Abstract
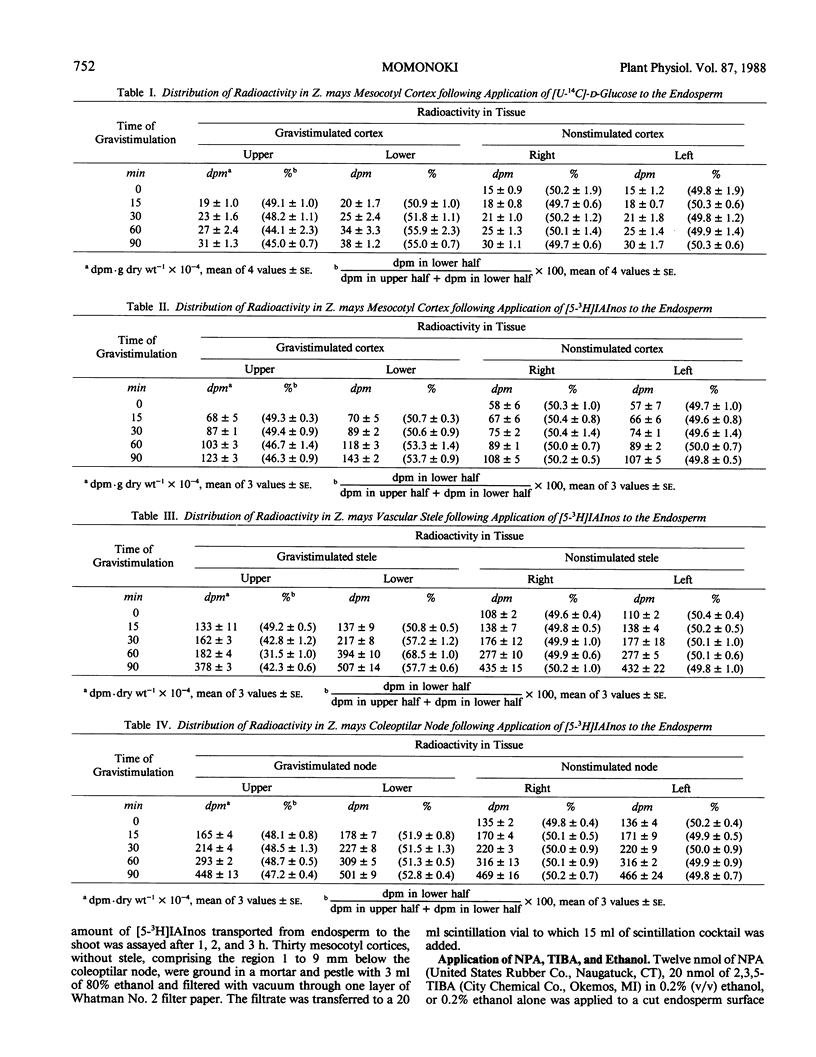
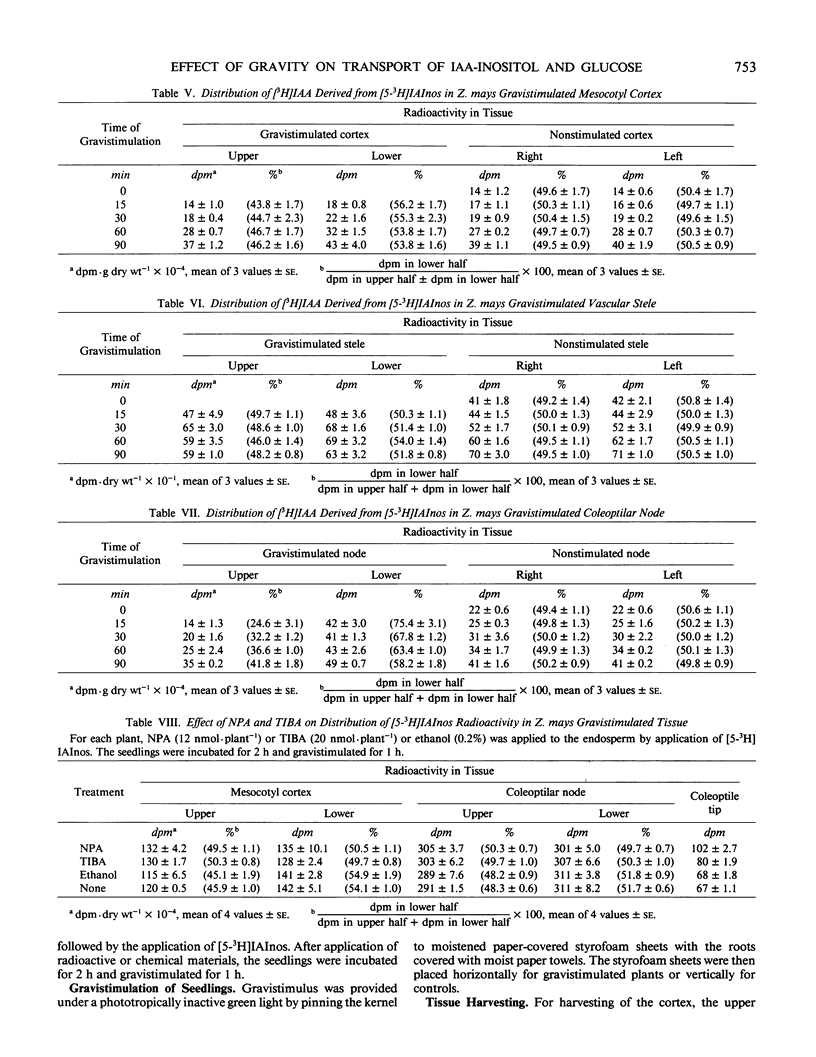
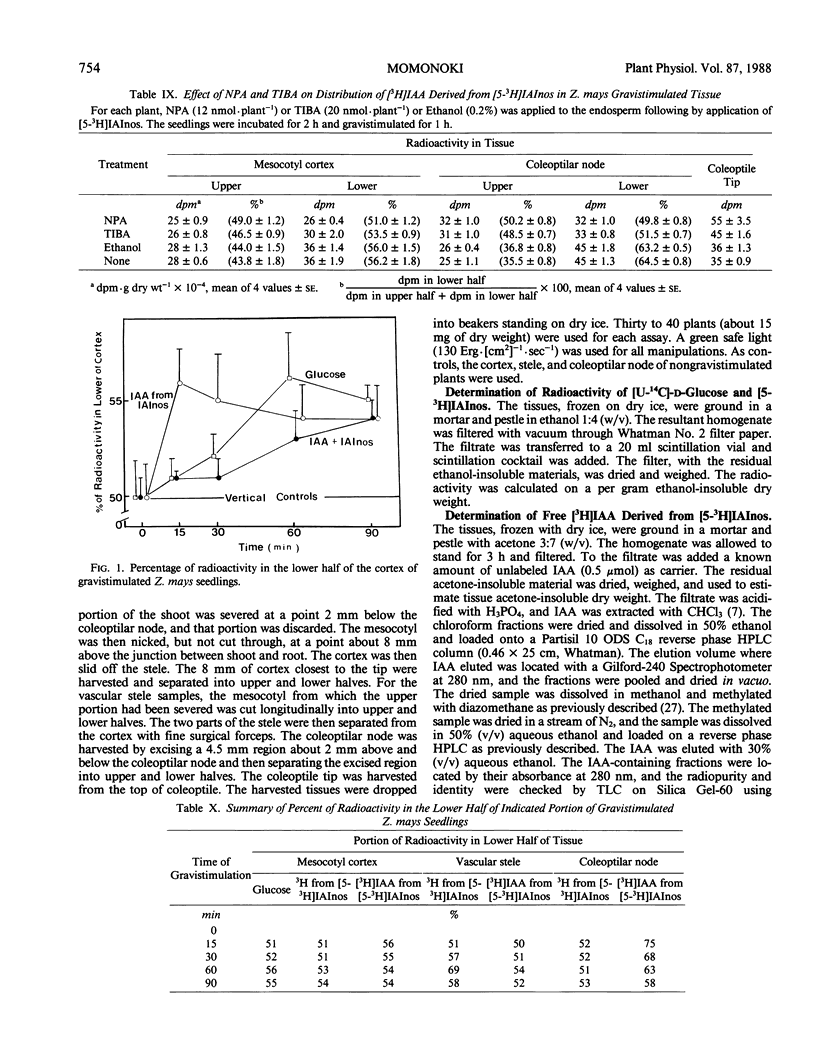
Indole-3-acetyl-myo-inositol occurs in both the kernel and vegetative shoot of germinating Zea mays seedlings. The effect of a gravitational stimulus on the transport of [3H]-5-indole-3-acetyl-myo-inositol and [U-14C]-d-glucose from the kernel to the seedling shoot was studied. Both labeled glucose and labeled indole-3-acetyl-myo-inositol become asymmetrically distributed in the mesocotyl cortex of the shoot with more radioactivity occurring in the bottom half of a horizontally placed seedling. Asymmetric distribution of [3H]indole-3-acetic acid, derived from the applied [3H]indole-3-acetyl-myo-inositol, occurred more rapidly than distribution of total 3H-radioactivity. These findings demonstrate that the gravitational stimulus can induce an asymmetric distribution of substances being transported from kernel to shoot. They also indicate that, in addition to the transport asymmetry, gravity affects the steady state amount of indole-3-acetic acid derived from indole-3-acetyl-myo-inositol.