Abstract
Prosthetic joint infection (PJI) is one of the most devastating complications for a patient following arthroplasty.
This scoping review aims to evaluate the burden of PJI on individual patients and the healthcare system regarding the mortality rate, patient-reported quality of life, and healthcare resource utilisation.
Patients with PJI have up to a five-fold higher mortality rate than those who have undergone an uninfected primary arthroplasty. There is an increased use of ambulatory aids and reduced joint function scores in patients with PJI. Global quality of life is poorer, specifically measured by the EQ-5D. Direct hospitalisation costs are two- to five-fold higher, attributed to surgery and prostheses, antibiotics, and a prolonged inpatient stay.
There is an immense clinical and health economic burden secondary to PJI worldwide. This is expected to rise exponentially due to the increasing number of primary procedures and an ageing population with comorbidities
Improving preventative and treatment strategies is imperative for patients and the healthcare system.
Keywords: prosthetic joint infection, arthroplasty, patient-reported outcome measure, health economic
Introduction
Prosthetic joint infection (PJI) is one of the most devastating post-surgical complications for a patient undergoing a total joint arthroplasty (TJA). As one of the leading causes of joint replacement failure, PJI causes implant loosening, tissue necrosis, and eventual loss of limb function, often leading to physical and emotional distress in patients (1). Patients with PJI often have prolonged hospital stays and require multiple reoperations (2). PJI can have long-term negative impacts on patients’ quality of life (QoL), even following successful clearance of the infection (3, 4, 5). Chronic infections may require multiple revision operations, and failure to control infection can lead to the need for joint fusion and even amputation (6).
Clinical management of PJI is costly. These patients require complex management by a multidisciplinary team with additional surgeries and prolonged antibiotic therapy. The delivery of this care results in direct costs of approximately US$46 000 in developed countries (United States and Australia) (7, 8).
The incidence of PJI is expected to increase substantially over the next few decades because of the ageing populations and changing demographic characteristics, including rates of obesity. The absolute number of total knee arthroplasties (TKAs) and total hip arthroplasties (THAs) performed for osteoarthritis annually in Australia is expected to be more than double by 2030 (9). Similarly, Kurtz et al. (10, 11) reported a gradually increasing incidence of PJI from 2.09% to 2.18% and predicted the absolute growth in the US to be 673% for TKA and 174% for THA from 2005 to 2030. With the growing demand for elective orthopaedic procedures and an increasing incidence, PJI cannot be ignored due to its significant clinical and economic impact. The aim of this scoping review is to outline the clinical outcomes (morbidity, mortality, QoL measures) and healthcare-associated costs of prosthetic joint infection globally.
Methods
Protocol and registration
The study protocol for this scoping review was drafted following Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines. The final version was registered on the Open Science Framework (https://osf.io/aus5f/).
Eligibility criteria
This review considered peer-reviewed studies published in English without time constraints. Included papers cited a validated diagnostic criterion for PJI (Centers for Disease Control and Prevention (CDC), Musculoskeletal Infection Society (MSIS), IDSA, International Classification of Disease (ICD) codes, and the European Bone and Joint Infection Society (EBJIS)) or cited a validated definition published by another author. However, these diagnoses may be impacted by the subjectivity of clinicians, which was infeasible to adjust for in this review. Included studies reported at least one of the following main outcomes: mortality rate, patient-reported QoL, or the economic impact of PJI such as direct and indirect costs.
Search strategy
The search strategy explored health-related outcomes and the economic impact of PJI in comparison to outcomes of uninfected arthroplasty. A search was conducted across the Ovid Medline, and Ovid Embase databases, using expanded keywords and index terms. Citation tracking of included studies was conducted to identify additional eligible studies.
Study selection
Two researchers (YX, TH) independently screened the titles and abstracts and assessed full texts for inclusion against the eligibility criteria on Covidence (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia). Disagreements were resolved through discussion between them. Where agreement could not be reached, the senior author (PFMC) was consulted.
Data extraction
The data collection process was performed by two independent researchers (YX, TH). An electronic data extraction sheet (Microsoft Excel; Redmond, WA, USA) was refined during the data extraction process. The key variables collected were author, publication year, country, sample size, surgery type, infection type, infection prevalence, and further outcomes of interest including mortality rate, cause of mortality, patient-reported outcomes, QoL assessment tools, cost perspective, total cost, and length of stay, all evaluated against an uninfected cohort.
