Abstract
Background
Toxicity and resistance may limit the use of HIV nucleoside reverse transcriptase inhibitors (NRTIs). We assessed the safety and activity of regimens that did not include an NRTI.
Methods and patients
We analysed NRTI-sparing regimens using pooled data from three cohorts in Australia and France where HIV RNA viral load, CD4 lymphocyte count and metabolic parameters are assessed prospectively. The inclusion criterion was the commencement of any antiretroviral combination excluding NRTIs.
Results
A total of 334 (3.9%) of 8477 patients were included in the present study for a median follow-up time of 105 weeks. Therapeutic combinations were one nonnucleoside reverse transcriptase inhibitor (NNRTI) plus one protease inhibitor (PI) (58%), two PIs (26%), one PI (16%), and one NNRTI plus two PIs (8%). At baseline, the median CD4 lymphocyte count was 264 cells/μL (interquartile range 164–446 cells/μL) and 25% of patients had plasma HIV RNA below 500 HIV-1 RNA copies/mL. In intent-to-treat analysis, 64% of patients had HIV RNA <500 copies/mL at 6 months and 68% at 24 months. The mean CD4 lymphocyte count increase was 60 cells/μL (95% confidence interval 41–76 cells/μL) at 6 months and 111 cells/μL (95% confidence interval 82–140 cells/μL) at 24 months. Prognostic factors for having HIV RNA <500 copies/mL at 6 months included independently having undetectable HIV RNA at baseline and being naïve for NNRTIs. The proportion of patients with triglycerides >2.3 mmol/L increased from 32% to 63% at 6 months and to 62% at 24 months (P-trend=0.002), and those with total cholesterol >6.2 mmol/L increased from 18% to 38% at 6 months and to 44% at 24 months (P-trend <0.001), with an increased risk for patients treated with NNRTI+PIs. Forty-one per cent of patients discontinued their NRTI-sparing regimen.
Conclusions
In these antiretroviral-experienced patients, NRTI-sparing therapy appeared to have satisfactory virological and immunological efficacy. However, hyperlipidaemia was frequent and requires monitoring of cardiovascular risk factors.
Keywords: antiretroviral regimen, cohort, nucleoside reverse transcriptase inhibitor
Introduction
International guidelines recommend starting antiretroviral therapy with a combination of three drugs including two nucleoside or nucleotide reverse transcriptase inhibitors (NRTIs) with either a protease inhibitor (PI) or a nonnucleoside reverse transcriptase inhibitor (NNRTI) [1]. The 2006 recommendations of the International AIDS Society-USA Panel suggest that changing a first regimen in experienced patients should be individualized from case to case according to the results of drug resistance testing and treatment history [1]. Many resistance mutations to NRTIs confer cross resistance. The tolerability and toxicity of NRTIs are other key issues when choosing a potentially life-long antiretroviral regimen, as these can compromise adherence. NRTIs can inhibit DNA γ-polymerase, and induce mitochondrial dysfunction, resulting in plasma hyperlactataemia and a large spectrum of illnesses: peripheral neuropathy, myopathies, steatohepatitis, pancreatitis, lipoatrophy, renal tubular acidosis, postnatal encephalopathy and lactic acidosis [2–5]. NRTI cessation is often required for these toxicities to improve or resolve. In these cases other antiretroviral combinations without NRTIs may have to be administered.
NRTI-sparing regimens have also been considered as an option for a first treatment [6] but, to date, very few data are available on their efficacy and their short- and long-term tolerance in clinical practice [7–9]. We hypothesized that, despite the lack of knowledge, NRTI-sparing regimens have been prescribed in clinical practice. Thus, we conducted an intercohort study to assess the use of these combinations.
Patients and Method
We included patients recruited in the ANRS CO3 Aquitaine Cohort, France, the Australian HIV Observational Database, and the Cohort of St Vincent’s Hospital, Sydney, Australia.
The Aquitaine Cohort database is the hospital-based information system of the Groupe d’Epidémiologie Clinique du SIDA en Aquitaine (GECSA). Anonymous data on a predefined set of demographic, laboratory and clinical variables are collected at each patient’s visit [10]. The Australian HIV Observational Database (AHOD) has prospectively collected data for HIV-infected adults at 27 sites throughout Australia since 1999. Anonymous data on a predefined set of demographic, laboratory (excluding metabolic data) and clinical variables are collected every 6 months [11]. The hospital-based cohort of St Vincent’s Hospital, Sydney, Australia collects epidemiological, clinical and biological data and therapeutic histories of HIV-infected adults followed in the clinic. Data are electronically recorded on the day of the visit by a research nurse and clinicians.
Data were pooled based on the most recent data merge (at the time of analysis) for each of the three cohorts (31 December 2004 for the Aquitaine Cohort, 1 March 2004 for AHOD, and 30 March 2005 for St Vincent’s Hospital).
The inclusion criterion in the present study was the commencement of any antiretroviral combination excluding NRTIs and tenofovir since 1 January 1997. NRTI-sparing regimens were categorized as protease inhibitor (PI) regimens (one or more PIs), or nonnucleoside reverse transcriptase inhibitor (NNRTI)+PI regimens (one NNRTI+one or more PIs). Ritonavir used as a booster was not counted as a PI. Data recorded up to 3 months prior to the start of an NRTI-sparing regimen were considered baseline data for the present analysis. Patients were categorized as lost to follow-up if no information was recorded for more than 12 months prior to the cut-off date. Reasons for starting or stopping NRTI-sparing regimens determined by the physician were: virological failure, toxicity (including lipodystrophy), poor adherence, patient choice, other and unknown. Mean change in biological variables [including HIV-1 RNA viral load, CD4 T-lymphocyte count, total cholesterol, high-density lipoprotein (HDL)-cholesterol and triglycerides] from baseline was determined for every 3-month period, up to 24 months. If not otherwise stated, all the analyses were conducted using the intent-to-treat approach (ITT): all analyses, including descriptive and endpoint statistics, included all patient follow-up data up to the time of censoring, regardless of their combination therapy at each time-point.
