Abstract
Objective:
Kenya is heralded as an example of declining HIV in Africa, while its tuberculosis (TB) numbers continue rising. We conducted a comparative investigation of TB–HIV co-dynamics in Africa to determine the likelihood of reported trends.
Methods and results:
Our mathematical modeling analysis exposes the notable incongruence of reported trends in Kenya because TB–HIV co-dynamics, tightly knit worldwide and most dramatically in sub-Saharan Africa, suggest that declining HIV trends should trigger reductions in TB trends. Moreover, a continental-scale analysis of TB–HIV trends places Kenya as an outlier in eastern and southern Africa, and shows TB outpacing HIV in western central Africa. We further investigate which TB processes across HIV stages have greater potential to reduce TB incidence via a sensitivity analysis.
Conclusions:
There are two parsimonious explanations: an unaccounted improvement in TB case detection has occurred, or HIV is not declining as reported. The TB–HIV mismatch could be compounded by surveillance biases due to spatial heterogeneity in disease dynamics. Results highlight the need to re-evaluate trends of both diseases in Kenya, and identify the most critical epidemiological factors at play. Substantial demographic changes have occurred in Kenya, including rapid urbanization accompanied by poor living conditions, which could disproportionately increase TB incidence. Other possible contributors include immune reconstitution due to the recent delivery of antiretrovirals, and an increased presence of the virulent Beijing/W TB genotype. Results support the importance of integrating information from closely interacting epidemics, because this approach provides critical insights unobtainable when components of generalized epidemics are considered individually.
Introduction
Worldwide, epidemiologists face great challenges when tracking temporal and spatial trends of infectious diseases (Walker et al., 2004; Ghys et al., 2006; Morgan et al., 2006; and Garnett et al., 2006a). Monitoring Kenya’s HIV epidemic has been particularly challenging. In this sub-Saharan country HIV prevalence declined from almost 10% in the late 1990s to 6.1% in 2005 (Supporting document), according to well supported recent estimates derived from a combination of different sources including annual sentinel surveillance in antenatal clinics since 1990 and Demographic and Health Surveys in 1993, 1998, and 2003 (García-Calleja et al., 2005, 2006; Cheluget et al., 2006; and UNAIDS, 2006). The resulting downward trend is heralded as an example of declining HIV in Africa (Shelton et al., 2006). Prior to these numbers, HIV prevalence in Kenya was estimated to have reached 15% in 2001 (Walker et al., 2003). A subsequent re-evaluation estimated prevalence peaking at 15.4% in 1999–2000, and decreasing to 9.5% in 2003 (Asamoah-Odei et al., 2004). In stark contrast to its HIV trends (Fig. 1), Kenya has reported a steady and substantial increase in the number of new TB cases and deaths since the early 1990s (Currie et al., 2003; and World Health Organization, 2006a). The divergent TB–HIV trends reported for Kenya raise serious concerns given the tight epidemiologic linkage typically exhibited by the two diseases, an association that is particularly dramatic in sub-Saharan Africa (De Cock and Chaisson, 1999; Corbett et al., 2003; and Nunn et al., 2005). Observed co-dynamics suggest strongly declining HIV trends should lead to declines in TB trends (Currie et al., 2003; Lawn et al., 2006; and Dye, 2006).
Fig. 1.
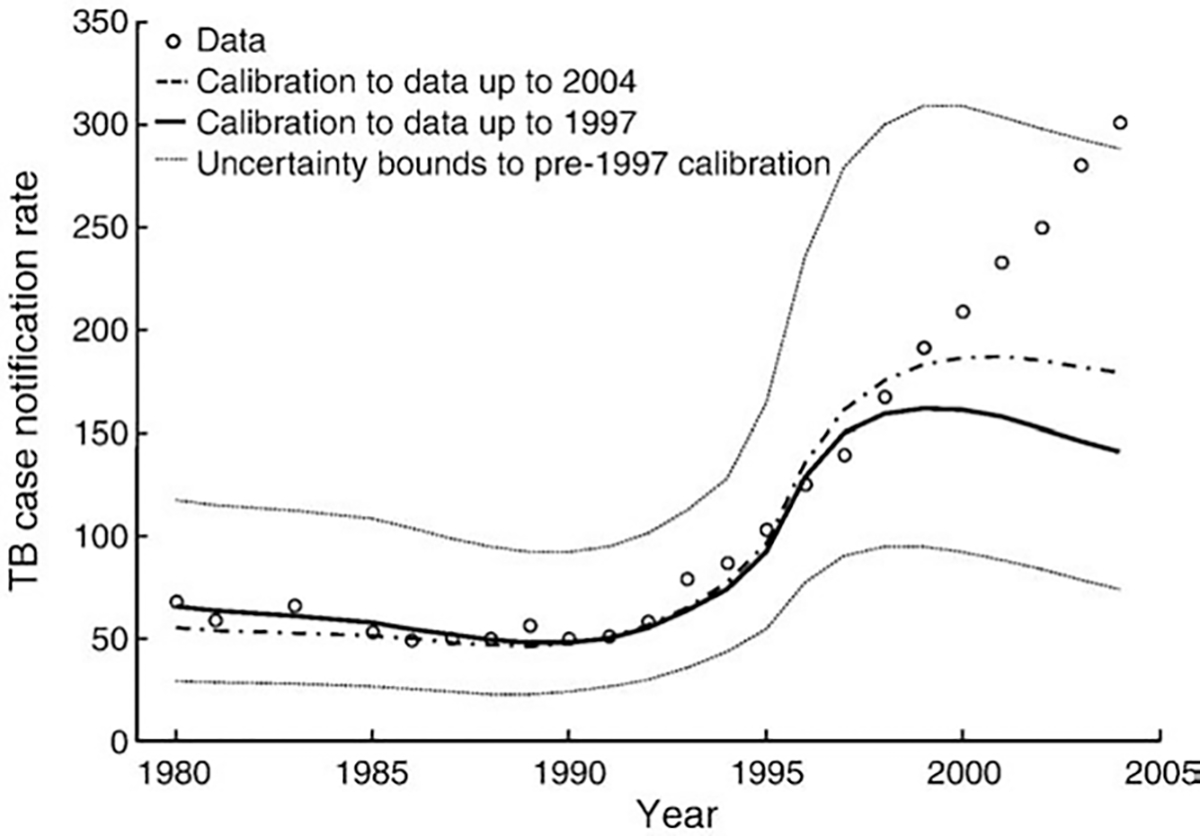
Reported TB case notifications per 100,000 persons in Kenya, and model output following reported HIV prevalence trends calibrated to various TB and joint TB–HIV measures for two different frames: 1980–1997, and 1980–2004. Note: we present the uncertainty bounds, as determined by the 1000 runs with the best goodness-of-fit (GF), for the calibration to data points up to 1997. We show TB case notifications because it is the measure with the longest time series, but similarly incongruent results were obtained for incidence and mortality.