Result synthesis
Studies were grouped according to the outcomes addressed. Health-related QoL and cost of care are largely reported in narrative and tabular forms. Health-related QoL measures include EuroQol Group 5 Dimension (EQ-5D),12 or 36 Short Form health survey (SF-12, SF-36). Joint pain and function scores include ambulatory outcomes, Oxford Hip Score (OHS), Oxford Knee Score (OKS), Harris Hip Score (HHS), or Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC).
Results
Search result
A total of 2073 records were identified using the search strategy; of these, 142 full texts were examined, and 30 articles were included in the review (Fig. 1).
Figure 1.
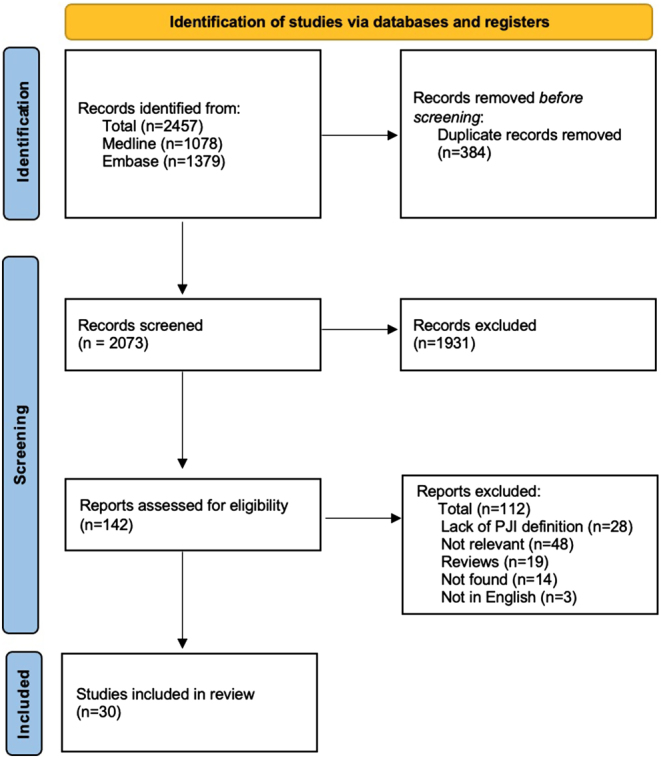
PRIMSA flow chart.
Among 30 eligible studies, 27 (90%) were conducted in high-income countries with the United States predominating (n = 12, 40%). The knee and hip were the most frequently studied joints with PJI (n = 20, 67%). Nine of the 30 studies addressed mortality, 8 investigated patient-reported outcomes, while 18 studied the economic impact (Supplementary Table S1 (see section on supplementary materials given at the end of this article)).
Mortality in septic revision, aseptic revision, and primary arthroplasty
Nine studies compared the mortality rate between patients with PJI, treated either medically or surgically, and an uninfected cohort who either had primary arthroplasty only or with aseptic revision (Supplementary Table S2). Mortality was highest in PJI, followed by aseptic revision, and lowest in primary arthroplasty without infection or revision. Compared with primary arthroplasty, the mortality risk for PJI at 1 year adjusted (for age, sex, and medical comorbidities) was 2.18. Compared with aseptic revision, the adjusted mortality risk for PJI at 1 year was 1.87 (12), whereas in the study by Boddapati et al. (13), no difference in mortality was found at 30 days. In the study by Zmistowski et al. (14), mortality in the knee and hip PJI was higher than in aseptic revision arthroplasty at any assessed time point, and it remained higher with time from a 90-day mortality of 3.7% (vs0.8%) to a 5-year mortality of 25.9% (vs 12.9%). All-cause 10-year mortality rate was notably higher in PJI patients, with an incidence of 45% compared to 29% in those without infection (3). However, the direct contribution of PJI to mortality was not provided by these studies.
Patient-reported outcome measures
Intergroup comparison in health-related quality of life
Seven of the eight studies assessed patients’ health-related QoL using a validated questionnaire (EQ-5D, SF-12, or SF-36). The most frequently used questionnaire was EQ-5D (4/8) (3, 5, 15, 16), followed by SF-12 (3/8) (17, 18, 19) and SF-36 (1/8) (5) (Supplementary Tables S3 and S4). Patients with PJI scored significantly more poorly on the EQ-5D questionnaire compared to uninfected patients in all studies. However, the three studies that used SF-12 reported no significant differences between the cohorts. Walter et al. (5) was the only study that used SF-36, and it reported a lower physical component score in patients with PJI at a median of 5 years following revision surgery. They also reported that 33.3% of PJI patients exceeded the threshold limit for at least one of the five psychiatric syndromes using the International Classification of Disease (ICD)-10-based symptom rating (ISR) (Table 1). The two studies conducted by Aboltins et al. (17, 18) noted no significant differences in the rates of improvement in SF-12 scores between the two cohorts.