Factors associated with virological response (defined as a viral load <500 HIV-1 RNA copies/mL plasma at 6 months) and with immunological response (defined as an increase of at least 20% in the CD4 T-lymphocyte count at 6 months compared with the baseline value) were examined using multivariate logistic regression models. Covariates examined at baseline included: age, gender, calendar period of initiation of NRTI-sparing regimen by quartile (1999 and before, 2000–2001, 2002, and 2003 and after), known duration of HIV infection (<10 years vs ≥10 years), AIDS stage, duration of antiretroviral therapy (<5 years vs ≥5 years), number of previous antiretroviral regimens (0–1, 2–3, 4–6 and >6), number of NRTIs previously received (0, 1–3 and >3 NRTIs), number of NNRTIs previously received (0 vs ≥1), number of PIs previously received (0, 1–2 and >2 PIs), type of NRTI-sparing regimen (PI only vs PI+NNRTI), HIV-1 RNA at the start of the NRTI-sparing regimen (≤500 vs >500 copies/mL plasma) and CD4 T-lymphocyte counts stratified at baseline (<50, 50–200, 201–350 and >350 cells/μL). The multivariate model was determined using the forward stepwise approach, considering only covariates that were significant at the 0.25 level in univariate analysis. A Cox proportional hazard model was used to assess the factors associated with NRTI-sparing regimen interruption.
General estimating equation (GEE) models accounting for repeated measures within individuals were used to assess trends in mean change in CD4 T-lymphocyte count from baseline to months 3, 6, 9, 12, 15, 18 and 24. GEE methods were also used to assess trends in the proportion of patients with HIV-1 RNA below 500 copies/mL plasma from baseline to 24 months and trends in the proportion of patients with total cholesterol, triglycerides and high-density lipoprotein (HDL) cholesterol above the cut-off for primary prevention [12]. All these analyses were adjusted by cohort. Analyses were performed using stata software version 8.0 [13].
Results
Patient characteristics
A total of 334 (3.9%) of 8477 patients in the three cohorts (82 from the St Vincent’s Hospital Cohort, 156 from the Aquitaine Cohort and 96 from AHOD) received an NRTI-sparing regimen during their follow-up and were included in the present analysis. Patient characteristics are shown in Table 1. NRTI-sparing regimens included the following combinations: one NNRTI plus one PI (50%), two PIs (26%), one PI (16%), and one NNRTI plus two PIs (8%). In NNRTI plus PI combinations (n=193) the most frequent regimen used was efavirenz plus lopinavir/r (n=38, 20%) followed by nevirapine plus indinavir (n=19, 10%), nevirapine plus lopinavir/r (n=18, 9%), and nevirapine plus saquinavir (n=16, 8%). The most common PI used as a single agent (n=55) was lopinavir/r (n=17, 31%), followed by indinavir (n=10, 18%) and saquinavir (n=7, 13%). In the double PI regimen (n=86), the most frequent combinations were saquinavir plus atazanavir (n=31, 36%), saquinavir plus lopinavir (n=20, 23%) and amprenavir plus lopinavir (n=19, 22%).
Table 1.
Epidemiological, biological and therapeutic characteristics of 334 patients starting an antiretroviral combination excluding a nucleoside reverse transcriptase inhibitor (NRTI)
| Characteristics | |
|---|---|
| Gender (% male) | 87 |
| Age (years) [median (IQR)] | 43.2 (38.1–50.2) |
| Transmission group (%) | |
| Men who have sex with men | 60 |
| Heterosexual contact | 16 |
| Injecting drug users | 7 |
| Blood recipients | 2 |
| Undetermined | 15 |
| CDC stage C (%) | 33 |
| Period of inception of NRTI-sparing regimen (%) | |
| 1999 and before | 21 |
| 2000–2001 | 15 |
| 2002 | 25 |
| 2003 and after | 39 |
| Known duration of HIV infection (years) [median (IQR)] | 9.6 (6.7–13.4) |
| Duration of antiretroviral therapy before NRTI-sparing regimen (years) [median (IQR)] | 5.2 (2.1–8.1) |
| Number of prior antiretroviral regimens [median (IQR)] | 4 (2–7) |
| Number of NNRTIs received [median (IQR)] | 1 (0–1) |
| Number of PIs received [median (IQR)] | 2 (1–3) |
| Reasons for stopping NRTI (%) | |
| Virological failure | 41 |
| Toxicity | 22 |
| Poor adherence | 10 |
| Patienťs choice | 2 |
| Others (protocol, pregnancy) | 12 |
| Unknown | 12 |
| NRTI-sparing combination therapy a (%) | |
| One NNRTI+one PI | 50 |
| Two PIs | 26 |
| One PI | 16 |
| One NNRTI+two Pis | 8 |
| Positive HCV antibodies (n=288) (%) | 15 |
| Positive HBs antigen (n=273) (%) | 5 |
| CD4 count (cells/μL) [median (IQR)] | 264 (164–446) |
| HIV-1 RNA (copies/mL) [median (IQR)] | 19550 (500–118650) |
| Total cholesterol (mmol/L) (n=143) [median (IQR)] | 5.0 (4.0–6.0) |
| Total cholesterol >6.2 mmol/L (%) | 18 |
| HDL-cholesterol (mmol/L) (n=90) [median (IQR)] | 1.0 (0.8–1.2) |
| HDL-cholesterol <0.9 mmol/L (%) | 31 |
| Triglycerides (mmol/L) (n=145) [median (IQR)] | 1.7 (1.2–3.1) |
| Triglycerides >2.3 mmol/L (%) | 32 |
•Ritonavir used as a booster is not counted as one PI in these combinations.
• CDC, Centers for Disease Control and Prevention; Hepatitis B HBs Ag CHBs, HCV, hepatitis C virus; HDL, high-density lipoprotein; IQR, interquartile range; PI, protease inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor.