Mathematical models provide an effective tool to explore epidemic interactions by integrating clinical knowledge on co-infections at the individual scale with mechanistic processes at the population scale. Furthermore, we can use long-term epidemiological trends from each epidemic, such as incidence and prevalence data, to cross-check anticipated effects (Currie et al., 2003; Williams and Dye, 2003; Salomon et al., 2006; Dye et al., 1998; and Murray and Salomon, 1998). In this regard, valuable insights have been obtained by contrasting epidemiological trends of interacting diseases. For example, comparing syphilis and gonorrhea trends across the United States established the importance of partial protective host immunity in determining a pattern of synchronized epidemics characteristic of syphilis but absent for gonorrhea (Grassly et al., 2005). Differential mortality in men and women due to the 1918 influenza epidemic explains subsequent changes in TB mortality trends in the first half of the 20th century (Noymer and Garenne, 2000). Co-infection with HIV and malaria may have enhanced the spread of both diseases in sub-Saharan Africa (Abu-Raddad et al., 2006) because HIV infected patients are more susceptible to malaria and, during febrile malaria episodes, HIV viral load increases. Integrating information on rates of curable sexually transmitted diseases (STD) and the timing of the HIV epidemic, together with sexual behavior differences, help explain the critically different outcomes of HIV/sexually transmitted intervention trials conducted in East Africa (White et al., 2004). Changes in STD incidence can be used to monitor the HIV epidemic because of the shared transmission route (Pinkerton et al., 2003).
We elaborated an existing TB–HIV model (Salomon et al., 2006) to include person–level interactions between HIV and TB across the four WHO HIV stages (Williams et al., 2005). The original purpose of our model was to investigate the potential impact of shortening TB treatment duration in high HIV prevalence areas (Sánchez et al., 2008). The HIV epidemic was treated as an external input, whereby HIV incidence and progression occurred as background processes that generated HIV prevalence levels reported for Kenya (Cheluget et al., 2006). We estimated disease parameters, including some that are very difficult to measure in clinical settings, using an iterative calibration process to simultaneously fit TB and joint TB–HIV epidemiological data from Kenya spanning 1980–2004 (Methods and Supporting document). Furthermore, our detailed model permitted us to investigate not only which disease processes can have a greater impact on TB incidence, but also the HIV stage at which we can intervene to cause a greater reduction in TB numbers.
The striking mismatch between TB and HIV trends reported for Kenya and those projected by the model led us to analyze TB–HIV trends for the whole of Africa to gain a wide-range perspective on TB–HIV co-epidemiology. Our comparative process provides the first example where reported HIV and TB trends cannot be reconciled given present-day knowledge on their co-dynamics, and demonstrates the value of monitoring epidemiological trends at broad spatial scales via the comparison of trends of tightly linked co-infections within a mathematical modeling framework. We further explore potential causes for the incongruent TB–HIV co-dynamics in Kenya.
Methods
Our compartmental model considers 42 TB categories and 5 HIV stages linked by disease processes (see Supporting document for a detailed explanation of the model structure). HIV affects TB rates characterizing infection, progression, and mortality. TB only influences HIV dynamics indirectly because it does not alter the rates of HIV infection or progression. We conducted all our computational analyses in Matlab®. The generation of the Latin Hypercube matrices and the sensitivity and uncertainty analyses (Blower and Dowlatabadi, 1994) were done in Simlab®.
Model formulation
Our model formulation is an extension of Salomon et al. (2006), the major difference between the two studies being the greater complexity characterizing the categories and processes representing the HIV epidemic in our model. The model consists of a set of discrete time difference equations with 1 month updates, which we have solved using a competing rates formulation to approximate continuous processes on time scales finer than a month (see Supporting document for our calculation method). This modeling scheme has the advantage that when a group of individuals is subjected to multiple processes simultaneously (e.g. individuals in the latent fast-progressor class can either die or break down to active disease) their outcome at any time step is determined in such a way that an artificial order of processes does not have to be defined because all processes act simultaneously. However, this approach does introduce the implicit assumption that each individual can only undergo one state transition per time step. More specifically, we assumed all TB processes and both background and HIV mortality operate as competing rates, while HIV infection and progression occurs independently from TB progression for those persons that do not die at each particular time step.
TB–HIV historical reconstruction
Each simulation reconstructs the history of the TB and HIV epidemics in Kenya through a series of historical phases (Supporting document). An epidemic of TB without treatment is run to equilibrium, generating conditions corresponding to 1958, when the first Kenyan national TB control program started (Odhiambo et al., 1999). This period includes partially-effective TB treatment and lasts 20 years. We then introduce HIV to match relevant increases in HIV prevalence beginning in the early 1980s. After 15 years DOTS begins and gradually increases its coverage over the next 2 years to replicate its official initiation in Kenya in 1993, and the achievement of nationwide coverage by 1996 (Hanson and Kibuga, 2000). We simulate DOTS for 10 years, until 2006.
Model calibration
In our calibration, we allowed the value of specified parameters to vary in order to identify the parameter set that generates the model trajectory with the closest fit to the epidemiological trends of interest. Calibrating a model is particularly valuable when the uncertainty in the numerical values of the parameters characterizing the different disease processes is large. We varied a total of 12 parameter types, of which nine were partitioned across HIV stage for a total of 47 parameters. In our selection we targeted those parameters that were most important in the sensitivity analysis of Salomon et al. (2006), and reflected core processes of TB natural history or the impact of TB control programs and HIV on TB dynamics. We first defined uniform parameter ranges for these chosen 47 parameters (Table S2) based on published literature, expert opinion, and several preliminary calibration rounds. We then proceeded to generate candidate parameter sets using the Latin Hypercube Sampling method, LHS (Blower and Dowlatabadi, 1994). All parameters had constant values throughout each run (i.e., throughout all the years corresponding to the historical reconstruction) except for two TB control parameters: the proportion of new cases entering the detectable pool and the proportion of cases (both smear-positive and negative) entering DOTS programs. To address the impact of decreased immunity as HIV infection progresses, we defined constraints for the relative magnitudes of specific parameters, satisfying the inequalities uninfected>stage I and stage II>stage III>stage IV or vice-versa. We did not constrain the relative magnitudes of parameters pertaining to stages I and II, because of the uncertainty on how the immune system is affected by early stage HIV infection in regards to TB infection and progression (Sonnenberg et al., 2005; and Fox et al., 2006). By leaving the relative magnitudes of these parameters unconstrained, we wanted to establish what individual-level TB progression patterns would emerge from the calibration of our model to country-level data.