Table 1.
Patient-reported QoL outcomes in each study.
| Reference | FUD (years) | QoL outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Infected cohort | Uninfected cohort | ||||||||
| EQ-5D | EQ-VAS | SF-12 | SF-36 | EQ-5D | EQ-VAS | SF-12 | SF-36 | ||
| Wildeman et al. (3) | 11* | 0.83 | 65† | 0.94 | 80† | ||||
| Poulsen et al. (15) | 8.2* | 0.85 | |||||||
| Non-reinfected group | 0.71 | 60.73 (ns) | |||||||
| Re-infected group | 0.6 | 50.64 (ns) | |||||||
| Hotchen et al. (16) | 1 | 0.782 | 77.9 | ||||||
| Uncomplicated PJI | 0.730 (ns) | 79.4 (ns) | |||||||
| Complex PJI | 0.515 | 68.4 | |||||||
| Limited options PJI | 0.333 | 60.2 | |||||||
| Romano et al. (19) | 4* | ||||||||
| PCS | 35.6 (ns) | 32.2 (ns) | |||||||
| MCS | 43.1 (ns) | 48.7 (ns) | |||||||
| Aboltins et al. (17) | 1 | ||||||||
| PCS | +10.9* (ns) | +15.7* (ns) | |||||||
| MCS | + ns* | +* ns | |||||||
| Aboltins et al. (18) | 1 | ||||||||
| PCS | +11.3* (ns) | +12.8* (ns) | |||||||
| MCS | +2.9* (ns) | +4.2* (ns) | |||||||
| Walter et al. (5) | 4.9* | 0.55 | 52.14 | 0.838 | 68.6 | ||||
| PCS | 24.82 | 48.36 | |||||||
| MCS | 46.16 (ns) | 50.87 (ns) | |||||||
| Mur et al. (20) | 1‡ | N/A | N/A | ||||||
*Mean values.
†Median value.
‡Minimum.
FUD, follow-up duration; MCS, mental component scores; PCS, physical component scores.
Intergroup comparison in joint pain and function
Six of the eight studies (3, 15, 16, 17, 19, 20) assessed the patients’ post-surgical joint pain and function using an appropriate joint function tool (ambulatory outcomes, OHS, OKS, HHS, or WOMAC). Wildeman et al. (3) and Mur et al. (20) reported a greater proportion of PJI patients requiring ambulatory aids and assisted living relative to uninfected patients (Table 2). Wildeman et al. (3) identified a worse OHS score in PJI patients. Aboltins et al. (17) noted lower HHS improvements in PJI patients at 1-year follow-up, but there were no significant differences in HHS between the two patient cohorts at 4 years. Romano et al. (19) were unable to identify any significant differences in the WOMAC score between the two cohorts.
Table 2.
Patient-reported pain and function outcomes in the included studies.
| Reference | Pain and function outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Infected cohort | Uninfected cohort | ||||||||
| AO | OHS | OKS | HHS | WOMAC | AO | OHS | HHS | WOMAC | |
| Wildeman et al. (3) | 36 | 44 | |||||||
| Assisted Living | 21% | 12% | |||||||
| Ambulatory aid | 65% | 42% | |||||||
| Poulsen et al. (15) | |||||||||
| Non-reinfected group | 31.15 (ns) | ||||||||
| Re-infected group | 23.22 (ns) | ||||||||
| Hotchen et al. (16) | |||||||||
| Uncomplicated PJI | 40.2 | 33.0 | |||||||
| Complex PJI | 32.3 | 22.9 | |||||||
| Limited options PJI | 19.5 | 23.7 | |||||||
| Romano et al. (19) | 71.2 (ns) | 74 (ns) | |||||||
| Function | 76.6 (ns) | 66.2 (ns) | |||||||
| Pain | 77.4 (ns) | 75.8 (ns) | |||||||
| Stiffness | 71.4 (ns) | 70.1 (ns) | |||||||
| Aboltins et al. (17) | +35.9* | +44.6* | |||||||
| Mur et al. (20) | |||||||||
| Requires 1 or 2 crutches | |||||||||
| TKA | 42.1% | 22.4% | |||||||
| THA | 63.4% | 25.6% | |||||||
*Mean values.