The median follow-up time until censoring date was 105 weeks [interquartile range (IQR) 39–196]. Fifty-nine per cent of patients were still on an NRTI-sparing regimen at the censoring date. Baseline characteristics did not differ between patients lost to follow up (n=34, 10%) and other patients (data not shown). Thirty-one patients (10%) experienced an AIDS-related illness (n=26: Kaposi sarcoma, n=5; disseminated Mycobacterium avium intracellulare infections, n=5; cytomegalovirus infection, n=4; non-Hodgkin lymphoma, n=2; recurrent pneumopathy, n=2; oesophageal candidiasis, n=2; toxoplasmosis, n=2; others, n=4) and/or death (n=14) during follow up.
Virological and immunological response
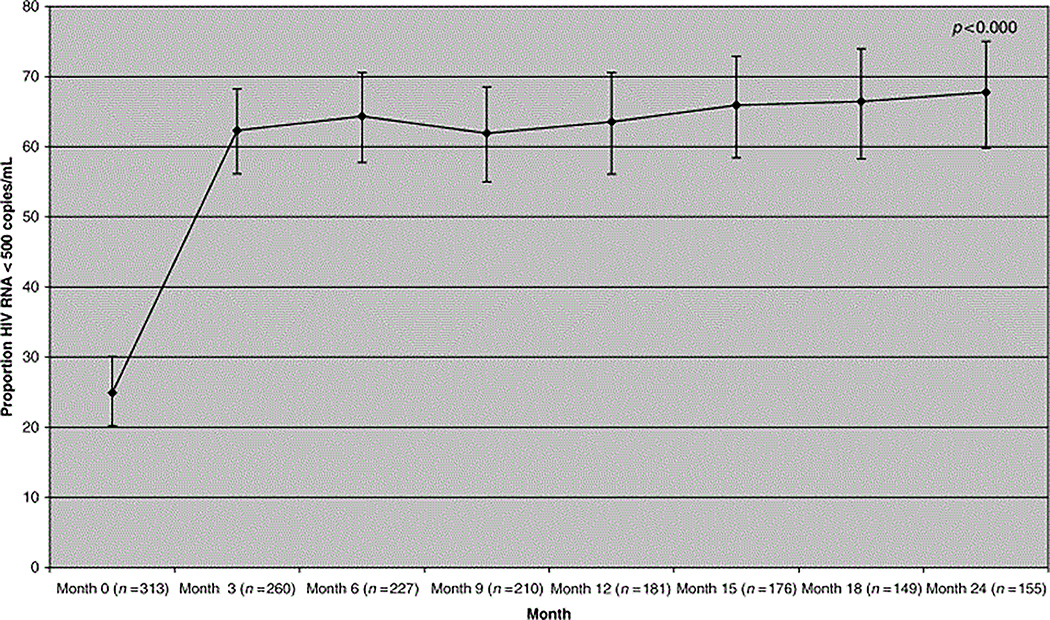
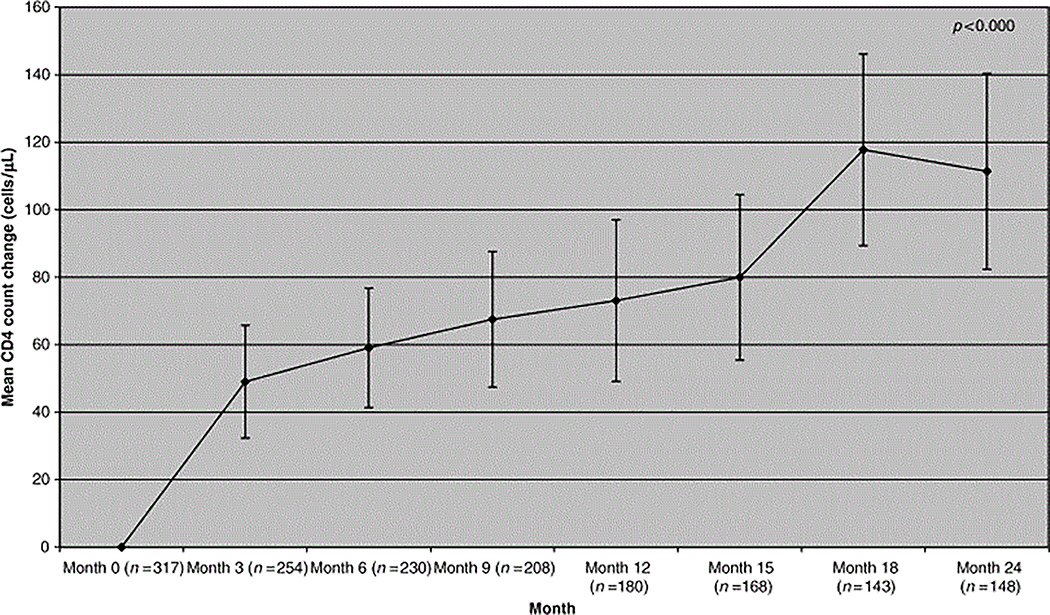
The proportion of patients with HIV RNA <500 copies/mL plasma rose from 25% at baseline to 64% at 6 months, 64% at 1 year and 68% at 2 years (Fig. 1). If patients were censored when NRTI-sparing therapy was stopped (as treated analysis), then the proportion of patients achieving plasma HIV RNA <500 copies/mL plasma on an NRTI-sparing therapy rose from 25% at baseline to 65% at 6 months, 66% at M12 and 79% at 24 months. Mean [95% confidence interval (CI)] CD4 T-lymphocyte count change was +60 cells/μL (41–77, n=230) at 6 months, 73 cells/μL cells at M12 (49–97, n=180) and 111 cells/μL cells at 24 months (82–140, n=148) (Fig. 2).
Figure 1.
Propotion of patients with HIV RNA <500 copies/mL after starting a nucleoside reverse transcriptase inhibitor (NRTI)-sparing regimen.
Figure 2.
Mean change in CD4 lymphocyte count from baseline in patients starting a nucleoside reverse transcriptase inhibitor (NRTI)-sparing regimen.