We defined the following goodness of fit measure:
where ‘observed’ corresponds to reported epidemiological data and ‘expected’ is the model output, which varies with each parameter set (Supporting document). The best-fit trajectory is that one with the smallest .
We first evaluated the fit of simulated trends to the full TB and TB–HIV datasets in Kenya (1980–2004, 106 measures) reported in the WHO Global TB Database (World Health Organization, 2006b) and the WHO Global TB Reports from 2001 to 2006 (Supporting document) under the HIV prevalence series covering this time period (Cheluget et al., 2006; and UNAIDS, 2006). When the difficulty in fitting post-1997 trends became evident (see below), we conducted a second calibration to the ‘pre-1997’ TB and TB–HIV dataset (1980–1997, 44 measures) and the HIV prevalence series also until 1997. We conducted an additional calibration to establish if our model could capture the rise in TB case notifications throughout the full dataset (1980–2004) given different assumptions regarding HIV trends (see Supporting document).
Pan-African patterns
We calculated the relative average annual changes in TB case notifications between 1998 and 2004, and compared it to the relative average annual changes in HIV prevalence between 2003 and 2005 for those African countries recently reported by both WHO (World Health Organization, 2006b) and UNAIDS (UNAIDS, 2006). Owing to substantial revisions for many countries, HIV estimates from earlier years could not be used at the continental scale. We subtracted 1 from this ratio to obtain a measure centered at 0:
The only exception was Zambia, who did not report a TB case notification estimate in 1998; for this country we used the 1999 estimate and divided the ratio in the numerator by 5 instead of 6 (to accommodate for the 1-year shorter interval to 2004).
Sensitivity analysis
We determined which parameters caused greater changes in TB incidence over the 25 year projection period (2006–2030) at a stable 6.7% HIV prevalence level. We calculated the partial rank correlation coefficients (PRCC) with respect to TB incidence at the very beginning and end of this time period for the 47 parameters allowed to vary in the model calibration, using the 1000 parameter sets that provided the best fit to reported Kenyan trends (Fig. 1 and Supporting document).
Results
Model calibration
Throughout the model calibration, it was not possible to obtain a satisfactory reproduction of TB trends reported prior and subsequent to 1997 while incorporating the reported HIV prevalence drop after 1997. Fig. 1 shows how beyond 1997 the poor fit arises because HIV prevalence declines strongly while TB measures continue to increase linearly. We note that the best-fitting values of the parameters for HIV stages I and II were ordered according to a progressively deteriorating immune system for all parameter types in the pre-1997 calibration, and for all but two types in the full dataset calibration (Supporting document).
The TB case notifications could be matched by the model if we allowed the HIV epidemic to increase monotonically to a prevalence of 22.7% in 2004 (Supporting document). Under this calibration the fit to the TB case notification trends improved by more than 5 times as compared to that of the best-fitting parameter set to reported HIV trends, while the overall fit to the full dataset worsened by less than 20%. The latter occurred mainly because of a worsening of the fit to combined TB–HIV measures, particularly in later years.
Pan-African patterns
When comparing country level TB–HIV co-dynamics across Africa (Fig. 2), Kenya registers as an extreme instance of TB increasing disproportionately to HIV compared to other countries with substantial HIV prevalence (chiefly those in eastern and southern Africa). Moreover, when considering all countries in Africa for which both UNAIDS and WHO provide official HIV and TB yearly estimates, Kenya’s ratio of relative annual changes is third only to those of Cameroon and Nigeria. Our analysis further reveals that TB case notification rates are growing at a faster pace relative to HIV prevalence in the western section of central Africa, as compared to other African regions. Fig. 3 highlights the notable increase in Kenyan TB notification rates as compared to Uganda, which has recently experienced an important drop in HIV levels.
Fig. 2.
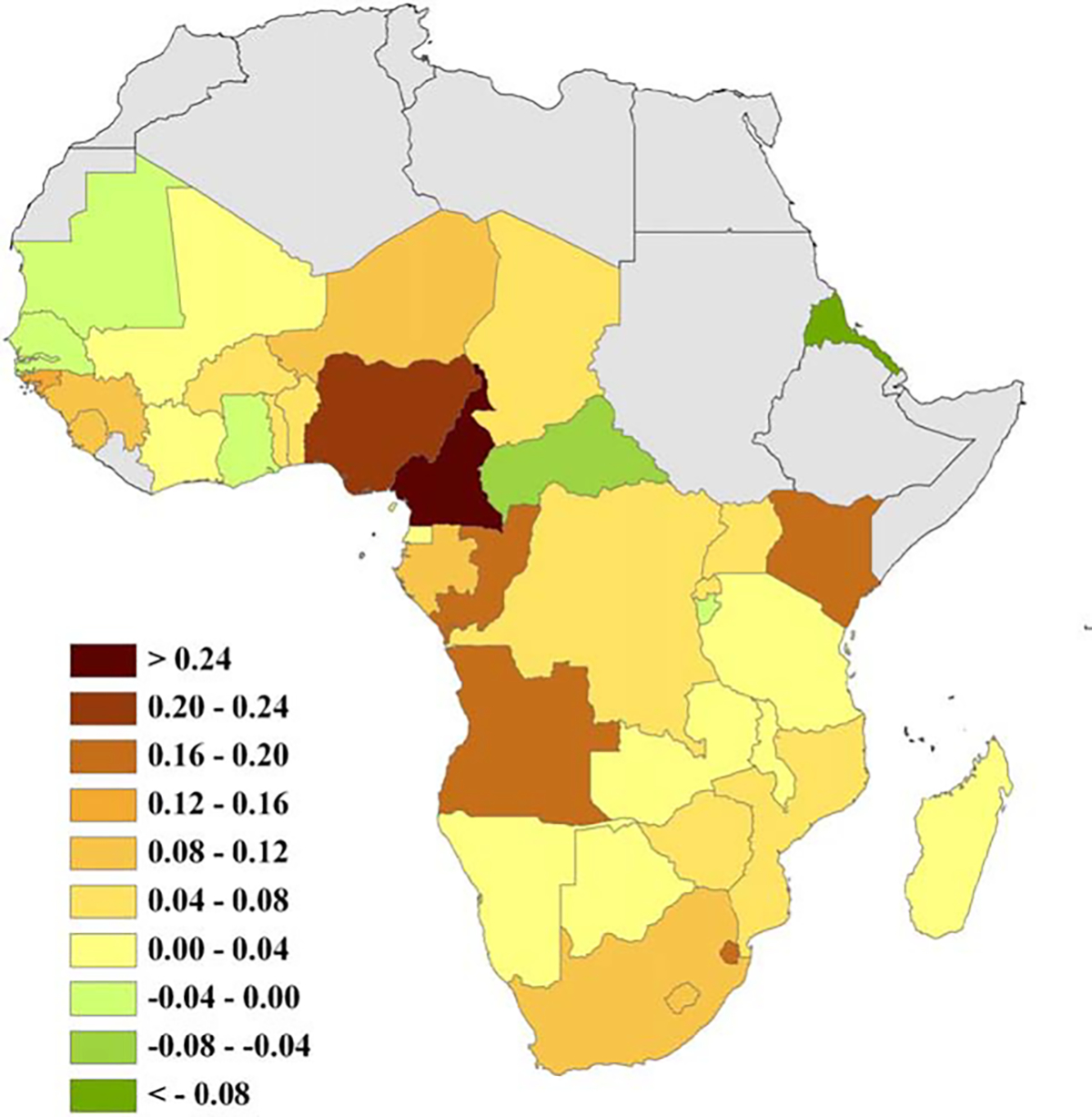
Ratio of relative annual changes in TB case notifications per 100,000 (1998–2004) and HIV prevalence (2003–2005) in Africa. Note: positive values indicate TB increases relative to HIV, zero suggests both diseases change at comparable rates, and negative values imply TB declines relative to HIV.