AO, ambulatory outcomes; ns, non-significant.
Length of stay
Lengths of hospitalisation were reported in 12 studies (Table 3). With the exception of Kapadia et al. (21), all studies reported a two-fold or longer hospitalisation period for PJI patients compared to uninfected patients. Two studies (22, 23) from Canada reported five- to ten-fold longer periods of hospitalisation for patients with PJI. Studies (11, 24, 25, 26) from the United States reported shorter lengths of hospitalisation for both patient cohorts relative to studies from other countries.
Table 3.
Overview of reported length of hospitalisation.
| Study | Country | Sample size (n) | Length of hospitalisation (days) | ||
|---|---|---|---|---|---|
| IFC | UIFC | IFC | UIFC | ||
| Morcos et al. (23) | Canada | 73 | 73 | 22.7 | 3.8 |
| Akindolire et al. (22) | Canada | 50 | 50 | 26.5 | 2.0 |
| Kasch et al. (30) | Germany | 35 | 71 | 34.0 | 15.0 |
| Puhto et al. (31) | Finland | 42 | 16.0 | ||
| Primary arthroplasty | 1708 | 4.0 | |||
| Aseptic revision | 18 | 14.0 | |||
| Peel et al. (7) | Australia | 21 | 42 | 31.6 | 7.9 |
| Alp et al. (27) | Turkey | 16 | 654 | 49.0 | 7.0 |
| Iqbal et al. (28) | Pakistan | 27 | 27 | 11.0 | 5.0 |
| Kapadia et al. (24) | USA | 21 | 21 | 5.3 | 3.0 |
| Brochin et al. (25) | USA | 70 011* | 395 198* | 6.0 | 3.0 |
| Kapadia et al. (29) | USA | 16 | 32 | 7.6 | 3.3 |
| Kurtz et al. (11) | USA | NIS data* | NIS data* | ||
| Hip | |||||
| 1990 | 27.5 | 10.3 | |||
| 2004 | 9.2 | 4.3 | |||
| Knee | |||||
| 1990 | 23.5 | 9.7 | |||
| 2004 | 8.8 | 3.9 | |||
| Padegimas et al. (26) | USA | 808* | 81690* | 4.0 | 2.0 |
*Data derived from the United States National Inpatient Sample (NIS) database.
IFC, infected cohort; UIFC, uninfected cohort.
Hospital resource use of PJI
Fourteen studies reported the hospital resource use cost of PJI as compared to the total cost for the uninfected cohort (7, 8, 11, 19, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31) (Supplementary Table S5). All focused on the direct cost incurred during in-hospital management. Total hospital charges commonly comprised operating room services (prosthesis, procedure cost, surgical supplies, etc.), hospital bed charge, medications, diagnostic tests, ICU stay, and physiotherapy. In general, the total cost for patients with hip PJI was remarkably higher, incurring a total cost 2–5.6 times the total cost for the uninfected cohort, regardless of whether they underwent primary or revision arthroplasties. Similarly, treating knee PJI caused a 1.6- to 4-fold increase. Shoulder PJI treatment was more costly than primary shoulder arthroplasty and hemiarthroplasty, but less costly than reverse shoulder arthroplasty (26). Indirect societal costs, including productivity loss and wage loss, were not reported in any of the reviewed studies.
Projected cost
Four studies estimated the future economic burden of PJI on the healthcare system. Chang et al. (32) estimated the hospital costs for PJI in Taiwan based on the average cost between 2006 and 2013. PJI costs were predicted to increase from NT$117M in 2014 to NT$569M in 2035 (US$3.8 to US$18.6 based on the current conversion rate). The number of PJIs was projected to be 5944 in Korea in 2030, leading to an increase in the total annual cost from US$18.0 million in 2018 to exceed US$57.0 million by 2030 (33). In the United States, the total annual number of hip and knee PJIs was estimated to be 25 928 and 40 096, respectively, in 2030, resulting in a combined annual cost of US$1.85 billion (34). All these estimates confirm the enormous magnitude of the challenge faced worldwide.
Discussion
The key findings of this review were that patients with PJI scored significantly lower compared to uninfected patients on the EQ-5D and SF-36 questionnaires following recovery and generally required greater ambulatory assistance. PJI incurred a greater hospital expenditure, and the patients with PJI experienced longer lengths of hospitalisation across all studies.