In patients starting NRTI-sparing therapy with HIV RNA <500 copies/mL, 89%, 93% and 87% had undetectable HIV RNA at 6, 12 and 24 months of follow-up, respectively.
In patients starting NRTI-sparing therapy with HIV RNA >500 copies/mL who were NNRTI naïve, 68%, 57% and 69% had undetectable HIV RNA at 6, 12 and 24 months, respectively. Among patients who had already received NNRTI in a previous regimen, 50%, 59% and 58% had undetectable HIV RNA at 6, 12 and 24 months, respectively. In patients starting NRTI-sparing therapy with HIV RNA >500 copies/mL and with PI Nonotherapy on bitherapy 44%, 57% and 46% had undetectable HIV RNA at 6, 12 and 24 months, respectively.
Factors associated with HIV RNA <500 copies/mL at 6 months are shown in Table 2. Starting NRTI-sparing regimen after 2000, (P=0.001), being NNRTI naïve at baseline (P=0.001) and having undetectable viraemia at baseline (P<0.001) were independently associated with a HIV RNA <500 copies/mL at 6 months.
Table 2.
Factors associated with virological response (HIV RNA <500 copies/mL at 6 months) in patients starting a nucleoside reverse transcriptase inhibitor (NRTI)-sparing regimen (logistic regression)
| Characteristic | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-value (P-trend) | OR (95% CI) | P-value (P-trend) | |
| Age >42 years | 0.95 (0.55–1.65) | 0.85 | ||
| Female gender | 1.05 (0.47–2.31) | 0.91 | ||
| Duration of antiretroviral therapy >5 years | 1.81 (1.01–3.25) | 0.05 | 1.70 (0.83–3.48) | 0.15 |
| Duration of HIV seropositivity >10 years | 1.03 (0.60–1.78) | 0.91 | ||
| Non-AIDS stage | 0.52 (0.29–0.91) | 0.02 | 0.57 (0.31–1.07) | 0.08 |
| Number of previous antiretroviral regimens | ||||
| 0–1 | 1.00 | 1.00 | ||
| 2–3 | 0.53 (0.20–1.41) | 0.20 (0.07) | 0.60 (0.19–1.84) | 0.37 (0.48) |
| 4–6 | 0.56 (0.22–1.46) | 0.24 | 0.86 (0.28–2.65) | 0.79 |
| >6 | 0.40 (0.16–1.00) | 0.05 | 0.59 (0.20–1.78) | 0.35 |
| Number of previous NRTIs | ||||
| 0 | 1.00 | |||
| 1–3 | 0.54 (0.14–2.13) | 0.38 (0.04) | ||
| >3 | 0.50 (0.13–1.86) | 0.30 | ||
| Number of previous PIs | ||||
| 0 | 1.00 | 1.00 | ||
| 1–2 | 0.97 (0.43–2.20) | 0.94 (0.01) | 1.26 (0.51–3.16) | 0.61 (0.10) |
| >2 | 0.40 (0.18–0.90) | 0.03 | 0.57 (0.22–1.48) | 0.25 |
| Previous NNRTIs | ||||
| 0 | 1.00 | |||
| 1–2 | 0.51 (0.29–0.90) | 0.02 | 0.31 (0.16–0.61) | 0.001 |
| NRTI-sparing regimen | ||||
| PI only | 1.00 | 1.00 | ||
| NNRTI+PI | 1.60 (0.91–2.81) | 0.10 | 1.60 (0.80–3.23) | 0.19 |
| CD4 count (cells/μL) | ||||
| <50 | 1.00 | 1.00 | ||
| 50–199 | 1.82 (0.85–3.90) | 0.12 (0.03) | 2.00 (0.86–4.64) | 0.11 (0.31) |
| 200–349 | 1.42 (0.68–2.95) | 0.35 | 1.74 (0.77–3.96) | 0.19 |
| ≥350 | 0.17 (0.06–0.48) | 0.001 | 0.27 (0.08–0.88) | 0.03 |
| Missing | 3.40 (0.38–30.66) | 0.28 | 1.55 (0.01–169.43) | 0.86 |
| HIV RNA (copies/mL) | ||||
| <500 | 1.00 | 1.00 | ||
| >500 | 0.17 (0.06–0.45) | 0.001 (<10−3) | 0.14 (0.05–0.40) | 0.001 (<10−4) |
| Missing | 0.39 (0.06–2.51) | 0.32 | 0.53 (0.07–4.28) | 0.55 |
| Calendar period | ||||
| 1997–1999 | 1.00 | 1.00 | ||
| 2000–2001 | 3.51 (1.63–7.53) | 0.001 (<10−2) | 6.09 (2.54–14.64) | 0.001 (<10−3) |
| 2002 | 4.65 (2.08–10.37) | 0.001 | 7.41 (2.94–18.66) | 0.001 |
| 2003–2004 | 2.94 (1.06–8.18) | 0.038 | 4.12 (1.28–13.29) | 0.002 |
• CI, confidence interval; OR, odds ratio; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Factors associated with an immunological response, defined as an increase of at least 20% in the CD4 T-lymphocyte count at 6 months, were assessed. Patients starting an NRTI-sparing regimen with a baseline CD4 count between 50 and 200 cells/μL [odds ratio (OR) 5.33; 95% CI 2.40–11.85; P<0.001], between 201 and 350 cells/μL (OR 8.44; 95% CI 3.75–19.00; P<0.001), and above 350 cells/μL (OR 5.65; 95% CI 2.07–15.39; P<0.001) had better immune reconstitution than patients starting an NRTI-sparing regimen at <50 cells/μL. HIV RNA >500 copies/mL at baseline was also associated with a CD4 increase (OR 4.38; 95% CI 1.95–9.86; P<0.001). When immune reconstitution was defined as a 40% increase in CD4 count, the same associations were found (data not shown).
Safety assessment
One hundred and thirty-seven patients (41%) stopped the NRTI-sparing regimen during their follow-up: 30% for toxicity, 16% for virological failure, 14% because of poor adherence, 8% because it was the patient’s choice, 6% for inclusion in therapeutic protocol, and 27% for unknown reasons. In patients starting an NRTI-sparing regimen with undetectable viral load, the main reason for stopping NRTIs was toxicity (36%).