Fig. 3.
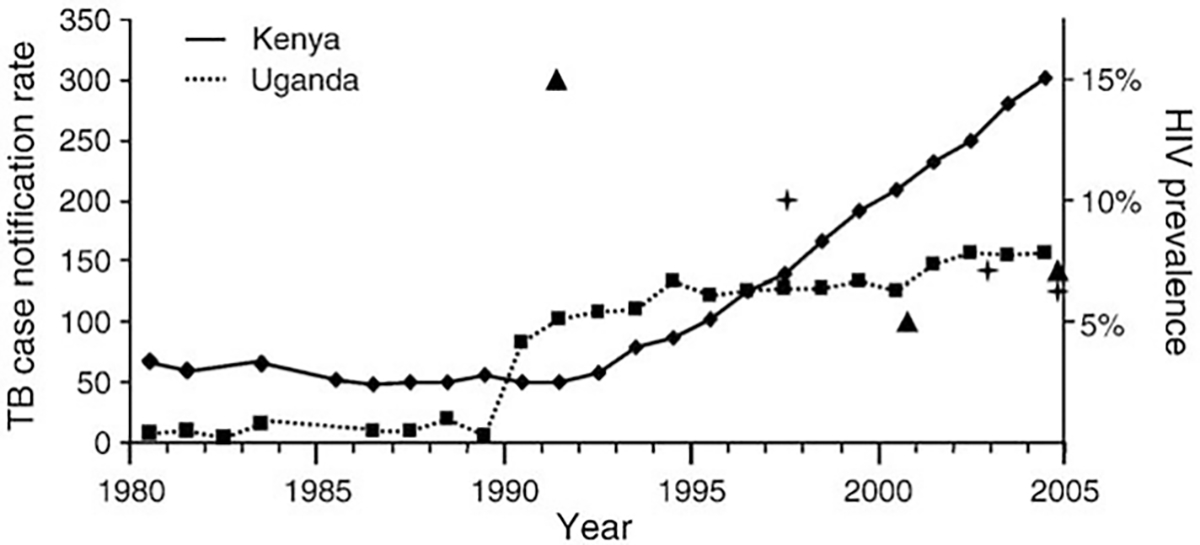
Yearly TB case notification rates per 100,000 in Kenya and Uganda for 1980–2004. Most relevant years in HIV prevalence are indicated by triangles for Uganda, and by crosses for Kenya (Uganda peaked at 15% in the early 1990s, Kenya at 10% in the late 1990s).
Sensitivity analysis
Fig. 4 summarizes the most influential parameters on TB incidence throughout the projection period for both the calibrations to the 1980–2004 and the 1980–1997 datasets. With minor exceptions, parameters with PRCC>0.2 in magnitude were the same and had similar magnitudes for the two calibrations (Supporting document).
Fig. 4.
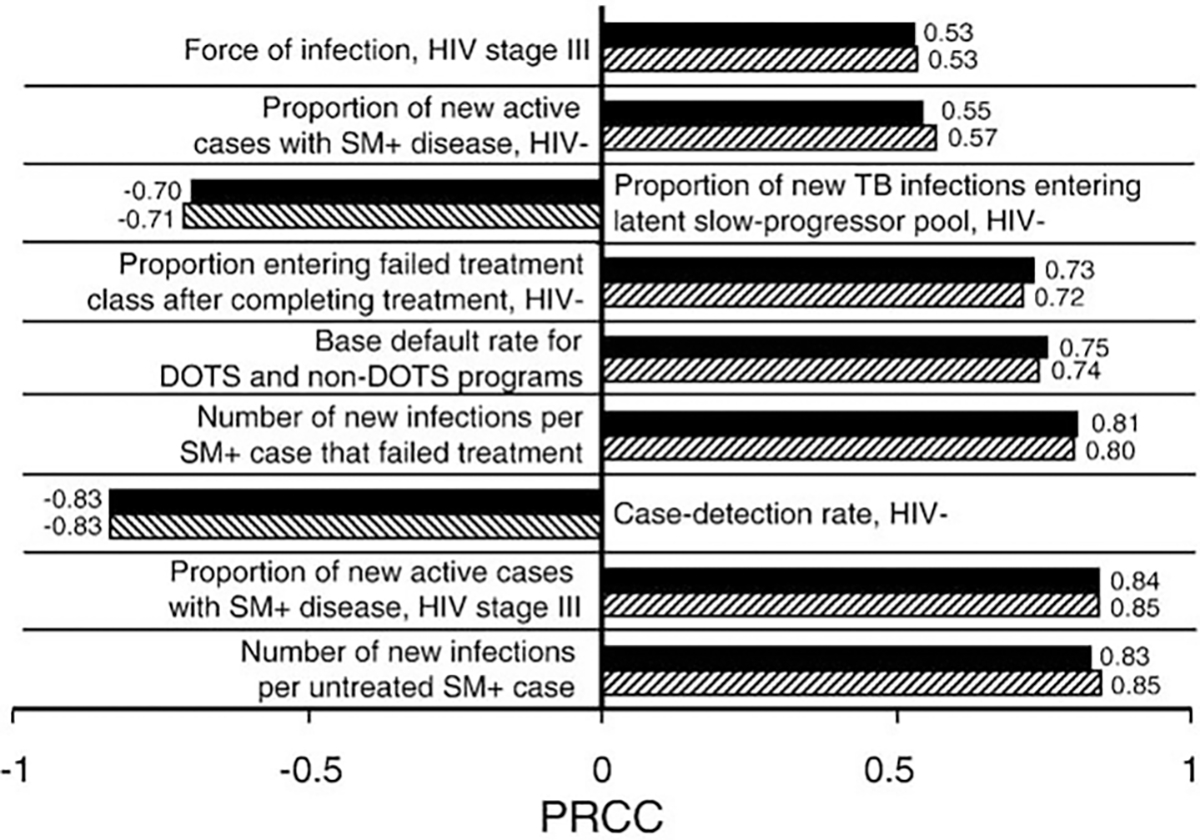
Parameters of greatest impact with regard to changes inTB incidence over a 25 year projection period (2006–2030) at a stable 6.7% HIV prevalence, according to the partial rank correlation coefficient analysis (PRCC). Solid bars represent the calibration to the full time series (1980–2004), and hatched bars represent the calibration to values reported up to, and including, 1997. HIV−: HIV uninfected; SM+: smear-positive TB case.