Mortality
Suffering a PJI seems to be associated with a higher mortality rate compared to the uninfected cohort. Polymicrobial infection was a significant independent predictor of mortality in PJI (12, 13, 35, 36). Other studies found Gram-negative organisms and enterococci resulted in a two- or three-fold increase, respectively, in the 1-year mortality risk (18). As causative pathogens of PJI were not the focus of this review, no conclusion can be drawn concerning the association between certain organisms and mortality risks.
Quality of life outcomes
Individual studies revealed that PJI tended to inflict negative impacts on QoL outcomes, but it was difficult to compare between studies given the heterogenicity of study populations, surgery types (one-stage revision, two-stage revision, debridement, antibiotics and implant retention (DAIR)), and surgery numbers. Participants between studies varied in age, co-morbidities, and reasons for surgery. The contribution of each surgery type to the clinical outcomes also remained uncertain in this review, although it was previously argued that DAIR could be a successful alternative to two-stage revision for knee PJI within 2 weeks of onset (37). Additionally, some studies did not properly match the infected and uninfected cohorts. For example, Walter et al. (5) matched only age between the two cohorts. Other important confounding factors such as sex, comorbidities, and BMI were not considered. Comorbidities were also overlooked in the study by Mur et al. (20). This reduces the quality of their findings. Propensity score matching can be a suitable approach in retrospective studies to minimise these confounding effects, as performed by Wildeman et al. (3, 38). An increase in properly powered, prospective multi-centre studies is required to produce more generalisable and accurate results.
The use of different QoL assessment tools may also influence the patient-reported outcomes. PJI patients reported significantly lower general QoL in studies that used EQ-5D but not SF-12. This may be because the EQ-5D surveys were generally conducted at much longer follow-ups compared to SF-12 and SF-36 in included studies. The discrepancies between the two questionnaires could also reflect the poor discriminatory validity of both tools in assessing patients with chronic joint pain (39). It is nevertheless tempting to speculate that the EQ-5D may provide superior validity in assessing the general health of PJI patients, as four of its subdomains (self-care, mobility, activities, and anxiety/depression) arguably have cross-over with tools that did report significant differences in the other studies. For example, Wildeman et al. (3) and Mur et al. (20) both reported increased requirements for ambulatory and living assistance in PJI patients, with clear implications on the self-care, mobility, and activities subdomains of EQ-5D. The subdomains in SF-12 do not appear to have the same level of cross-over potential.
Length of stay
PJI patients had a longer in-patient stay compared to uninfected patients, which is consistent with the higher hospital costs in PJI management. Although longer hospitalisations are known to be correlated with costs, the extent of PJI as the main cause of this increased expenditure was difficult to disentangle from the studies included in this review. Despite studies in the United States reporting slightly shorter hospital stays compared to studies conducted in the other countries, they had the highest PJI management expenditure. This is consistent with the exceptionally high cost of healthcare in the United States that has been observed and discussed by other authors (40).
Hospital resource use cost
Many of the reviewed studies evaluating the costs associated with PJI have small sample sizes and employ various approaches to estimating costs, which resulted in a wide range of findings presented. Data from the United States National Inpatient Sample (NIS) database did not include other resource use such as the surgeon’s and physician’s fees or costs of further rehabilitation, and studies that used NIS data or did not consider indirect costs likely underestimated the episode of care costs (26). Studies may also benefit from specifying patient out-of-pocket costs vsgovernment or other payer-funded burden as knowing how PJI management is funded may help explain whether PJI is associated with the greater economic burden on the healthcare system, PJI patients, or both.
Strengths and limitations
Although arthroplasty is considered highly successful and cost-effective (41), uncommon but detrimental complications such as PJI can cause an enormous burden on patients and health systems. These results provide further insight into the major health-related and economic burden of PJI that is rising with the increasing volume of primary arthroplasty. This review demonstrates the tremendous societal burden of PJI and identifies knowledge gaps for future studies, yet it has several limitations. First, it was difficult to directly compare costs between analyses conducted in different countries due to the heterogeneity of currencies used and the different periods of time involved. Second, variations in surgical treatment for PJI between studies could have influenced the outcomes reported here in an unpredictable manner. Mortality rates associated with these procedures vary slightly, and this variability may not be fully attributable to PJI but instead to multiple other factors affecting the surgical outcome, such as the number and duration of procedures. Studies with no appropriately matched cohorts would not have considered pre-existing conditions such as immune deficiency, which have deleterious effects on patients’ recovery from infection and subsequently affect their QoL and mortality risk. Given the nature of a scoping review, none of these additional variables were adjusted or analysed. Finally, the findings in this review may not be generalisable to other complications or procedures.