The median duration of NRTI-sparing regimen was 52 (IQR 23–118) weeks before stopping or censoring. Analysis of prognostic factors for ceasing an NRTI-sparing regimen showed that a patient starting an NRTI-sparing regimen because of lipodystrophy or NRTI-related toxicity was less likely to stop this regimen (OR 0.62; 95% CI 0.39–1.00; P=0.05). No other predictors for cessation of this regimen were found either in univariate or in multivariate analysis (data not shown). There was no difference in the time to stopping the NRTI-sparing regimen between patients on NNRTI plus PI regimens and those on PI-only regimens (data not shown).
Lipids, hepatic transaminases and glucose levels were assessed every 3 months up to 24 months in the St Vincent’s and Aquitaine Cohorts. Glucose levels and liver enzymes did not significantly change over time (Table 3). However, we observed a significant increase in the proportion of patients with lipid levels above the (National Cholesterol Education Program NCEP) cut-offs for primary prevention: the percentage of patients with total cholesterol above the threshold of 6.2 mmol/L was 18% at baseline (n=143), rising to 37% at 3 months (n=140) and to 44% at 24 months (n=78) (P-trend <0.001). The proportion of patients with HDL-cholesterol below 0.9 mmol/L increased slightly from 31% at baseline to 44% at 6 months, with a decrease to 21% at 24 months (P-trend=0.009). Hypertriglyceridaemia, defined as levels of triglycerides above 2.3 mmol/L, increased from 32% (n=145) at baseline to 62% at 3 months (n=140) and to 62% at 24 months (n=76) (P-trend=0.002).
Table 3.
Evolution of percentage of patients with metabolic abnormalities after starting a nucleoside reverse transcriptase inhibitor (NRTI)-sparing regimen (Aquitaine and St Vincenťs Cohorts) (n=238)
| Month 0 | Month 3 | Month 6 | Month 9 | Month 12 | Month 15 | Month 18 | Month 24 | |
|---|---|---|---|---|---|---|---|---|
| Cholesterol | ||||||||
| Available data | 143 | 140 | 132 | 111 | 92 | 89 | 76 | 78 |
| ≤6.2 mmol/L (%) | 82.5 | 62.9 | 62.1 | 70.3 | 62.0 | 57.3 | 59.2 | 56.4 |
| >6.2 mmol/L (%) | 17.5 | 37.1 | 37.9 | 29.7 | 38.0 | 42.7 | 40.8 | 43.6 |
| HDL-cholesterol | ||||||||
| Available data | 90 | 72 | 69 | 53 | 54 | 53 | 52 | 52 |
| <0.9 mmol/L (%) | 31.1 | 44.4 | 43.5 | 28.3 | 29.6 | 37.7 | 38.5 | 21.2 |
| ≥0.9 mmol/L (%) | 68.9 | 55.6 | 56.5 | 71.7 | 70.4 | 62.3 | 61.5 | 78.9 |
| Triglycerides | ||||||||
| Available data | 145 | 140 | 132 | 110 | 92 | 89 | 78 | 76 |
| ≤2.3 mmol/L (%) | 67.6 | 37.9 | 37.1 | 47.3 | 37.0 | 42.7 | 32.0 | 38.2 |
| >2.3 mmol/L (%) | 32.4 | 62.1 | 62.9 | 52.7 | 63.0 | 57.3 | 68.0 | 61.8 |
| Glucose | ||||||||
| Available data | 155 | 138 | 120 | 106 | 95 | 37 | 75 | 77 |
| ≤11 mmol/L (%) | 97.4 | 97.8 | 97.5 | 98.1 | 96.8 | 100 | 98.7 | 98.7 |
| >11 mmol/L (%) | 2.6 | 2.2 | 2.5 | 1.9 | 3.2 | 0 | 1.3 | 1.3 |
| AST | ||||||||
| Available data | 180 | 157 | 136 | 116 | 104 | 102 | 84 | 81 |
| ≤100 IU/L (%) | 91.1 | 95.5 | 92.7 | 94.8 | 95.2 | 96.1 | 95.2 | 93.8 |
| >100 IU/L (%^) | 8.9 | 4.5 | 7.5 | 5.2 | 4.8 | 3.9 | 4.8 | 6.2 |
• The P-trend values are: P<0.001 for cholesterol, P=0.009 for HDL-cholesterol, P=0.001 for triglycerides, P=0.711 for glucose, and P=0.123 for SGOT
• HDL, high-density lipoprotein; AST, (aspartate aminotransferase).
The proportions of patients with a rise in total cholesterol and/or triglycerides above threshold values were significantly higher in the group of patients treated with NNRTI plus PI than in patients treated with PI alone: the proportion of patients with total cholesterol >6.2 mmol/L increased from 14% at baseline to 21% at 6 months and 20% at 24 months in patients treated with PI alone, and from 21% at baseline to 47% at 6 months and 49% at 24 months in patients treated with NNRTI plus PI (P=0.005); the proportion of patients with triglycerides >2.3 mmol/L increased from 32% at baseline to 47% at 6 months and 40% at 24 months in patients treated with PI alone and from 33% at baseline to 72% at 6 months and 67% at 24 months in patients treated with NNRTI plus PI (P=0.046).
Discussion
This study assessed the clinical, virological and immunological outcomes of NRTI-sparing regimens in clinical practice. Overall, our data suggest that the use of NRTI-sparing regimens may be virologically effective in pretreated patients, as 64% of patients with available data were under the threshold of 500 copies/mL at 6 months of follow up and 68% at 24 months. Ninety-one per cent of patients starting an NRTI-sparing regimen with HIV RNA <500 copies/mL still had undetectable HIV RNA at 6 months. Similarly, an NRTI-sparing regimen led to immune restoration even in these heavily experienced patients. However, a significant increase in the proportion of patients with lipid levels above the NCEP threshold, particularly in patients treated with a combination therapy including NNRTI and PI, was observed.