Discussion
The process of building and calibrating a model to investigate the impact of HIV on TB control (Sánchez et al., 2008) uncovered how in Kenya the recent trends of these two diseases were at odds. Hence, in order to further our understanding of TB–HIV co-dynamics, we examined the joint epidemiological trends of TB and HIV across Africa at different spatial scales. At the community level, the detailed field study of Lawn et al. (2006) provides valuable corroboration of how changes in HIV trends will lead to changes in TB trends. In a South African township, TB case notification rates tapered off in 2002–2004 after a gradual decrease in the HIV prevalence increase rate during 1996–2004. At larger geographic scales, Uganda is the only African country where HIV prevalence has undergone a decrease comparable to that reported for Kenya (UNAIDS, 2006; and Murphy et al., 2006). Ugandan TB case notification rates have been roughly constant since 1994, in sharp contrast to those of Kenya (Fig. 3). In Zimbabwe, HIV prevalence probably peaked in 1998 (Gregson et al., 2006; and Mahomva et al., 2006), and a reduction in TB case notifications started by 2003 (World Health Organization, 2006a). In our analysis Kenya is extraordinary among African countries in terms of relative TB and HIV dynamics (Fig. 2), even though the numbers officially reported by WHO and UNAIDS (UNAIDS, 2006; and World Health Organization, 2006a) do not provide the most optimal time frames for comparison because they do not capture the main drop in HIV prevalence occurring in Kenya in the late 1990s (i.e., our measure disproportionately captures changes in TB trends compared with those of the causal factor, HIV, therefore underestimating the potential impact of Kenya’s substantial HIV decline). Furthermore, Cameroon and Nigeria, the only two countries with a higher rate of TB growth as compared to that of HIV, have much lower TB case notification rates and lower HIV prevalence levels than Kenya (UNAIDS, 2006; and Murphy et al., 2006). The fast TB growth in relation to HIV trends observed in the western section of central Africa also merits further investigation.
In consequence, we found that the lack of congruency in Kenya is supported not only because reported trends cannot be reconciled within the realm of our general understanding of TB–HIV co-epidemiology (Fig. 1), but also because the joint patterns in Kenya constitute an extreme example of TB–HIV co-dynamics in Africa (Figs. 2 and 3). Below we present a discussion of our exploration into the potential causes for the mismatch. For a discussion on the various limitations of our modeling exercise, please refer to the Supporting document.
TB case detection
The most parsimonious explanation for the incongruent TB and HIV trends is that TB case detection has improved in Kenya. No major changes in DOTS coverage have been reported in Kenya since 1995 (World Health Organization, 2006a). If the increasing TB case notifications do not reflect improvements in DOTS coverage, then an increase in the case detection rate from the 1990s to the 2000s, compounded by underreporting in earlier years, could be responsible for our inability to fit the data with our model. Moreover, HIV could have exacerbated the difference by making public health workers more aware and likely to detect new TB cases (see above). Indeed, Mansoer et al. (in press) found that the increase in TB case notifications (all forms and smear-positive TB) between 2001 and 2006 coincides with increases in the number of TB diagnostic units, general health units, TB suspects examination rates, and staffing levels. Their analysis leads them to conclude that the case detection rate had probably increased from approximately 57% in 1996 to around 70% in 2006 (Mansoer et al., in press). In comparison, new smear-positive TB cases increased from around 90 cases per 100,000 persons to around 140 cases per 100,000 persons between 2000 and 2006, and total (smear-positive and smear-negative) notifications increased from around 200 to around 300 per 100,000 persons between 2000 and 2004 (Fig. 1). Given the respective magnitude of these changes, even though an increase in the detection rate can certainly explain some of the trend, it is unlikely to be the sole cause of the TB–HIV mismatch.
HIV trends
An alternative, simple explanation for the mismatch is that HIV prevalence has not decreased as reported, and instead the decreasing trend reflects the change in HIV surveillance methodology. Garnett et al. (2006b) note that the strong decline in HIV prevalence is not predicted by reported changes in risk behavior in Kenya. When using a different modeling formulation, Currie et al. (2003) also obtained the critical result where declining HIV trends trigger a decline in TB trends. Most significantly, UNAIDS is at present evaluating new data from a population based survey together with data from other sources to provide updated HIV estimates for Kenya. Even though at the moment only provisional ranges are provided by UNAIDS, preliminary analyses do indicate HIV numbers in Kenya could be higher than those reported in previous years (UNAIDS, 2008).
