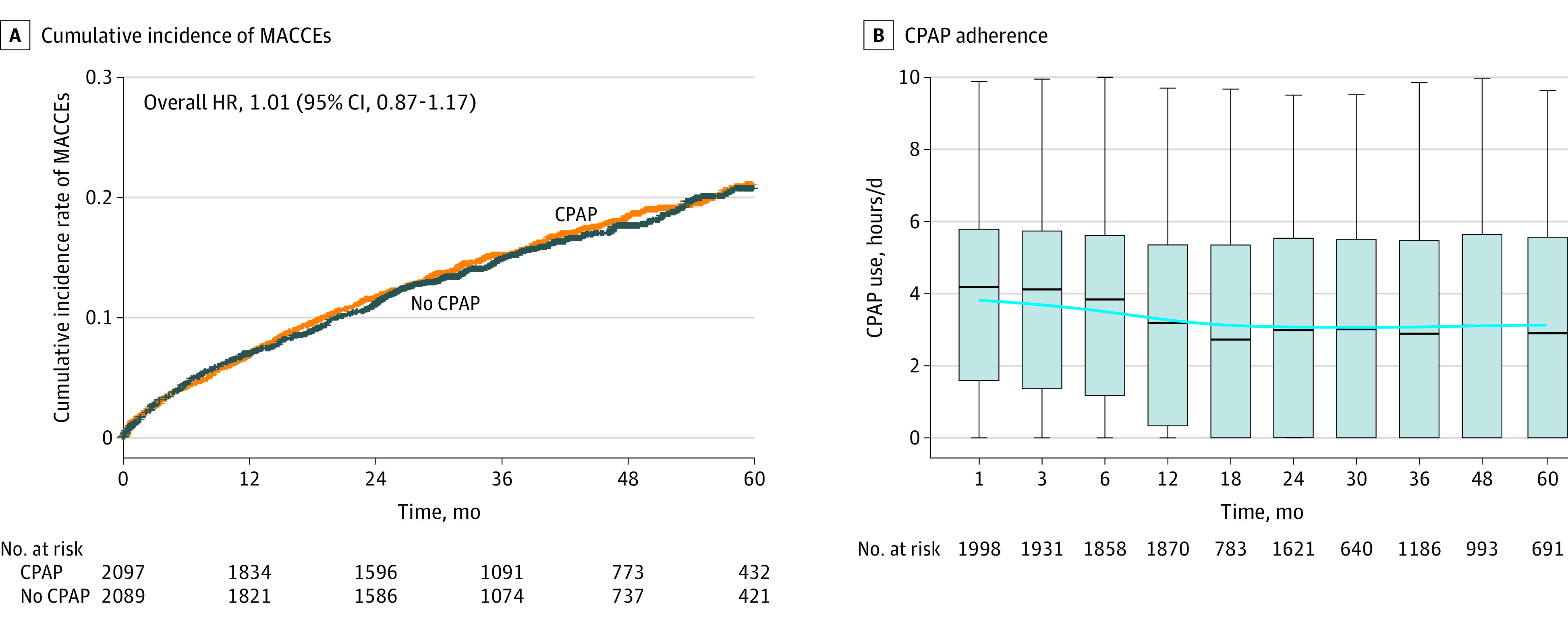
A, Cumulative incidence from the 1-stage individual participant data meta-analysis of a first primary major adverse cardiac and cerebrovascular events (MACCEs); a composite of cardiovascular death, nonfatal acute myocardial infarction, nonfatal stroke, hospital admission for heart failure, and new hospitalization for unstable angina or transient ischemic attack) in the group that received continuous positive airway pressure (CPAP) and in the group with no CPAP. Mixed-effects Cox proportional hazards model adjusted by clinical trial as random effects. The median follow-up time was 37 months (IQR, 24.1-56.2). B, CPAP adherence (hours/d) during follow-up. Box-and-whiskers plot showing median (IQR), 1.5 times the IQR. Blue smooth line over time was performed using generalized additive model.
Figure 3. Overall Hazard Ratio Forest Plot From the 2-Stage IPD Meta-Analysis for MACCEs and Individual Components.