The use of NRTI-sparing regimens increased after 2000, but the number of patients is still small. Only 334 patients on an NRTI-sparing regimen were identified out of over 8000 included in three large cohorts since 1997 (3.9%). The typical patient was treatment experienced with moderately advanced and long-standing HIV disease, 33% of them having a prior AIDS-defining illness.
There are several limitations to this study. First, all analyses were based on data from three different cohorts, with variations in the amount of patient follow-up and disease stage. However, wherever possible, all analyses were stratified by cohort in an attempt to minimize any bias. Secondly, as this cohort analysis was entirely observational, patients on an NRTI-sparing regimen would have self-selected this regimen, with varying reasons for commencing an NRTI-sparing regimen and with numerous NRTI-sparing regimen combinations. Thirdly, there were no standardized guidelines for reporting reasons for stopping or commencing treatment, across or even within cohorts.
Our results are consistent with the limited data already published. The most frequent regimen in our patients was a PI in combination with an NNRTI. This regimen has been studied previously in naïve patients in a large randomized trial comparing three arms (unboosted indinavir plus efavirenz, efavirenz plus zidovudine-lamivudine, and unboosted indinavir plus zidovudine-lamivudine). The percentage of patients with plasma viral load <400copies/mL at 48 weeks was 53% in the group assigned to indinavir plus efavirenz, as opposed to 70% in the group assigned to efavirenz plus two NRTIs [14]. Indinavir, however, is now rarely used as a single PI in naïve or experienced patients. Our study shows that most patients with an NNRTI-containing regimen used lopinavir/ritonavir (LPV/r). A pilot study recently demonstrated a satisfactory outcome in 21 experienced (but NNRTI naïve) and 65 naïve patients on LPV/r plus efavirenz: 73% of patients achieved a plasma viral load <400 copies/mL [9]. A trial of efavirenz plus LPV/r and efavirenz plus two NRTIs following a first suppressive three- or four-drug regimen with a median follow-up time of 110 weeks suggested a trend, although not statistically significant, towards a higher rate of virological failure in the LPV/r plus efavirenz arm (P=+0.088) [15]. The combination of LPV/r and nevirapine was studied in naïve patients and results at 48 weeks showed that 11 of the 14 naïve patients had undetectable viraemia at week 48 [16]. Overall, NNRTI plus PI combinations have been found to be effective in some small-scale comparative trials in naïve or pretreated patients. Very recently, the results of a large randomized, open-label, prospective trial comparing three class-sparing regimens (two NRTIs plus LPV/r vs two NRTIs plus efavirenz vs LPV/r plus efavirenz) for naïve subjects have been made available: compared with a standard regimen of efavirenz plus two NRTIs, the NRTI-sparing regimen of LPV/r plus efavirenz had a similar virological outcome and safety [17].
Different combinations of double-PI boosted regimens have been studied, mostly in pretreated subjects. In the LOPSAQ study, a combination of LPV/r with saquinavir without an NRTI backbone was given to 121 patients with advanced HIV disease and multiple regimen failure [18]. Preliminary 24-week results were encouraging, with immune reconstitution and the median viral load decreasing from 5.2 to 2.1 log10 copies/mL. Smaller studies performed on heavily treated patients using LPV/r plus indinavir [19] or a LPV/r plus amprenavir [20] regimens have shown similar results.
Our results for a wide range of NRTI-sparing regimens in 334 mostly heavily pretreated patients (of whom 25% only had undetectable viraemia at baseline) compare well with those obtained even in naïve patients with NNRTI plus PI or double boosted PIs and suggest a use for these regimens in specific situations. However, the main predictive factor of virological response at 6 months was being naïve to NNRTIs, suggesting the importance of this class in such a combination.
The indication to start an NRTI-sparing regimen was reported to be virological failure in 40% of patients. Twenty-five per cent of patients starting an NRTI-sparing regimen were virologically suppressed at the start of the NRTI-sparing regimen, suggesting that, for these patients at least, toxicity was a leading cause of stopping NRTIs.
Among patients identified as having started an NRTI-sparing regimen, the rate of discontinuation was 41%. Reasons for stopping are sometimes difficult to assess: clinicians may choose between competing reasons, and the assessment is subjective. However, reasons for stopping the NRTI-sparing regimens were reported to be toxicity for 30% of patients and virological failure for 16%. This high rate of discontinuation is consistent with previously reported data. Allavena et al. reported a 24% rate of discontinuation in patients in their study, and a third of the patients on a LPV/r-containing regimen were reported to stop for toxicity reasons [9]. Staszewski et al. found an even higher discontinuation rate of 50% in patients on an efavirenz/LPV/r regimen [14]. However, we have shown that patients with previous NRTI toxicity were less likely to discontinue an NRTI-sparing regimen and thus could represent a target population for these combinations.
An increase of lipid values was observed in patients treated with an NRTI-sparing regimen. The percentage of patients with total cholesterol >6.2 mmol/L increased from 18% at baseline to 44% at 24 months, and the percentage of patients with triglycerides >2.3 mmol/L increased from 32% at baseline to 62% at 24 months. Furthermore, patients on a combination of PI plus NNRTI were more likely to have an atherogenic lipid profile than patients on PI only. Such findings have been reported by others studying NRTI-sparing regimens, including the previously mentioned open-label study of 86 patients on LPV/r and efavirenz [9]. In that study, the authors observed a rapid rise in lipid levels during the first 8 weeks of treatment; later, lipids levels remained stable up to 48 weeks. In another open-label study, patients failing NRTIs were switched to indinavir/r 800/100 mg twice a day (bid) plus efavirenz 600 mg once a day [8]. This regimen gave a durable virological response, but a pro-atherogenic metabolic profile developed and nephrotoxicity occurred, requiring indinavir dose reductions. In lipoatrophic patients, small pilot nonpublished studies have evaluated the benefit of a switch to a PI-containing/NRTI-sparing regimen compared with a maintenance NRTI-containing regimen and showed that the combination lopinavir/efavirenz was associated with a significant improvement in body fat [21], but also with a greater increase in triglycerides and total cholesterol, compared with the NRTI arm [22].