In order to reproduce reported TB trends in Kenya, our model required a monotonically increasing HIV epidemic as the driver. Moreover, while notification rates for the years 2005 and 2006 for smear-positive TB patients have declined in Kenya, those of smear-negative and extra-pulmonary patients have continued rising, and the proportion of pulmonary TB patients that are smear-positive is declining steadily since 1995 (Mansoer et al., in press). Because HIV-uninfected persons are more likely to be smear-positive as compared to HIV-infected persons (Supporting document) this rate of decrease in the smear-positive fraction does not support a decrease in HIV prevalence among the TB-infected in Kenya. However, these trends may reflect a recent improved diagnosis of TB in HIV-infected patients as compared to previous years (see above). In any case, present day limitations to estimating HIV prevalence (Morgan et al., 2006; and Dye et al., 2005) are not likely to generate an underestimation of the magnitude that our model required to match the TB case notification rates in Kenya, (i.e., 6.7% HIV prevalence reported in 2004 vs. 22.7% estimated). Furthermore, because of the inherent difficulties in disease monitoring and the uncertainties regarding the parameter values characterizing TB–HIV interactions, our calibration process is not sufficiently refined to permit us to estimate the true HIV prevalence in Kenya even if reported TB trends are accurate. Note that we did not conduct this fit as a means to estimate HIV prevalence in Kenya. Rather this fit was intended to show there is no inherent problem with the model that made it incapable of tracking the TB trends officially reported for Kenya. By altering inputs to the calibration process, the TB numbers could be reconciled with HIV epidemics that increase to prevalence levels <20%, but these changes will require further assumptions regarding recent changes in TB and HIV dynamics in Kenya. HIV prevalence in Kenya is therefore likely to be considerably <22.7%, and other causes besides the underestimation of HIV trends are responsible, or must be contributing, to the TB–HIV discordance.
Spatial heterogeneity
Additional confounding factors could have contributed to the reported co-dynamics. Spatial heterogeneity in disease dynamics and surveillance is important in both HIV and TB epidemiology (Cheluget et al., 2006; UNAIDS, 2006; Abu-Raddad et al., 2006; Dye et al., 2005; Hallett et al., 2006; Glynn et al., 2006; and Gagneux et al., 2006a,b), and may greatly affect the interpretation of temporal patterns (Brown et al., 2006). Averaging across a country can lead to biases not only in our understanding of the trends of the individual diseases, but also in our interpretation of their co-epidemiology; HIV and TB numbers may predominantly reflect trends from distinct parts of the country, such as rural and urban areas. As such, important differences in TB–HIV trends exist across districts in Kenya. For example, during 1990–2004 Kisumu had a much higher average HIV prevalence than Nairobi (the former attained 35% in 2000, while the latter never reached 18% (Cheluget et al., 2006). However, the TB case notification rates of these two districts were very similar (Mansoer et al., in press): in 1996 both had close to 300 new TB cases per 100,000 persons, and this proportion rose to approximately 700 per 100,000 persons in 2004–2005. Moreover, important demographic changes have recently occurred in Kenya that could have contributed to the spatial heterogeneity in disease dynamics. A rapid urbanization rate, particularly in Nairobi, led to a dramatic increase in the number of people living in urban slums, and overcrowded unsanitary conditions are prime TB habitat. These factors can generate a positive feedback loop for TB, which may further facilitate the spread of bacterial strains that are at a transmission disadvantage with respect to the wild type strain, such as drug resistant strains, see below (Gagneux et al., 2006a, c). Further investigations may clarify the existence of frequency- and density-threshold effects acting in synergy with HIV and/or human demography to alter TB dynamics.
Antiretroviral therapy, TB genetics, and disease progression
Increases in antiretroviral therapy (ARVT) coverage in Kenya—from 3% in 2003 to 19.7% in 2005 (UNAIDS, 2006)—could also have contributed to the reported increase in TB case notifications in recent years, because patients starting ARVT undergo a process of immune reconstitution that can lead to the development of TB symptoms. Additionally, the Beijing/W Mycobacterium tuberculosis genotype, which has been increasing globally (Glynn et al., 2006), was recently identified in Nairobi (Githui et al., 2004). If this genotype has higher virulence and transmissibility than previous Kenyan strains, we expect to see a corresponding increase in TB cases (Glynn et al., 2006; Gagneux et al., 2006b,c; Lan et al., 2003; Marais et al., 2006; López et al., 2003; Malik and Godfrey-Faussett, 2005; and Lawn and Wilkinson, 2006).
The temporal dynamics of the TB–HIV co-epidemic, and as such of our model results, will be directly impacted by the time of progression to active TB upon infection with HIV. We would like to point out that a recent study (Wandel et al., 2008) indicates these could potentially be slower than the estimates we used in our model formulation (Morgan et al., 2002) (see Supporting document), thus delaying the onset of the predicted drop in TB rates as a consequence of the decrease in HIV prevalence. In any case, the studies discussed above, together with Fig. 3, indicate that other sites have undergone a more rapid response of the TB epidemic to decreasing trends in the HIV epidemic than what Kenya is showing.
Sensitivity analysis: TB processes across HIV stages with greatest impact on TB
It is imperative to establish priorities for control of the twin epidemics, and a sensitivity analysis can help us determine which TB life history and control processes could cause a greater reduction in TB incidence if intervened, and at what HIV stage(s) we can intervene to maximize TB control benefits. In turn, these processes should represent those measures most crucial to determine accurately from the data. Our sensitivity analysis yields remarkably consistent results for the two model calibrations (the pre-1997 and the full dataset, Fig. 4), indicating the robustness of these conclusions to the model formulation and specified parameter values. The high-impact parameters corresponded mostly to the HIV uninfected and to HIV stage III, and to a lesser extent to HIV stage I. This pattern is likely due to HIV uninfected people comprising the majority of the population, as well as to the relative durations and degrees of immunocompetence of the HIV stages. The longest lasting HIV stage is III followed by I (5.5 and 2 years, respectively). The relative importance of stage I may also be influenced by the fact that all infected patients in the simulation will have passed (or be in) the first stage—whereas only part of them will already have reached later stages. Additional insights are provided in the Supporting document.
Conclusion
Our study represents the first comparative analysis exposing anomalies in the temporal trends of HIV and TB in sub-Saharan Africa, thus identifying key issues for public health planners to resolve. Because HIV is such a strong driver of TB dynamics, it cannot have had such a dramatic country-level decrease in Kenya without triggering a change in TB. Therefore, there should be a continued emphasis on understanding past and present HIV–TB co-epidemiology. One or a combination of the explanations presented above could explain the mismatch between empirical data and model output. If HIV has not decreased, this conveys bad news regarding the HIV epidemic. On the other hand, this would make TB trends less worrisome because if HIV is decreasing, and if not all the increase in TB is due to an increase in case detection and/or spatial heterogeneity in HIV and TB surveillance, then some other unidentified phenomenon is responsible for the increase in TB trends. In this regard, recent re-evaluations of the TB case detection rate cannot fully account for the mismatch.