Forest plot from the 2-stage individual participant data (IPD) meta-analysis showing the hazard ratio (95%CI) for the major adverse cardiac and cerebrovascular events (MACCEs) and for individual components of the MACCEs. Repeat revascularization was excluded because it was only included in the RICADSSA trial. The area of the squares is proportional to the study weight and diamonds represent pooled hazard ratios (95% CIs). The dashed line depicts a null effect. ISAACC indicates Impact of Sleep Apnea Syndrome in the Evolution of Acute Coronary Syndrome7; RICCADSA, Randomized Intervention With CPAP in Coronary Artery Disease and Sleep Apnea8; and SAVE, Sleep Apnea Cardiovascular Endpoints.6
In the CPAP group, the overall median adherence was 3.03 hours per day (IQR, 0.75-5.10), measured and downloaded by means of the machines’ internal clocks, and 38.5% of patients treated with CPAP had good adherence (mean adherence of ≥4 hours per day). On-treatment analysis adjusted by time-varying and baseline confounding factors using a marginal structural Cox model revealed a decreased event risk associated with good CPAP adherence (HR, 0.69 [95% CI, 0.52-0.92]; P = .01). A sensitivity analysis censoring information of follow-up periods after a change in adherence was performed. Baseline characteristics of the patients according to adherence groups were compared (eTable 4 in Supplement 1). This sensitivity analysis showed that good CPAP adherence was associated with a significant event risk reduction (HR, 0.55 [95% CI, 0.36-0.84]; P = .005).
Discussion
The results of this meta-analysis provide a rigorous basis for recommendations about the types of patients with OSA and established cardiovascular disease most likely to benefit from CPAP treatment and contribute to the wider debate about how patient heterogeneity influences treatment effectiveness. In this study, the first IPD meta-analysis of the effect of CPAP treatment on major cardiovascular and cerebrovascular adverse events in adults with cardiovascular disease is reported. This analysis revealed that adequate adherence to CPAP is associated with a reduction in the risk of cardiovascular recurrence. These novel results indicate the potential positive effect of CPAP-adherent treatment on secondary cardiovascular prevention.
Several well-conducted observational studies identified OSA as an independent risk factor for coronary disease, stroke, hypertension, cardiac arrythmia, atherosclerosis, and mortality.20,21,22 Moreover, observational studies have reported a decrease in the cumulative incidence of cardiovascular events in patients with severe OSA treated with CPAP compared with those with severe OSA not using CPAP.23,24 While numerous observational studies have indicated a close relationship between the severity of OSA and the incidence of cardiovascular events, together with a positive association of CPAP users with decreased cardiovascular episodes, interventional RCTs have not demonstrated a positive effect of allocation to OSA treatment with CPAP in secondary cardiovascular prevention. The main feature identified as being associated with the no effect of CPAP treatment on secondary cardiovascular prevention is poor adherence to treatment with CPAP. The hypothesis of this study was that treatment with CPAP would protect the patient only during the periods in which the treatment was in use. Such treatment would decrease the pathophysiological consequences associated with OSA, such as increases in sympathetic activity or mechanical stress in the vascular wall, which would increase plaque instability.7 Previous studies have reported that CPAP withdrawal leads to a rapid reoccurrence of OSA and is associated with impaired endothelial function, increased levels of urinary catecholamines, enhanced blood pressure, surges in heart rate,25,26 and cardiac dysrhythmias.27 Thus, this hypothesis is that the effect of treatment with CPAP is produced only during its use, implying that periods of nonadherence would be associated with the reestablishment of pathophysiological cardiovascular features of OSA. Thus, it could be argued that the effectiveness of treatment with CPAP in secondary cardiovascular prevention would be limited to adherent-treatment periods.