Overall, NRTI-sparing regimens show encouraging long-term results in terms of virological and immunological safety. However, long-term toxicity, particularly regarding the metabolic profile and cardiovascular outcome, remains a concern and may depend on the type of regimen used.
Appendix: composition of the three cohort study groups
The Aquitaine Cohort study group
Organization and methodology:
G. Chêne, F. Dabis, R. Thiebaut and R. Salamon.
Clinical coordination:
D. Lacoste, D. Malvy, I. Pellegrin, J. F. Moreau, M. Dupon, P. Morlat, J. L. Pellegrin and J. M. Ragnaud.
Participating hospital departments (participating physicians):
Bordeaux University Hospital: J. Beylot (P. Morlat, N. Bernard, D. Lacoste and F. Bonnet), C. Beylot (M. S. Doutre), C. Conri (J. Constans), P. Couzigou and H. Fleury (B. Masquelier and I. Pellegrin), M. Dupon (I. Chossat), J. L. Pellegrin (P. Mercie), M. Le Bras (D. Malvy, F. Djossou and J. P. Pivetaud), J. F. Moreau (J. L. Taupin), J. M. Ragnaud (C. De La Taille, H. Dutronc and D. Neau); Dax Hospital: M. Loste (I. lanchard, L. Caunègre and A. Pons); Bayonne Hospital: F. Bonnal (Y. Blanchard, S. Farbos and M. C. Gemain); Libourne Hospital: J. Ceccaldi, B. Darpeix and P. Legendre; Villeneuve sur Lot Hospital: E. Buy.
Data management and analysis:
S. Lawson-Ayayi, E. Balestre, G. Palmer and D. Touchard.
Data collection:
M. J. Blaizeau, M. Decoin, S. Delveaux, A. M. Formaggio, M. Pontgahet and B. Uwamaliya.
The AHOD study group
New South Wales:
M. Gotowski, S. Taylor and L. Stuart-Hill, Bligh Street Clinic, Tamworth. E. Jackson, D. Hunter and L. Lewis, Blue Mountains Sexual Health and HIV Clinic, Katoomba. M. Block, D. Austen, D. Quan, A. Gowers and C. Anderson, Holdsworth House General Practice, Darlinghurst. K. Brown and N. Skobalj, Illawarra Sexual Health, Warrawong. C. O’Connor and B. Allam, Livingstone Road Sexual Health Centre, Marrickville. D. Templeton, Macquarie Sexual Health Centre, Dubbo. M. T. Liang, Nepean Sexual Health and HIV Clinic, Penrith. D. Allen and B. Strazdinis, Holden Clinic, Gosford. D. Smith and J. Armishaw, SHAIDS, Lismore. D. Cooper, A. Carr and M. Lacey, St Vincents Hospital, Sydney. B. Donovan, C. Pell and B. Judd, Sydney Sexual Health Centre, Sydney. R. Finlayson and R. Richardson, Taylor Square Private Clinic, Darlinghurst. D. Ellis, The Medical and Vein Centre, Coffs Harbour. D. Baker*, J. Kidd, R. McFarlane and R. Vale, 407 Bourke Street, Surry Hills. P. Canavan,* National Association of People Living with HIV/AIDS (NAPWA). P. Rawstorne,* National Centre in HIV Social Research, University of New South Wales, Sydney. B. Mulhall,* University of Sydney, Sydney. M. Law,* K. Petoumenos* and D. Smith,* NCHECR, Darlinghurst.
Northern Territory:
B. Hughes, H. Lyttle and P. Knibbs, Communicable Disease Centre, Royal Darwin Hospital, Darwin.
South Australia:
G. Rogers, S. Markinson, C. Sullivan, F. Downey, M. Curry, J. Oddy and J. Thompson, The Care and Prevention Programme, Adelaide University, Adelaide.
Queensland:
M. Kelly and H. Magon, AIDS Medical Unit. D, Brisbane. Sowden and A. Walker, Blackall Terrace Specialist Centre, Blackall Terrace. D. Orth, G. Lister and D. Youds, Gladstone Road Medical Centre, Highgate Hill. J. Chuah,* N. Wendt, W. Fankhauser and B. Dickson, Gold Coast Sexual Health Clinic, Miami. D. Russell, J. Leamy and C. D’arcy Evans, Sexual Health Program, Cairns Base Hospital, Cairns.
Victoria:
T. Reid and J. Laing, Melbourne Sexual Health Centre, Carlton. I. Woolley, T. Korman, A. Padiglione and K. Visvanathan, Monash Medical Centre, Clayton. N. Roth,* B. Eu, S. Strecker, D. Russell and H. Wood, Prahran Market Clinic, South Yarra. A. Mijch,* J. Hoy, A. Pierce, M. Bryant, C. McCormack and K. Watson, The Alfred Hospital, Prahran. J. Anderson,* R. Moore, D. Russell, G. McGovern, R. McNair and K. Lowe, The Carlton Clinic, Carlton. N. Medland, The Centre Clinic, St Kilda.
Western Australia:
S. Mallal,* M. French, A. Cain, J. Skett and C. Moore, Department of Clinical Immunology, Royal Perth Hospital, Perth.
*Steering committee member.
The St Vincent’s Hospital Cohort study group
Participating physicians: D. Cooper, A. Carr, S. Pett, P. Mallon, A. Winston, C. Wheatherall, S. Millikan, B. Brew and A. Calmy.
Database manager: K. Hesse.