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandurski R. S., Schulze A., Dayanandan P., Kaufman P. B. Response to gravity by Zea mays seedlings. I. Time course of the response. Plant Physiol. 1984;74:284–288. doi: 10.1104/pp.74.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurski R. S., Schulze A., Domagalski W. Possible effects of organelle charge and density on cell metabolism. Adv Space Res. 1986;6(12):47–54. doi: 10.1016/0273-1177(86)90065-7. [DOI] [PubMed] [Google Scholar]
- Chisnell J. R. Myo-inositol esters of indole-3-acetic acid are endogenous components of Zea mays L. shoot tissue. Plant Physiol. 1984;74:278–283. doi: 10.1104/pp.74.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. D., Schulze A. Double-standard isotope dilution assay. I. Quantitative assay of indole-3-acetic acid. Anal Biochem. 1981 Apr;112(2):249–257. doi: 10.1016/0003-2697(81)90290-6. [DOI] [PubMed] [Google Scholar]
- Dayanandan P., Kaufman P. B. Analysis and significance of gravity-induced asymmetric growth in the grass leaf-sheath pulvinus. Ann Bot. 1984;53:29–44. doi: 10.1093/oxfordjournals.aob.a086668. [DOI] [PubMed] [Google Scholar]
- Ehmann A. The van urk-Salkowski reagent--a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr. 1977 Feb 11;132(2):267–276. doi: 10.1016/s0021-9673(00)89300-0. [DOI] [PubMed] [Google Scholar]
- Gillespie B., Thimann K. V. Transport & Distribution of Auxin during Tropistic Response. I. The Lateral Migration of Auxin in Geotropism. Plant Physiol. 1963 Mar;38(2):214–225. doi: 10.1104/pp.38.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacki J., Bandurski R. S. Myo-Inositol Esters of Indole-3-acetic Acid as Seed Auxin Precursors of Zea mays L. Plant Physiol. 1980 Mar;65(3):422–427. doi: 10.1104/pp.65.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke D. M., Bandurski R. S. Metabolic conversion of 14C-indole-3-acetic acid to 14C-oxindole-3-acetic acid. Biochem Biophys Res Commun. 1981 Nov 30;103(2):429–433. doi: 10.1016/0006-291x(81)90470-8. [DOI] [PubMed] [Google Scholar]
- Reinecke D. M., Bandurski R. S. Oxindole-3-acetic Acid, an Indole-3-acetic Acid Catabolite in Zea mays. Plant Physiol. 1983 Jan;71(1):211–213. doi: 10.1104/pp.71.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]