Unraveling what role these various factors may have played in shaping the reported TB and HIV trends will require increased coordination and exchange of information among organizations and institutions managing each disease (Nunn et al., 2005). Our study highlights the need for a long-term interplay between data collection at the individual and population levels at multiple spatial scales, and for the methodical integration of TB, HIV, and other co-infection data. Results further support the importance of integrating information from closely interacting epidemics within a theoretical modeling framework, which provides critical insights unobtainable when components of generalized epidemics are considered individually.
Supplementary Material
Acknowledgments
We thank anonymous reviewers and David Bangsberg, Jason Barbour, Frank Cobelens, Kathryn DeRiemer, Judy Hahn, John Hargrove, Philip Hopewell, Gwynne Oosterbaan, Perry de Valpine, Francois Venter, and members of Wayne Getz’s lab, for their help and orientation. We also thank the Kenyan public health authorities and all persons participating in the data collection. This research was supported by NIH-NIDA (MSS and WMG), the James S. McDonnell Foundation 21st Century Science Initiative (WMG), the Global Alliance for TB Drug Development (WMG), and SACEMA, the South African Center for Epidemiological Modeling and Analysis (WMG).
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.epidem.2008.08.001.
References
- Abu-Raddad LJ, Patnaik P, Kublin JG, 2006. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science 314, 1603–1606. [DOI] [PubMed] [Google Scholar]
- Asamoah-Odei E, García-Calleja JM, Boerma JT, 2004. HIV prevalence and trends in sub-Saharan Africa: no decline and large subregional differences. Lancet 364, 35–40. [DOI] [PubMed] [Google Scholar]
- Blower SM, Dowlatabadi H, 1994. Sensitivity and uncertainty analysis of complex-models of disease transmission—an HIV model, as an example. Int. Stat. Rev. 62, 229–243. [Google Scholar]
- Brown T, Grassly NC, Garnett G, Stanecki K, 2006. Improving projections at the country level: the UNAIDS estimation and projection package 2005. Sex. Transm. Infect. 82, III34–III40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheluget B, Baltazar G, Orege P, Ibrahim M, Marum LH, Stover J, 2006. Evidence for population level declines in adult HIV prevalence in Kenya. Sex. Transm. Infect. 82, I21–I26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. , 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163, 1009–1021. [DOI] [PubMed] [Google Scholar]
- Currie CS, Williams BG, Cheng RC, Dye C, 2003. Tuberculosis epidemics driven by HIV: is prevention better than cure? AIDS 17, 2501–2508. [DOI] [PubMed] [Google Scholar]
- De Cock KM, Chaisson RE,1999. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int. J. Tuberc. Lung Dis. 3, 457–465. [PubMed] [Google Scholar]
- Dye C, 2006. Global epidemiology of tuberculosis. Lancet 367, 938–940. [DOI] [PubMed] [Google Scholar]
- Dye C, Garnett GP, Sleeman K, Williams BG, 1998. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Directly observed short-course therapy. Lancet 352, 1886–1891. [DOI] [PubMed] [Google Scholar]
- Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC, 2005. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA-J. Am. Med. Assoc. 293, 2767–2775. [DOI] [PubMed] [Google Scholar]
- Fox J, Weber J, Fidler S, 2006. Primary HIV. Sex. Transm. Infect. 82, 267–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneux S, Burgos MV, DeRiemer K, Enciso A, Munoz S, Hopewell PC, et al. , 2006a. Impact of bacterial genetics on the transmission of isoniazid-resistant Mycobacterium tuberculosis. Plos Pathogens 2, 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, Narayanan S, et al. , 2006b. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 103, 2869–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneux S, Long CD, Small PM, Van T, Schoolnik GK, Bohannan BJM, 2006c. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science 312, 1944–1946. [DOI] [PubMed] [Google Scholar]
- García-Calleja JM, Marum LH, Carcamo CP, Kaetano L, Muttunga J, Way A, 2005. Lessons learned in the conduct, validation, and interpretation of national population based HIV surveys. AIDS 19 (Suppl. 2), S9–S17. [DOI] [PubMed] [Google Scholar]
- García-Calleja JM, Gouws E, Ghys PD, 2006. National population based HIV prevalence surveys in sub-Saharan Africa: results and implications for HIV and AIDS estimates. Sex. Transm. Infect. 82 (Suppl. 3), iii64–iii70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett GP, García-Calleja JM, Rehle T, Gregson S, 2006a. Behavioural data as an adjunct to HIV surveillance data. Sex. Transm. Infect. 82, I57–I62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett GP, Gregson S, Stanecki KA, 2006b. Criteria for detecting and understanding changes in the risk of HIV infection at a national level in generalised epidemics. Sex. Transm. Infect. 82, I48–I51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghys PD, Kufa E, George MV, 2006. Measuring trends in prevalence and incidence of HIV infection in countries with generalised epidemics. Sex. Transm. Infect. 82, I52–I56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githui WA, Jordaan AM, Juma ES, Kinyanjui P, Karimi FG, Kimwomi J, et al. , 2004. Identification of MDR-TB Beijing/W and other Mycobacterium tuberculosis genotypes in Nairobi, Kenya. Int. J. Tuberc. Lung Dis. 8, 352–360. [PubMed] [Google Scholar]
- Glynn JR, Kremer K, Borgdorff MW, Rodríguez MP, van Soolingen D, 2006. Beijing/W genotype Mycobacterium tuberculosis and drug resistance. Emerg. Infect. Dis. 12, 736–743.16704829 [Google Scholar]
- Grassly NC, Fraser C, Garnett GP, 2005. Host immunity and synchronized epidemics of syphilis across the United States. Nature 433, 417–421. [DOI] [PubMed] [Google Scholar]
- Gregson S, Garnett GP, Nyamukapa CA, Hallett TB, Lewis JJ, Mason PR, et al. , 2006. HIV decline associated with behavior change in eastern Zimbabwe. Science 311, 664–666. [DOI] [PubMed] [Google Scholar]
- Hallett TB, Aberle-Grasse J, Boulos LM, Cayemittes MPA, Cheluget B, Chipeta J, et al. , 2006. Declines in HIV prevalence can be associated with changing sexual behaviour in Uganda, urban Kenya, Zimbabwe, and urban Haiti. Sex. Transm. Infect. 82, I1–I8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson C, Kibuga D, 2000. Effective tuberculosis control and health sector reforms in Kenya: challenges of an increasing tuberculosis burden and opportunities through reform. Int. J. Tuberc. Lung Dis. 4, 627–632. [PubMed] [Google Scholar]