References
- 1.Hammer SM, Saag M, Schechter M et al. Treatment for HIV adult infection. Recommendations of the International AIDS Society-USA Panel. J Am Med Assoc 2006; 296: 828–843. [Google Scholar]
- 2.Cote HC, Brumme ZL, Craib KJ et al. Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. N Engl J Med 2002; 346: 811–820. [DOI] [PubMed] [Google Scholar]
- 3.Cossarizza A, Moyle G. Antiretroviral nucleoside and nucleotide analogues and mitochondria. AIDS 2004; 18: 137–151. [DOI] [PubMed] [Google Scholar]
- 4.Lonergan JT, Behling C, Pfander H et al. Hyperlactatemia and hepatic abnormalities in 10 human immunodeficiency virusinfected patients receiving nucleoside analogue combination regimens. Clin Infect Dis 2000; 31: 162–166. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet F, Bonarek M, Morlat P et al. Risk factors for lactic acidosis in HIV-infected patients treated with nucleoside reverse-transcriptase inhibitors: a case-control study. Clin Infect Dis 2003; 36: 1324–1328. [DOI] [PubMed] [Google Scholar]
- 6.Joly V, Yeni P. Nucleoside analogue-sparing strategy for the treatment of chronic HIV infection: potential interest and clinical experience. Antiviral Ther 2005; 10: 29–40. [PubMed] [Google Scholar]
- 7.Tebas P, Zhang J, Yarasheski K et al. Switch to a protease inhibitor-containing/nucleoside reverse transcriptase inhibitorsparing regimen increases appendicular fat and serum lipid levels without affecting glucose metabolism or bone mineral density. The results of a prospective randomized trial, ACTG 5125s. 12th Conference on Retroviruses and Opportunistic Infections. Boston, MA, February 2005. [Abstract 40]. [Google Scholar]
- 8.Boyd MA, Siangphoe U, Duncombe CJ et al. Indinavir/ritonavir 800/100mg bid and efavirenz 600mg qd in patients with combination nucleoside failure: 96 week outcomes of HIV-NAT 009. XV International AIDS Conference. Bangkok, Thailand, July 2004. [Abstract MoOrB1084]. [Google Scholar]
- 9.Allavena C, Ferre V, Brunet-Francois C et al. Efficacy and tolerability of a nucleoside reverse transcriptase inhibitorsparing combination of lopinavir/ritonavir and efavirenz in HIV-1 infected patients. J Acquir Immune Defic Syndr 2005; 39: 300–306. [DOI] [PubMed] [Google Scholar]
- 10.Chene G, Binquet C, Moreau JF et al. Changes in CD41cell count and the risk of opportunistic infection or death after highly active antiretroviral treatment. AIDS 1998; 12: 2313–2320. [DOI] [PubMed] [Google Scholar]
- 11.Petoumenos K, Australian HIV Observational Database. The role of observational data in monitoring trends in antiretroviral treatment and HIV disease stage: results from the Australian HIV observational database. J Clin Virol 2003; 26: 209–222. [DOI] [PubMed] [Google Scholar]
- 12.Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Final Report. Circulation 2002; 106: 3143–3420. [PubMed] [Google Scholar]
- 13.Stata. Stata Statistical Software, Release 8.0. College Station, TX: Stata Corporation, 2003. [Google Scholar]
- 14.Staszewski S, Morales-Ramirez J, Tashima KT et al. The Study 006 Team. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. N Engl J Med 1999; 341: 1865–1873. [DOI] [PubMed] [Google Scholar]
- 15.Fischl M, Bassett R, Collier A et al. Randomised, controlled trial of lopinavir/ritonavir1efavirenz versus efavirenz12 nucleoside reverse transcriptase inhibitors following a first suppressive 3- or 4-drug regimen in advanced HIV disease. 12th Conference on Retroviruses and Opportunistic Infections.Boston, MA, February 2005. [Abstract 162]. [Google Scholar]
- 16.Harris M, Ochoa C, Allavena C et al. NRTI Sparing Trial (CTN 177): antiviral and metabolic effects of nevirapine1lopinavir/ ritonavir versus zidovudine/lamivudine1nevirapine. 3rd International AIDS Conference. Rio de Janeiro, Brazil, July 2005. [Abstract WePe63C14]. [Google Scholar]
- 17.Riddler SA, Haubrich R, DiRienzo G et al. A prospective,randomized, phase III trial of NRTI-, PI-, and NNRTI-sparing regimens for initial treatment of HIV infection – ACTG 5142. XVI World AIDS Conference. Toronto, Canada August 2006. [Abstract THLB02]. [Google Scholar]
- 18.Staszewski S, Dauer B, Von Hentig N et al. The LOPSAQ study: 24 week analysis of the double protease inhibitor salvage regimen containing lopinavir plus saquinavir without any additional antiretroviral therapy. 2nd International AIDS Conference. Paris, France July 2003. [Abstract 853]. [Google Scholar]
- 19.Staszewski S, Dauer B, Gute P et al. The CrixiLop Cohort study: preliminary results from a salvage study of HIV positive patients treated with indinavir and lopinavir/ritonavir without the addition of reverse transcriptase inhibitors. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Chicago, IL, September 2003. [Abstract H-853]. [Google Scholar]
- 20.Raguin G, Chene G, Morand-Joubert L et al. Puzzle 1 Study group. Salvage therapy with amprenavir, lopinavir and ritonavir 200 mg/d or 400 mg/d in HIV-infected patients in virological failure. Antiviral Ther 2004; 9: 615–625. [PubMed] [Google Scholar]
- 21.Murphy R, Zhang J, Hafner R et al. AACTG 5110 Study Team. Switching from thymidine analog-sparing or a nucleosidesparing regimen improves lipoatrophy: 24 weeks results of a prospective randomized clinical trial, AACTG 5110. 12th Conference on Retroviruses and Opportunistic Infections. Boston, MA, February 2005. [Abstract LB45]. [Google Scholar]
- 22.Tebas P, Zhang J, Yarakeshi K et al. and the Adult AIDS Clinical Trial Group (AACTG). Switch to a protease inhibitor containing/nucleoside reverse transcriptase inhibitor sparing regimen increases appendicular fat and serum lipid levels without affecting glucose metabolism or bone mineral density. The results of a prospective randomized trial, ACTG 5125. 12th Conference on Retroviruses and Opportunistic Infections. Boston, MA, February 2005. [Abstract LB40]. [Google Scholar]