Conclusion
In this scoping review, there were a relatively lower number of identified studies that reported QoL outcomes and mortality rates, compared to costs of PJI. Nevertheless, the review revealed that PJI is associated with an enormous humanistic burden due to its higher mortality risk and negative impacts on QoL and joint function. With the increasing volume of elective arthroplasty, PJI also inflicted a growing economic burden on healthcare systems via immense hospital resource use including hospital stay charges and operation costs. Future comparison of PJI-related clinical and economic impacts in different arthroplasty procedures would assist in further exploration of new knowledge.
Supplementary Materials
ICMJE Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding statement
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author contribution statement
Y Xu, PFM Choong, and MA Schuetz developed and formulated the research goals. Y Xu and TB Huang developed the research methodology. Y Xu and TB Huang conducted screening, data extraction, and wrote the initial manuscript. The ICARAUS group managed and coordinated the research activity and reviewed and edited the manuscript. PFM Choong and MA Schuetz supervised the research execution.
Acknowledgement
We thank Patrick Condron, senior librarian, from Melbourne Medical School, University of Melbourne, for assisting the search strategy design. ICARAUS group: The following are the members of ICARAUS (Infection Collaboration in trAuma, oRthopAedics and bUrnS): Steven M McPhail PhD, Andrew L Foster, Silvia Manzanero PhD, Michelle M Dowsey MEpi, PhD, Sina Babazadeh MBBS, PhD, Andrej Trampuz, Kevin D Tetsworth MD, FRACS.
References
- 1.Li C Renz N & Trampuz A. Management of periprosthetic joint infection. Hip Pelvis 201830138–146. ( 10.5371/hp.2018.30.3.138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peel TN Cheng AC Lorenzo YP Kong DCM Buising KL & Choong PFM. Factors influencing the cost of prosthetic joint infection treatment. Journal of Hospital Infection 201385213–219. ( 10.1016/j.jhin.2013.07.012) [DOI] [PubMed] [Google Scholar]
- 3.Wildeman P Rolfson O Soderquist B Wretenberg P & Lindgren V. What are the long-term outcomes of mortality, quality of life, and hip function after prosthetic joint infection of the hip? A 10-year Follow-up from Sweden. Clinical Orthopaedics and Related Research 20214792203–2213. ( 10.1097/CORR.0000000000001838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helwig P Morlock J Oberst M Hauschild O Hubner J Borde J Sudkamp NP & Konstantinidis L. Periprosthetic joint infection: effect on quality of life. International Orthopaedics 2014381077–1081. ( 10.1007/s00264-013-2265-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walter N Rupp M Hierl K Koch M Kerschbaum M Worlicek M & Alt V. Long-term patient-related quality of life after knee periprosthetic joint infection. Journal of Clinical Medicine 2021101–9. ( 10.3390/jcm10050907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hungerer S Kiechle M von Rüden C Militz M Beitzel K & Morgenstern M. Knee arthrodesis versus above-the-knee amputation after septic failure of revision total knee arthroplasty: comparison of functional outcome and complication rates. BMC Musculoskeletal Disorders 201718443. ( 10.1186/s12891-017-1806-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peel TN Dowsey MM Buising KL Liew D & Choong PFM. Cost analysis of debridement and retention for management of prosthetic joint infection. Clinical Microbiology and Infection 201319181–186. ( 10.1111/j.1469-0691.2011.03758.x) [DOI] [PubMed] [Google Scholar]
- 8.Stambough JB Nam D Warren DK Keeney JA Clohisy JC Barrack RL & Nunley RM. Decreased hospital costs and surgical site infection incidence with a universal decolonization protocol in primary total joint arthroplasty. Journal of Arthroplasty 201732728–734.e1. ( 10.1016/j.arth.2016.09.041) [DOI] [PubMed] [Google Scholar]
- 9.Ackerman IN Bohensky MA Zomer E Tacey M Gorelik A Brand CA & de Steiger R. The projected burden of primary total knee and hip replacement for osteoarthritis in Australia to the year 2030. BMC Musculoskeletal Disorders 20192090. ( 10.1186/s12891-019-2411-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtz SM Lau E Watson H Schmier JK & Parvizi J. Economic burden of periprosthetic joint infection in the united states. Journal of Arthroplasty 201227(8) 61–5.e1. ( 10.1016/j.arth.2012.02.022) [DOI] [PubMed] [Google Scholar]
