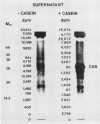
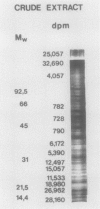
Abstract
An enzyme activity, found for the first time in plants, mainly located in the 22,000g supernatant of the crude extract of sprout apices of Helianthus tuberosus L. cv OB1 tubers, is able in vitro to covalently bind polyamines to endogenous substrates of different molecular weights. The major assay parameters, such as pH, dithiothreitol, and extract concentrations were optimized. The time course of the reaction, the dependence on putrescine concentration, its competition with histamine, the capacity to bind spermidine and spermine better than putrescine, the stability of the reaction product and analysis of the latter by sodium dodecyl sulfate polyacrylamide gel electrophoresis and thin-layer chromatography suggest that putrescine is linked to endogenous substrates by means of an enzyme reaction that shows some similarities with transglutaminase activities detected in animals. However, the activities of the crude extract and of a fraction eluted from a Sephadex G25 column were not affected by CaCl2 concentrations lower or equal to 5 millimolar; 4 or 10 millimolar EGTA caused a very small reduction; higher concentrations of CaCl2 and 15 millimolar or more of EDTA were inhibitory. N,N′-dimethylcasein was not recognized as a substrate. These data indicate that this activity also displays some characteristics which are different from those of animal transglutaminases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambron R. T., Kremzner L. T. Post-translational modification of neuronal proteins: evidence for transglutaminase activity in R2, the giant cholinergic neuron of Aplysia. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3442–3446. doi: 10.1073/pnas.79.11.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestreri E., Cioni P., Romagnoli A., Bernini S., Fissi A., Felicioli R. Mechanism of polyamine inhibition of a leaf protease. Arch Biochem Biophys. 1987 Jun;255(2):460–463. doi: 10.1016/0003-9861(87)90415-2. [DOI] [PubMed] [Google Scholar]
- Beninati S., Piacentini M., Argento-Cerù M. P., Russo-Caia S., Autuori F. Presence of di- and polyamines covalently bound to protein in rat liver. Biochim Biophys Acta. 1985 Jul 26;841(1):120–126. doi: 10.1016/0304-4165(85)90281-8. [DOI] [PubMed] [Google Scholar]
- Birckbichler P. J., Orr G. R., Patterson M. K., Jr Differential transglutaminase distribution in normal rat liver and rat hepatoma. Cancer Res. 1976 Aug;36(8):2911–2914. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Canellakis Z. N., Bondy P. K., Infante A. A. Spermidine is bound to a unique protein in early sea urchin embryos. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7613–7615. doi: 10.1073/pnas.82.22.7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang D. M. Beta-adrenergic receptor internalization and processing: role of transglutaminase and lysosomes. Mol Cell Biochem. 1984;58(1-2):79–89. doi: 10.1007/BF00240607. [DOI] [PubMed] [Google Scholar]
- Delcros J. G., Roch A. M., Quash G. The competitive inhibition of tissue transglutaminase by alpha-difluoromethylornithine. FEBS Lett. 1984 Jun 11;171(2):221–226. doi: 10.1016/0014-5793(84)80492-5. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Chung S. I. Molecular and catalytic properties of transglutaminases. Adv Enzymol Relat Areas Mol Biol. 1973;38:109–191. doi: 10.1002/9780470122839.ch3. [DOI] [PubMed] [Google Scholar]
- Folk J. E. Transglutaminases. Annu Rev Biochem. 1980;49:517–531. doi: 10.1146/annurev.bi.49.070180.002505. [DOI] [PubMed] [Google Scholar]
- Haddox M. K., Russell D. H. Increased nuclear conjugated polyamines and transglutaminase during liver regeneration. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1712–1716. doi: 10.1073/pnas.78.3.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juprelle-Soret M., Wattiaux-De Coninck S., Wattiaux R. Presence of a transglutaminase activity in rat liver lysosomes. Eur J Cell Biol. 1984 Jul;34(2):271–274. [PubMed] [Google Scholar]
- Korner G., Bachrach U. Intracellular distribution of active and inactive transglutaminase in stimulated cultured C6 glioma cells. J Cell Physiol. 1987 Jan;130(1):44–50. doi: 10.1002/jcp.1041300108. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Campbell-Wilkes L. K., Cooperstein L. A filter paper assay for transamidating enzymes using radioactive amine substrates. Anal Biochem. 1972 Dec;50(2):623–631. doi: 10.1016/0003-2697(72)90074-7. [DOI] [PubMed] [Google Scholar]
- Lorand L., Conrad S. M. Transglutaminases. Mol Cell Biochem. 1984;58(1-2):9–35. doi: 10.1007/BF00240602. [DOI] [PubMed] [Google Scholar]
- Lorand L., Parameswaran K. N., Stenberg P., Tong Y. S., Velasco P. T., Jönsson N. A., Mikiver L., Moses P. Specificity of guinea pig liver transglutaminase for amine substrates. Biochemistry. 1979 May 1;18(9):1756–1765. doi: 10.1021/bi00576a019. [DOI] [PubMed] [Google Scholar]
- Paonessa G., Metafora S., Tajana G., Abrescia P., De Santis A., Gentile V., Porta R. Transglutaminase-mediated modifications of the rat sperm surface in vitro. Science. 1984 Nov 16;226(4676):852–855. doi: 10.1126/science.6149619. [DOI] [PubMed] [Google Scholar]
- Pohjanpelto P., Virtanen I., Hölttä E. Polyamine starvation causes disappearance of actin filaments and microtubules in polyamine-auxotrophic CHO cells. Nature. 1981 Oct 8;293(5832):475–477. doi: 10.1038/293475a0. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. Relation of protein synthesis and transglutaminase activity to formation of the cross-linked envelope during terminal differentiation of the cultured human epidermal keratinocyte. J Cell Biol. 1978 Mar;76(3):705–711. doi: 10.1083/jcb.76.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch A. M., Quash G., Ripoll H., Mardon M. Differences in polyamine availability and insertion into fibronectins released from normal and transformed cells. Biochem J. 1983 Jan 15;210(1):137–144. doi: 10.1042/bj2100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. H. Posttranslational modification of ornithine decarboxylase by its product putrescine. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1167–1172. doi: 10.1016/0006-291x(81)90741-5. [DOI] [PubMed] [Google Scholar]
- Schrode J., Folk J. E. Transglutaminase-catalyzed cross-linking through diamines and polyamines. J Biol Chem. 1978 Jul 25;253(14):4837–4840. [PubMed] [Google Scholar]
- Williams-Ashman H. G., Wilson J., Beil R. E., Lorand L. Transglutaminase reactions associated with the rat semen clotting system: modulation by macromolecular polyanions. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1192–1198. doi: 10.1016/0006-291x(77)91132-9. [DOI] [PubMed] [Google Scholar]