The availability of IPD provides greater statistical power for investigating interactions between treatments and covariates. In the present analysis, an inverse probability of treatment weighting analysis was performed to assess the effect of good adherence to CPAP that included the longitudinal evolution of CPAP adherence over the follow-up. The inverse probability of treatment weighting model revealed a positive effect of adherent CPAP treatment on the risk of recurrent cardiovascular events. This on-treatment model accounted for the fact that the weights are estimated rather than known with certainty. In this model, the weights of CPAP adherence during follow-up were computed together with the longitudinal changes in body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) and daytime sleepiness, well-known contributing risk factors for OSA. The results of this analysis suggest that good adherence to CPAP (mean adherence ≥4 hours per day) was associated with a significantly decreased risk of cardiovascular recurrence. Identification of the main contributors to CPAP adherence have been a topic of investigation. These studies identify aspects related to OSA symptoms (mainly daytime somnolence) and/or positive initial experiences with CPAP use.28,29 It has also been argued that the clinical setting of recruitment is a critical factor for CPAP adherence because patients who attend sleep clinics are commonly more symptomatic and present with snoring and witnessed apneas, though may not have symptoms of daytime somnolence.4 In contrast, participants in the SAVE, ISAACC, and RICCADSA (Randomized Intervention With CPAP in Coronary Artery Disease and Sleep Apnea) trials were recruited in stroke or cardiac clinics, presented a different profile, were older (mean of >10 years), had established cardiovascular disease at baseline, and had minimal knowledge regarding OSA. Based on the analysis of CPAP use patterns in the SAVE study, early CPAP adherence is most informative of subsequent CPAP adherence,30 although some indicators of disease severity (loud snoring and required level of CPAP pressure) are also predictors of better adherence. Additionally, increased BMI has been largely associated with increased severity of OSA. Changes in BMI are likely to be informative of potential changes in apnea-hypopnea index,31 as decrease in BMI may be associated with decreased apnea-hypopnea index, that could affect adherence to the treatment. Moreover, previous studies suggested that BMI changes could affect adherence to therapies32 and a potential relationship between CPAP use and BMI changes has been reported.33 BMI has therefore been proposed to be a driver of CPAP adherence. Finally, OSA symptoms, mainly daytime sleepiness, have been largely described as a major driver of CPAP adherence. Although patients included in the present study were nonsleepy, modest but potentially clinically significant changes in Epworth Sleepiness Scale scores were observed and could affect CPAP adherence. Adherence to treatment is complex to determine and there are other potential factors that could affect patient adherence, such as health education, motivation, attitude, self-efficacy, psychosocial factors, and other health care system–related features. The results of this study that identify adherence to treatment as the main contributor to the positive effect of CPAP should make implementation of specific and personalized actions that improve adherence to treatment a priority.
Limitations
This study had some limitations. First, this IPD meta-analysis evaluated CPAP as the criterion-standard treatment for OSA. Nevertheless, future studies including the effect of alternative treatments to OSA, such as mandibular advancement devices, hypoglossal nerve stimulation, or positional therapy, that have demonstrated efficacy should be performed.34
Second, the present study evaluated the effect of CPAP treatment on a composite cardiovascular end point that provides statistical efficiency and is supported by epidemiological and mechanistic studies of an association of OSA with cardiovascular outcomes.35 Nevertheless, there was insufficient statistical power to assess the on-treatment effect on secondary end points such as components of the composite outcome reported.
Third, the present study included a lower number of women, among whom OSA has been recently36 associated with an increased risk of cardiovascular recurrence and where it would be necessary to assess the potential positive effect of CPAP treatment in reducing the risk of cardiovascular recurrence.
Fourth, patients included in these trials were diagnosed with OSA mostly based on home sleep apnea tests, so this study may not apply to patients who underwent in-laboratory polysomnography.
Fifth, on-treatment analysis is not protected against selection bias because these types of studies are susceptible to this form of bias. It should be acknowledged that patients who were adherent to CPAP may have other differences in health status. The marginal structural model approach applied in this study was specifically designed to overcome selection bias. Moreover, a comparison of baseline characteristics was conducted for one of the analyses included in the study. The results did not show a profile with lower comorbidity in the good adherence group, suggesting a lack of association with “healthy users.” However, it should be noted that relying solely on these baseline variables is insufficient to address the potential selection bias inherent in this type of study. Additionally, other potential baseline and time-varying confounding factors were considered. The marginal structural model included baseline adjustments for age, sex, antihypertensive drugs, and trial study as well as time-varying BMI and daytime sleepiness. It is possible that there are additional factors that could be associated with adherence and other outcomes that were not measured in these studies. This model should be confirmed in future studies and additional confounding factors could be considered in the relationship between adherence to CPAP and cardiovascular risk.
Conclusions
Adherence to CPAP was associated with a reduction in the risk of cardiovascular event recurrence, suggesting that CPAP adherence is a key factor in secondary cardiovascular prevention in patients with OSA.
Educational Objective: To identify the key insights or developments described in this article.
-
The authors conducted an individual participant data meta-analysis of 3 trials to assess the potential value of continuous positive airway pressure (CPAP) for the prevention of cardiovascular events in patients with cardiovascular disease. Why did they choose to conduct an individual participant data meta-analysis rather than a traditional meta-analysis that uses aggregated data?
Examination of the individual data lengthens the potential follow-up interval and allows for longer-term outcome assessment.