- 11.Kurtz SM Lau E Schmier J Ong KL Zhao K & Parvizi J. Infection burden for hip and knee arthroplasty in the United States. Journal of Arthroplasty 200823984–991. ( 10.1016/j.arth.2007.10.017) [DOI] [PubMed] [Google Scholar]
- 12.Gundtoft PH Pedersen AB Varnum C & Overgaard S. Increased mortality after prosthetic joint infection in primary THA. Clinical Orthopaedics and Related Research 20174752623–2631. ( 10.1007/s11999-017-5289-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boddapati V Fu MC Mayman DJ Su EP Sculco PK & McLawhorn AS. Revision total knee arthroplasty for periprosthetic joint infection is associated with increased postoperative morbidity and mortality relative to noninfectious revisions. Journal of Arthroplasty 201833521–526. ( 10.1016/j.arth.2017.09.021) [DOI] [PubMed] [Google Scholar]
- 14.Zmistowski B Karam JA Durinka JB Casper DS & Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. Journal of Bone and Joint Surgery. American Volume 2013952177–2184. ( 10.2106/JBJS.L.00789) [DOI] [PubMed] [Google Scholar]
- 15.Poulsen NR, Mechlenburg I, Soballe K, Troelsen A, Lange J, Troelsen A, Zawadski A, Kjersgaard AG, Heine C, Riis J, et al. Improved patient-reported quality of life and hip function after cementless 1-stage revision of chronic periprosthetic hip joint infection. Journal of Arthroplasty 2019342763–2769.e1. ( 10.1016/j.arth.2019.06.010) [DOI] [PubMed] [Google Scholar]
- 16.Hotchen AJ Wismayer MG Robertson-Waters E McDonnell SM Kendrick B Taylor A Alvand A & McNally M. The Joint-Specific BACH classification: a predictor of outcome in prosthetic joint infection. EClinicalmedicine 202142101192. ( 10.1016/j.eclinm.2021.101192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aboltins C Dowsey MM Peel T Lim WK Parikh S Stanley P & Choong PF. Early prosthetic hip joint infection treated with debridement, prosthesis retention and biofilm-active antibiotics: functional outcomes, quality of life and complications. Internal Medicine Journal 201343810–815. ( 10.1111/imj.12174) [DOI] [PubMed] [Google Scholar]
- 18.Aboltins C Dowsey M Peel T Lim WK & Choong P. Good quality of life outcomes after treatment of prosthetic joint infection with debridement and prosthesis retention. Journal of Orthopaedic Research 201634898–902. ( 10.1002/jor.23089) [DOI] [PubMed] [Google Scholar]
- 19.Romano CL Romano D Logoluso N & Meani E. Septic versus aseptic hip revision: how different? Journal of Orthopaedics and Traumatology: Official Journal of the Italian Society of Orthopaedics and Traumatology 201011167–174. ( 10.1007/s10195-010-0106-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mur I Jordan M Rivera A Pomar V Gonzalez JC Lopez-Contreras J Crusi X Navarro F Gurgui M & Benito N. Do prosthetic joint infections worsen the functional ambulatory outcome of patients with joint replacements? A retrospective matched cohort study. Antibiotics 202091–15. ( 10.3390/antibiotics9120872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapadia BH Johnson AJ Issa K & Mont MA. Economic evaluation of chlorhexidine cloths on healthcare costs due to surgical site infections following total knee arthroplasty. Journal of Arthroplasty 2013281061–1065. ( 10.1016/j.arth.2013.02.026) [DOI] [PubMed] [Google Scholar]
- 22.Akindolire J Morcos MW Marsh JD Howard JL Lanting BA & Vasarhelyi EM. The economic impact of periprosthetic infection in total hip arthroplasty. Canadian Journal of Surgery. Journal Canadien de Chirurgie 202063E52–E56. ( 10.1503/cjs.004219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morcos MW Kooner P Marsh J Howard J Lanting B & Vasarhelyi E. The economic impact of periprosthetic infection in total knee arthroplasty. Canadian Journal of Surgery. Journal Canadien de Chirurgie 202164E144–E148. ( 10.1503/cjs.012519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapadia BH McElroy MJ Issa K Johnson AJ Bozic KJ & Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. Journal of Arthroplasty 201429929–932. ( 10.1016/j.arth.2013.09.017) [DOI] [PubMed] [Google Scholar]