- Lan NTN, Lien HTK, Tung LB, Borgdorff MW, Kremer K, van Soolingen D, 2003. Mycobacterium tuberculosis Beijing genotype and risk for treatment failure and relapse, Vietnam. Emerg. Infect. Dis. 9, 1633–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn SD, Wilkinson R, 2006. Extensively drug resistant tuberculosis. BMJ 333, 559–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R, 2006. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clin. Infect. Dis. 42, 1040–1047. [DOI] [PubMed] [Google Scholar]
- López B, Aguilar D, Orozco H, Burger M, Espitia C, Ritacco V, et al. , 2003. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 133, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahomva A, Greby S, Dube S, Mugurungi O, Hargrove J, Rosen D, et al. , 2006. HIV prevalence and trends from data in Zimbabwe, 1997–2004. Sex. Transm. Infect. 82 (Suppl. 1), i42–i47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik AN, Godfrey-Faussett P, 2005. Effects of genetic variability of Mycobacterium tuberculosis strains on the presentation of disease. Lancet, Infect. Dis. 5, 174–183. [DOI] [PubMed] [Google Scholar]
- Mansoer J, Scheele S, Floyd K, Dye C, Sitienei J, Williams BG, New Methods for Estimating the Tuberculosis Case Detection Rate in High-HIV Prevalence Countries: The Example of Kenya. Bulletin of the World Health Organization, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais BJ, Victor TC, Hesseling AC, Barnard M, Jordaan A, Brittle W, et al. , 2006. Beijing and Haarlem genotypes are overrepresented among children with drug-resistant tuberculosis in the Western Cape Province of South Africa. J. Clin. Microbiol. 44, 3539–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Mahe C, Mayanja B, Okongo JM, Lubega R, Whitworth JAG, 2002. HIV-1 infection in rural Africa: is there a difference in median time to AIDS and survival compared with that in industrialized countries? AIDS 16, 597–603. [DOI] [PubMed] [Google Scholar]
- Morgan M, Walker N, Gouws E, Stanecki KA, Stover J, 2006. Improved plausibility bounds about the 2005 HIV and AIDS estimates. Sex. Transm. Infect. 82, III71–III77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EM, Greene ME, Mihailovic A, Olupot-Olupot P, 2006. Was the “ABC” approach (abstinence, being faithful, using condoms) responsible for Uganda′s decline in HIV? PLoS Med. 3, e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Salomon JA, 1998. Modeling the impact of global tuberculosis control strategies. Proc. Natl. Acad. Sci. U. S. A 95, 13881–13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noymer A, Garenne M, 2000. The 1918 influenza epidemic′s effects on sex differentials in mortality in the United States. Popul. Dev. Rev. 26, 565–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn P, Williams B, Floyd K, Dye C, Elzinga G, Raviglione M, 2005. Tuberculosis control in the era of HIV. Nat. Rev. Immunol. 5, 819–826. [DOI] [PubMed] [Google Scholar]
- Odhiambo JA, Borgdorff MW, Kiambih FM, Kibuga DK, Kwamanga DO, Ng’ang’a L, et al. , 1999. Tuberculosis and the HIV epidemic: increasing annual risk of tuberculous infection in Kenya, 1986–1996. Am. J. Public Health 89, 1078–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton SD, Layde PM, DiFranceisco W, Chesson HW, 2003. All STDs are not created equal: an analysis of the differential effects of sexual behaviour changes on different STDs. Int. J. STD AIDS 14, 320–328. [DOI] [PubMed] [Google Scholar]
- Salomon JA, Lloyd-Smith JO, Getz WM, Resch S, Sánchez MS, Porco TC, et al. , 2006. Prospects for advancing tuberculosis control efforts through novel therapies. PLoS Med. 3, e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez MS, Lloyd-Smith JO, Porco TC, Williams BG, Borgdorff MW, Mansoer J, et al. , 2008. Impact of HIV on novel therapies for tuberculosis control. AIDS 22, 963–972. [DOI] [PubMed] [Google Scholar]
- Shelton JD, Halperin DT, Wilson D, 2006. Has global HIV incidence peaked? Lancet 367, 1120–1122. [DOI] [PubMed] [Google Scholar]
- Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S, 2005. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J. Infect. Dis. 191, 150–158. [DOI] [PubMed] [Google Scholar]
- UNAIDS, UNAIDS Annual Report: 2006 Report on the global AIDS epidemic: A UNAIDS 10th anniversary special edition. Geneva, 2006. [Google Scholar]
- UNAIDS, 2008 Report on the global AIDS epidemic. Geneva, 2008. [Google Scholar]
- Walker N, Stanecki KA, Brown T, Stover J, Lazzari S, García-Calleja JM, et al. , 2003. Methods and procedures for estimating HIV/AIDS and its impact: the UNAIDS/WHO estimates for the end of 2001. AIDS 17, 2215–2225. [DOI] [PubMed] [Google Scholar]
- Walker N, Grassly NC, Garnett GP, Stanecki KA, Ghys PD, 2004. Estimating the global burden of HIV/AIDS: what do we really know about the HIV pandemic? Lancet 363, 2180–2185. [DOI] [PubMed] [Google Scholar]
- Wandel S, Egger M, Rangsin R, Nelson KE, Costello C, Lewden C, et al. , 2008. Duration from seroconversion to eligibility for antiretroviral therapy and from ART eligibility to death in adult HIV-infected patients from low and middle-income countries: collaborative analysis of prospective studies. Sex. Transm. Infect. 84 (Suppl. 1), i31–i36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RG, Orroth KK, Korenromp EL, Bakker R, Wambura M, Sewankambo NK, et al. , 2004. Can population differences explain the contrasting results of the Mwanza, Rakai, and Masaka HIV/sexually transmitted disease intervention trials? A modeling study. J. Acquir. Immune Defic. Syndr. 37, 1500–1513. [DOI] [PubMed] [Google Scholar]
- Williams BG, Dye C, 2003. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science 301, 1535–1537. [DOI] [PubMed] [Google Scholar]
- Williams BG, Granich R, Chauhan LS, Dharmshaktu NS, Dye C, 2005. The impact of HIV/AIDS on the control of tuberculosis in India. Proc. Natl. Acad. Sci. U. S. A. 102, 9619–9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2006a. Global Tuberculosis Control: Surveillance, Planning, Financing: WHO report 2006. World Health Organization, Geneva. [Google Scholar]
- World Health Organization, 2006b. WHO Global TB Database. Available at: http://www.who.int/tb/country/global_tb_database/en/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