Examining individual data renders imbalances in baseline characteristics and missing data statistically unimportant.
Individual data allow assessment of the intervention (CPAP) in clinically important subgroups.
-
What were the results with regard to major adverse cardiac or cerebrovascular events (MACCEs)?
Even marginal CPAP adherence (1 to <2 hours/day) was protective against MACCEs, though not as protective as better adherence (≥2 hours/day).
Only the group with good CPAP adherence (≥4 hours/day) demonstrated a statistically meaningful reduction in MACCEs.
The CPAP group experienced more MACCEs overall and in every subgroup analysis.
-
What do the authors suggest is an implication of their findings?
CPAP should not be offered to patients with characteristics that predict inconsistent use.
Implementation of specific and personalized actions to improve patient adherence to treatment should be prioritized.
The value of CPAP in secondary prevention of cardiovascular events is clinically very limited.
eMethods. Methodological Details Regarding Marginal Structural Models
eTable 1. Excluded Articles and Reason
eTable 2. Additional Trial Characteristics
eTable 3. One-Stage IPD Meta-Analysis for the Individual Components of the Primary Endpoint
eTable 4. Baseline Characteristics According to CPAP Compliance Groups in Sensitivity Analysis Population
eFigure 1. Direct Acyclic Graph of the Inverse Probability of Treatment Weighted Model to Assess the Effect of CPAP Adherence on the Risk of Recurrent Cardiovascular Event
eFigure 2. Risk of Bias Assessment: Assessments Regarding Each Risk of Bias Item for Each Randomized Clinical Trial Included
eAppendix. Methodological Details Regarding Literature Search Strategy
eReferences
Data Sharing Statement
References
- 1.Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687-698. doi: 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sánchez-de-la-Torre M, Campos-Rodriguez F, Barbé F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013;1(1):61-72. doi: 10.1016/S2213-2600(12)70051-6 [DOI] [PubMed] [Google Scholar]
- 3.Hu X, Fan J, Chen S, Yin Y, Zrenner B. The role of continuous positive airway pressure in blood pressure control for patients with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich). 2015;17(3):215-222. doi: 10.1111/jch.12472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. ; Spanish Sleep and Breathing Network . Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161-2168. doi: 10.1001/jama.2012.4366 [DOI] [PubMed] [Google Scholar]
- 5.Labarca G, Schmidt A, Dreyse J, et al. Efficacy of continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA) and resistant hypertension (RH): systematic review and meta-analysis. Sleep Med Rev. 2021;58:101446. doi: 10.1016/j.smrv.2021.101446 [DOI] [PubMed] [Google Scholar]
- 6.McEvoy RD, Antic NA, Heeley E, et al. ; SAVE Investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919-931. doi: 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 7.Sánchez-de-la-Torre M, Sánchez-de-la-Torre A, Bertran S, et al. ; Spanish Sleep Network . Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med. 2020;8(4):359-367. doi: 10.1016/S2213-2600(19)30271-1 [DOI] [PubMed] [Google Scholar]
- 8.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea: the RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613-620. doi: 10.1164/rccm.201601-0088OC [DOI] [PubMed] [Google Scholar]
- 9.Labarca G, Dreyse J, Drake L, Jorquera J, Barbe F. Efficacy of continuous positive airway pressure (CPAP) in the prevention of cardiovascular events in patients with obstructive sleep apnea: systematic review and meta-analysis. Sleep Med Rev. 2020;52:101312. doi: 10.1016/j.smrv.2020.101312 [DOI] [PubMed] [Google Scholar]
- 10.Stewart GB, Altman DG, Askie LM, Duley L, Simmonds MC, Stewart LA. Statistical analysis of individual participant data meta-analyses: a comparison of methods and recommendations for practice. PLoS One. 2012;7(10):e46042. doi: 10.1371/journal.pone.0046042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221 [DOI] [PubMed] [Google Scholar]
- 12.Stewart LA, Clarke M, Rovers M, et al. ; PRISMA-IPD Development Group . Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA. 2015;313(16):1657-1665. doi: 10.1001/jama.2015.3656 [DOI] [PubMed] [Google Scholar]
- 13.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479-504. doi: 10.5664/jcsm.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shemilt I, Aluko P, Graybill E, et al. ; Campbell and Cochrane Economics Methods Group . Economic evidence. In: Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Wiley; 2019:507-523. doi: 10.1002/9781119536604.ch20 [DOI] [Google Scholar]