- 25.Brochin RL Phan K Poeran J Zubizarreta N Galatz LM & Moucha CS. Trends in periprosthetic hip infection and associated costs: a population-based study assessing the impact of hospital factors using national data. Journal of Arthroplasty 201833(7S) S233–S238. ( 10.1016/j.arth.2018.02.062) [DOI] [PubMed] [Google Scholar]
- 26.Padegimas EM Maltenfort M Ramsey ML Williams GR Parvizi J & Namdari S. Periprosthetic shoulder infection in the United States: incidence and economic burden. Journal of Shoulder and Elbow Surgery 201524741–746. ( 10.1016/j.jse.2014.11.044) [DOI] [PubMed] [Google Scholar]
- 27.Alp E Cevahir F Ersoy S & Guney A. Incidence and economic burden of prosthetic joint infections in a university hospital: a report from a middle-income country. Journal of Infection and Public Health 20169494–498. ( 10.1016/j.jiph.2015.12.014) [DOI] [PubMed] [Google Scholar]
- 28.Iqbal F, Shafiq B, Noor SS, Ali Z, Memon N & Memon N. Economic burden of periprosthetic joint infection following primary total knee replacement in a developing country. Clinics in Orthopedic Surgery 202012470–476. ( 10.4055/cios20037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapadia BH Banerjee S Cherian JJ Bozic KJ & Mont MA. The economic impact of periprosthetic infections after total hip arthroplasty at a specialized tertiary-Care Center. Journal of Arthroplasty 2016311422–1426. ( 10.1016/j.arth.2016.01.021) [DOI] [PubMed] [Google Scholar]
- 30.Kasch R Merk S Assmann G Lahm A Napp M Merk H & Flessa S. Comparative analysis of direct hospital care costs between aseptic and two-stage septic knee revision. PLoS One 201712e0169558. ( 10.1371/journal.pone.0169558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puhto T Puhto AP Vielma M & Syrjala H. Infection triples the cost of a primary joint arthroplasty. Infectious Diseases 201951348–355. ( 10.1080/23744235.2019.1572219) [DOI] [PubMed] [Google Scholar]
- 32.Chang CH Lee SH Lin YC Wang YC Chang CJ & Hsieh PH. Increased periprosthetic hip and knee infection projected from 2014 to 2035 in Taiwan. Journal of Infection and Public Health 2020131768–1773. ( 10.1016/j.jiph.2020.04.014) [DOI] [PubMed] [Google Scholar]
- 33.Kim HS Park JW Moon SY Lee YK Ha YC & Koo KH. Current and future burden of periprosthetic joint infection from national claim database. Journal of Korean Medical Science 202035e410. ( 10.3346/jkms.2020.35.e410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Premkumar A Kolin DA Farley KX Wilson JM McLawhorn AS Cross MB & Sculco PK. Projected economic burden of periprosthetic joint infection of the hip and knee in the United States. Journal of Arthroplasty 2021361484–1489.e3. ( 10.1016/j.arth.2020.12.005) [DOI] [PubMed] [Google Scholar]
- 35.Choi HR & Bedair H. Mortality following revision total knee arthroplasty: a matched cohort study of septic versus aseptic revisions. Journal of Arthroplasty 2014291216–1218. ( 10.1016/j.arth.2013.11.026) [DOI] [PubMed] [Google Scholar]
- 36.Shahi A Tan TL Chen AF Maltenfort MG & Parvizi J. In-hospital mortality in patients with periprosthetic joint infection. Journal of Arthroplasty 201732948–952.e1. ( 10.1016/j.arth.2016.09.027) [DOI] [PubMed] [Google Scholar]
- 37.Qasim SN Swann A Ashford R & The DAIR. The DAIR (debridement, antibiotics and implant retention) procedure for infected total knee replacement – a literature review. SICOT-J 201732. ( 10.1051/sicotj/2016038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behavioral Research 201146399–424. ( 10.1080/00273171.2011.568786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tawiah AK Al Sayah F Ohinmaa A & Johnson JA. Discriminative validity of the EQ-5D-5 L and SF-12 in older adults with arthritis. Health and Quality of Life Outcomes 20191768. ( 10.1186/s12955-019-1129-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papanicolas I Woskie LR & Jha AK. Health care spending in the United States and other high-income countries. JAMA 20183191024–1039. ( 10.1001/jama.2018.1150) [DOI] [PubMed] [Google Scholar]
- 41.Daigle ME Weinstein AM Katz JN & Losina E. The cost-effectiveness of total joint arthroplasty: a systematic review of published literature. Best Practice and Research. Clinical Rheumatology 201226649–658. ( 10.1016/j.berh.2012.07.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a