- 15.Tierney JF, Vale C, Riley R, et al. Individual participant data (IPD) meta-analyses of randomised controlled trials: guidance on their use. PLoS Med. 2015;12(7):e1001855. doi: 10.1371/journal.pmed.1001855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burke DL, Ensor J, Riley RD. Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Stat Med. 2017;36(5):855-875. doi: 10.1002/sim.7141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 18.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 19.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2018. [Google Scholar]
- 20.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071-1078. [PMC free article] [PubMed] [Google Scholar]
- 21.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283(14):1829-1836. doi: 10.1001/jama.283.14.1829 [DOI] [PubMed] [Google Scholar]
- 22.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447-1451. doi: 10.1164/rccm.200505-702OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marin JM, Carrizo SJ, Vicente E, Agustí AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046-1053. doi: 10.1016/S0140-6736(05)71141-7 [DOI] [PubMed] [Google Scholar]
- 24.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-González C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156(2):115-122. doi: 10.7326/0003-4819-156-2-201201170-00006 [DOI] [PubMed] [Google Scholar]
- 25.Kohler M, Stoewhas AC, Ayers L, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011;184(10):1192-1199. doi: 10.1164/rccm.201106-0964OC [DOI] [PubMed] [Google Scholar]
- 26.Schwarz EI, Schlatzer C, Stehli J, et al. Effect of CPAP withdrawal on myocardial perfusion in OSA: a randomized controlled trial. Respirology. 2016;21(6):1126-1133. doi: 10.1111/resp.12798 [DOI] [PubMed] [Google Scholar]
- 27.Rossi VA, Stoewhas AC, Camen G, et al. The effects of continuous positive airway pressure therapy withdrawal on cardiac repolarization: data from a randomized controlled trial. Eur Heart J. 2012;33(17):2206-2212. doi: 10.1093/eurheartj/ehs073 [DOI] [PubMed] [Google Scholar]
- 28.Chai-Coetzer CL, Luo YM, Antic NA, et al. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. Sleep. 2013;36(12):1929-1937. doi: 10.5665/sleep.3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehrtash M, Bakker JP, Ayas N. Predictors of continuous positive airway pressure adherence in patients with obstructive sleep apnea. Lung. 2019;197(2):115-121. doi: 10.1007/s00408-018-00193-1 [DOI] [PubMed] [Google Scholar]
- 30.Van Ryswyk E, Anderson CS, Antic NA, et al. Predictors of long-term adherence to continuous positive airway pressure in patients with obstructive sleep apnea and cardiovascular disease. Sleep. 2019;42(10):zsz152. doi: 10.1093/sleep/zsz152 [DOI] [PubMed] [Google Scholar]
- 31.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015-3021. doi: 10.1001/jama.284.23.3015 [DOI] [PubMed] [Google Scholar]
- 32.Boye KS, Shinde S, Kennedy-Martin T, Robinson S, Thieu VT. Weight change and the association with adherence and persistence to diabetes therapy: a narrative review. Patient Prefer Adherence. 2022;16:23-39. doi: 10.2147/PPA.S328583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Benseñor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70(3):258-264. doi: 10.1136/thoraxjnl-2014-205361 [DOI] [PubMed] [Google Scholar]
- 34.Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314(21):2280-2293. doi: 10.1001/jama.2015.16303 [DOI] [PubMed] [Google Scholar]
- 35.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing: In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118(10):1080-1111. doi: 10.1161/CIRCULATIONAHA.107.189375 [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Fan J, Guo R, et al. Association of obstructive sleep apnoea with cardiovascular events in women and men with acute coronary syndrome. Eur Respir J. 2023;61(1):2201110. doi: 10.1183/13993003.01110-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Methodological Details Regarding Marginal Structural Models
eTable 1. Excluded Articles and Reason
eTable 2. Additional Trial Characteristics
eTable 3. One-Stage IPD Meta-Analysis for the Individual Components of the Primary Endpoint
eTable 4. Baseline Characteristics According to CPAP Compliance Groups in Sensitivity Analysis Population
eFigure 1. Direct Acyclic Graph of the Inverse Probability of Treatment Weighted Model to Assess the Effect of CPAP Adherence on the Risk of Recurrent Cardiovascular Event
eFigure 2. Risk of Bias Assessment: Assessments Regarding Each Risk of Bias Item for Each Randomized Clinical Trial Included
eAppendix. Methodological Details Regarding Literature Search Strategy
eReferences
Data Sharing Statement